An Overview of Recent Progress in Engineering Three-Dimensional Scaffolds for Cultured Meat Production
Abstract
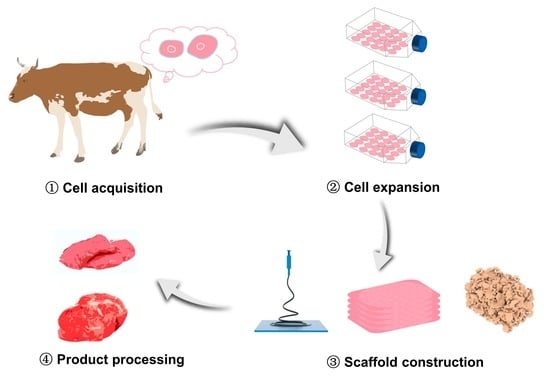
1. Introduction
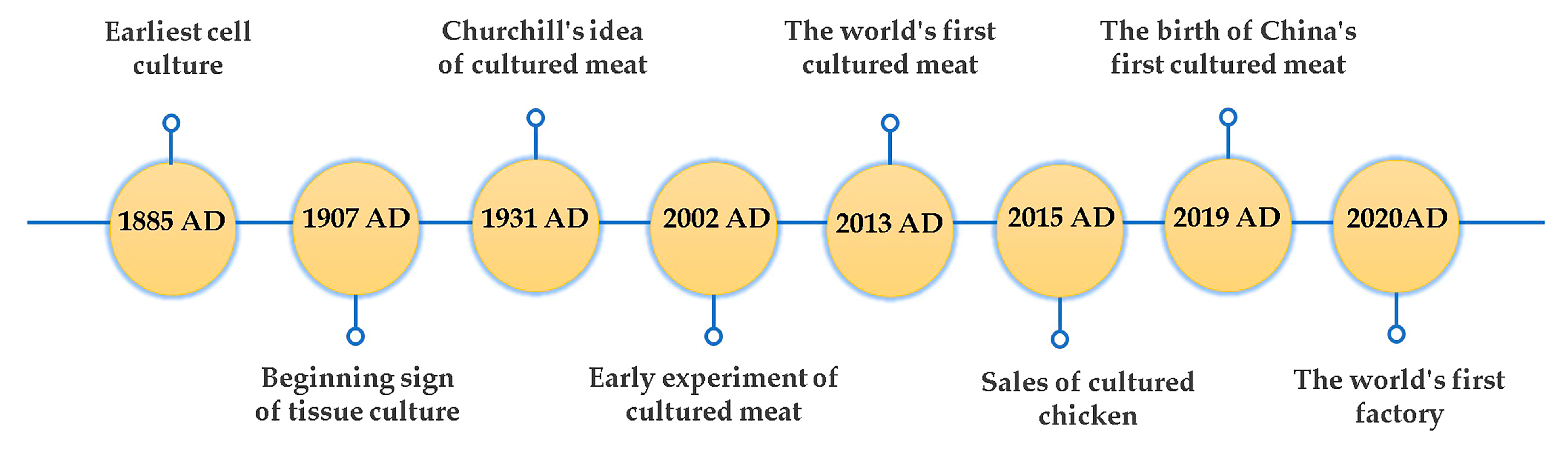
2. Historical Perspective of Cultured Meat
3. Scaffolding Biomaterials for Cultured Meat
3.1. Animal-Derived Biomaterials
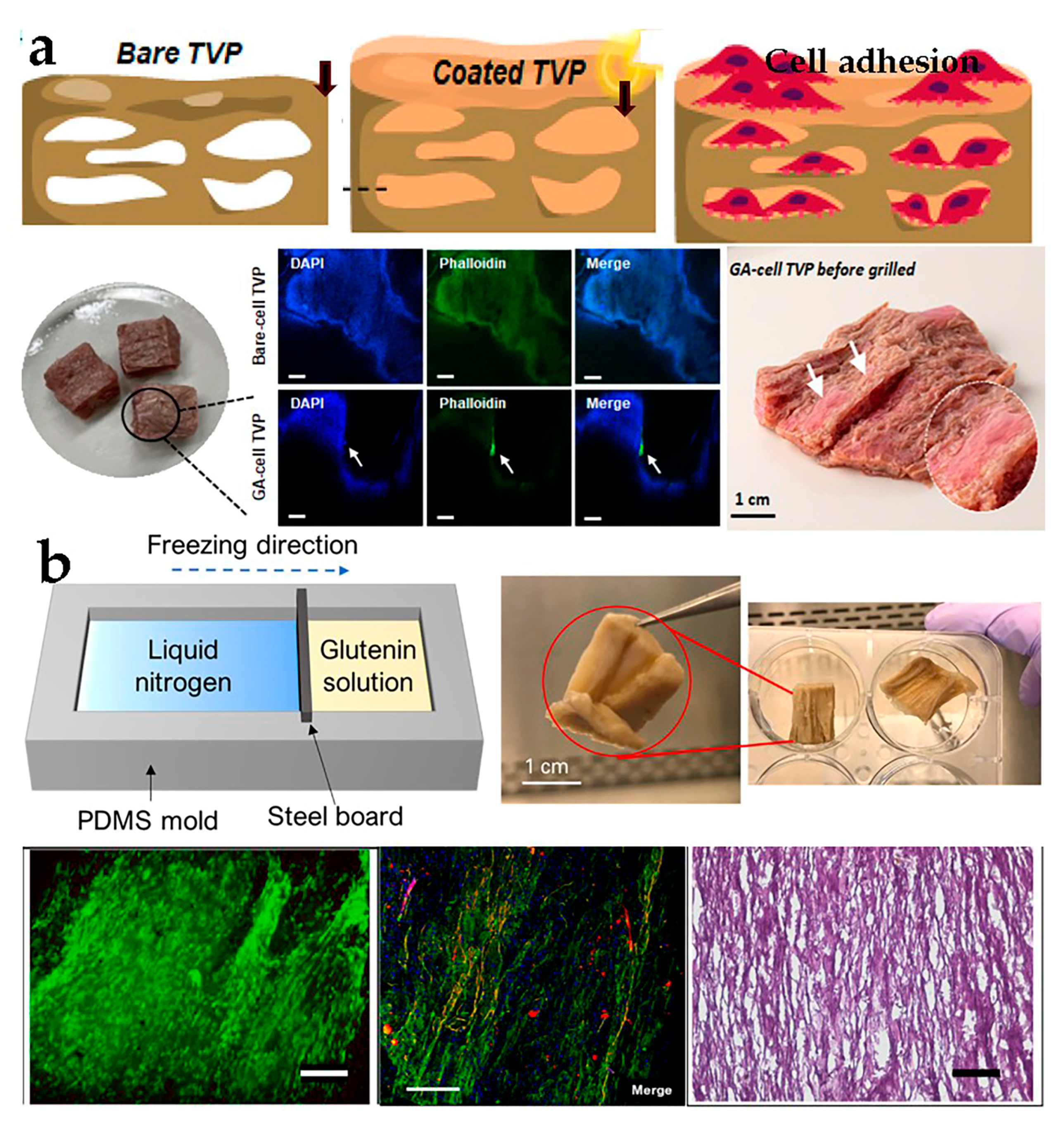
3.2. Plant-Derived Biomaterials
3.3. Synthetic Polymer Biomaterials
4. Three-Dimensional Scaffold Technologies
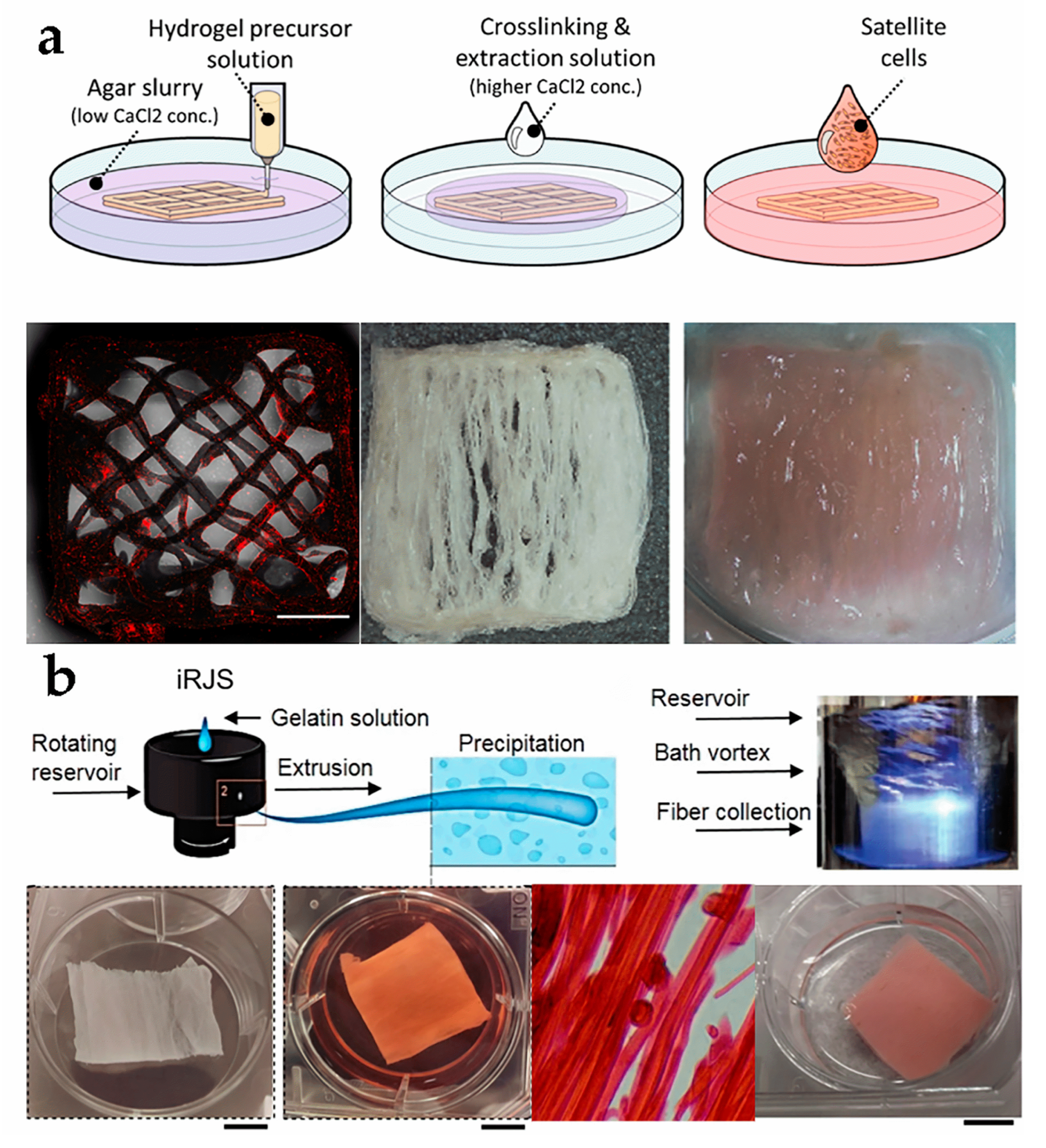
4.1. Three-Dimensional Printing
4.2. Electrospinning
4.3. Extrusion
4.4. Directional Freezing
4.5. Electric Field
4.6. Cell Microcarriers
4.7. Plant Tissue Decellularization
4.8. Cell Sheet Engineering
5. Potential Benefits and Challenges of the Cultured Meat
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; Food and Agricultural Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.N. Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elem. Sci Anth. 2018, 6, 52. [Google Scholar] [CrossRef]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.J.; Wassenaar, T.; Castel, V.; Rosales, M.; De haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agricultural Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Melzener, L.; Verzijden, K.E.; Buijs, A.J.; Post, M.J.; Flack, J.E. Cultured beef: From small biopsy to substantial quantity. J. Sci. Food Agric. 2021, 101, 7–14. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Pluhar, E.B. Meat and Morality: Alternatives to Factory Farming. J. Agric. Environ. Ethics 2010, 23, 455–468. [Google Scholar] [CrossRef]
- Levi, S.; Yen, F.; Baruch, L.; Machluf, M. Scaffolding technologies for the engineering of cultured meat: Towards a safe, sustainable, and scalable production. Trends Food Sci. Technol. 2022, 126, 13–25. [Google Scholar] [CrossRef]
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; de Graaf, C. Replacement of meat by meat substitutes. A survey on person- and product-related factors in consumer acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; de Mattos, M.J.T. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef]
- Lee, M.; Park, S.; Choi, B.; Kim, J.; Choi, W.; Jeong, I.; Han, D.; Koh, W.; Hong, J. Tailoring a Gelatin/Agar Matrix for the Synergistic Effect with Cells to Produce High-Quality Cultured Meat. ACS Appl. Mater. Interfaces 2022, 14, 38235–38245. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, e2102908. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Badar, I.H.; Xia, X.; Kong, B.; Chen, Q. Prospects of artificial meat: Opportunities and challenges around consumer acceptance. Trends Food Sci. Technol. 2021, 116, 434–444. [Google Scholar] [CrossRef]
- Dilworth, T.; McGregor, A. Moral Steaks? Ethical Discourses of In Vitro Meat in Academia and Australia. J. Agric. Environ. Ethics 2015, 28, 85–107. [Google Scholar] [CrossRef]
- Wilks, M.; Phillips, C.J.C. Attitudes to in vitro meat: A survey of potential consumers in the United States. PLoS ONE 2017, 12, e0171904. [Google Scholar] [CrossRef]
- Mancini, M.C.; Antonioli, F. Exploring consumers’ attitude towards cultured meat in Italy. Meat Sci. 2019, 150, 101–110. [Google Scholar] [CrossRef]
- Magdalena, J.-S. History of Cell Culture. In New Insights into Cell Culture Technology; Gowder, S.J.T., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 1. [Google Scholar]
- Ambrose, C.T. An amended history of tissue culture: Concerning Harrison, Burrows, Mall, and Carrel. J. Med. Biogr. 2017, 27, 95–102. [Google Scholar] [CrossRef]
- de Vries, R.B.M.; Leenaars, M.; Tra, J.; Huijbregtse, R.; Bongers, E.; Jansen, J.A.; Gordijn, B.; Ritskes-Hoitinga, M. The potential of tissue engineering for developing alternatives to animal experiments: A systematic review. J. Tissue Eng. Regen. Med. 2015, 9, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Churchill, W. Fifty Years Hence. In Teaching American History; The Strand Magazine: London, UK, 1931; pp. 24–27. [Google Scholar]
- Benjaminson, M.A.; Gilchriest, J.A.; Lorenz, M. In vitro edible muscle protein production system (mpps): Stage 1, fish. Acta Astronaut. 2002, 51, 879–889. [Google Scholar] [CrossRef]
- Post, M.J. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef]
- Zhou, G.; Ding, S.; Xu, X. Progress and Challenges in Cultured Meat. J. Chin. Inst. Food Sci. Technol. 2020, 20, 1–11. [Google Scholar]
- Sharma, S.; Thind, S.S.; Kaur, A. In vitro meat production system: Why and how? J. Food Sci. Technol. 2015, 52, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguin, Y.; Sanchez, E.; Acevedo, C.A. Edible Scaffolds Based on Non-Mammalian Biopolymers for Myoblast Growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Chen, X.; Chen, Y.; Ding, S.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; et al. Chitosan-sodium alginate-collagen/gelatin three-dimensional edible scaffolds for building a structured model for cell cultured meat. Int. J. Biol. Macromol. 2022, 209, 668–679. [Google Scholar] [CrossRef]
- Verbeke, W.; Spranghers, T.; De Clercq, P.; De Smet, S.; Sas, B.; Eeckhout, M. Insects in animal feed: Acceptance and its determinants among farmers, agriculture sector stakeholders and citizens. Anim. Feed Sci. Technol. 2015, 204, 72–87. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Wu, P.; Zhao, Y.; Suo, X.; Xiao, A.; Ke, M.; He, X.; Tong, Z.; Chen, Y. Green fabrication of seedbed-like Flammulina velutipes polysaccharides–derived scaffolds accelerating full-thickness skin wound healing accompanied by hair follicle regeneration. Int. J. Biol. Macromol. 2021, 167, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, T.; Levenberg, S. Tissue Engineering for Clean Meat Production. Front. Sustain. Food Syst. 2019, 3, 46. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ramji, K.; Shah, R.N. Electrospun soy protein nanofiber scaffolds for tissue regeneration. J. Biomater. Appl. 2014, 29, 411–422. [Google Scholar] [CrossRef]
- Xiang, N.; Yuen, J.S.K., Jr.; Stout, A.J.; Rubio, N.R.; Chen, Y.; Kaplan, D.L. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials 2022, 285, 121543. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Zhu, H.; Li, C.; Song, W.; Ding, S.; Zhou, G. Production of cultured meat by culturing porcine smooth muscle cells in vitro with food grade peanut wire-drawing protein scaffold. Food Res. Int. 2022, 159, 111561. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Sun, C.; Lu, W.; Fang, Y. Anisotropic Food Structures and the Related Biomimic Fabrications. J. Chin. Inst. Food Sci. Technol. 2019, 19, 1–12. [Google Scholar]
- Allan, S.J.; Ellis, M.J.; De Bank, P.A. Decellularized grass as a sustainable scaffold for skeletal muscle tissue engineering. J. Biomed. Mater. Res. A 2021, 109, 2471–2482. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhao, J.; Liu, C.; Song, X.; Yang, D.; Zhu, K.; Wang, S.; Zhang, Y.S.; Chen, A. Highly Porous Microcarriers for Minimally Invasive In Situ Skeletal Muscle Cell Delivery. Small 2019, 15, e1901397. [Google Scholar] [CrossRef]
- Mai, D.J.; Schroeder, C.M. 100th Anniversary of Macromolecular Science Viewpoint: Single-Molecule Studies of Synthetic Polymers. ACS Macro Lett. 2020, 9, 1332–1341. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission (CAC). General Standard for Food Additives; CAC: Rome, Italy, 2021. [Google Scholar]
- Ianovici, I.; Zagury, Y.; Redenski, I.; Lavon, N.; Levenberg, S. 3D printable plant protein-enriched scaffolds for cultivated meat development. Biomaterials 2022, 284, 121487. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 2021, 12, 5059. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle tissue engineering in fibrous gelatin: Implications for meat analogs. NPJ Sci. Food 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liao, L.; Zhang, X.; Chen, X.; Peng, S.; Zou, L.; Liang, R.; Liu, W. Electric field-driven fabrication of anisotropic hydrogels from plant proteins: Microstructure, gel performance and formation mechanism. Food Hydrocoll. 2023, 136, 108297. [Google Scholar] [CrossRef]
- Zernov, A.; Baruch, L.; Machluf, M. Chitosan-collagen hydrogel microparticles as edible cell microcarriers for cultured meat. Food Hydrocoll. 2022, 129, 107632. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Ding, S.; Deng, L.; Zhang, Y.; Li, J.; Shi, Z.; Wu, Z.; Liang, K.; Yan, X.; et al. Engineered meatballs via scalable skeletal muscle cell expansion and modular micro-tissue assembly using porous gelatin micro-carriers. Biomaterials 2022, 287, 121615. [Google Scholar] [CrossRef]
- Norris, S.C.P.; Kawecki, N.S.; Davis, A.R.; Chen, K.K.; Rowat, A.C. Emulsion-templated microparticles with tunable stiffness and topology: Applications as edible microcarriers for cultured meat. Biomaterials 2022, 287, 121669. [Google Scholar] [CrossRef]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized spinach: An edible scaffold for laboratory-grown meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Thyden, R.; Perreault, L.R.; Jones, J.D.; Notman, H.; Varieur, B.M.; Patmanidis, A.A.; Dominko, T.; Gaudette, G.R. An Edible, Decellularized Plant Derived Cell Carrier for Lab Grown Meat. Appl. Sci. 2022, 12, 5155. [Google Scholar] [CrossRef]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. Engineering Murine Adipocytes and Skeletal Muscle Cells in Meat-like Constructs Using Self-Assembled Layer-by-Layer Biofabrication: A Platform for Development of Cultivated Meat. Cells Tissues Organs 2022, 211, 304–312. [Google Scholar] [CrossRef]
- Park, S.; Jung, S.; Heo, J.; Koh, W.G.; Lee, S.; Hong, J. Chitosan/Cellulose-Based Porous Nanofilm Delivering C-Phycocyanin: A Novel Platform for the Production of Cost-Effective Cultured Meat. ACS Appl. Mater. Interfaces 2021, 13, 32193–32204. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Yan, W.; Lu, W.F.; Wang, C.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Del. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.; Reid, R.R. 3D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Forgacs, G. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, B.; Tucker, N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Yu, M.; Dong, R.; Yan, X.; Yu, G.; You, M.; Ning, X.; Long, Y. Recent Advances in Needleless Electrospinning of Ultrathin Fibers: From Academia to Industrial Production. Macromol. Mater. Eng. 2017, 302, 1700002. [Google Scholar] [CrossRef]
- Langelaan, M.L.P.; Boonen, K.J.M.; Polak, R.B.; Baaijens, F.P.T.; Post, M.J.; van der Schaft, D.W.J. Meet the new meat: Tissue engineered skeletal muscle. Trends Food Sci. Technol. 2010, 21, 59–66. [Google Scholar] [CrossRef]
- Uehara, T.M.; Paino, I.M.M.; Santos, F.A.; Scagion, V.P.; Correa, D.S.; Zucolotto, V. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym. Adv. Technol. 2020, 31, 1437–1443. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Nanostructured Biomaterials for Regeneration. Adv. Funct. Mater. 2008, 18, 3566–3582. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Qian, D.; Chen, L.; Mo, X.; Wang, L.; Wang, Y.; Cui, W. Electrospun fibrous sponge via short fiber for mimicking 3D ECM. J. Nanobiotechnol. 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.H.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Hwang, Y.; Joo, S. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Shi, A.; Hu, H.; Wang, Q. Research advances on food extrusion equipment, technology and its mechanism. Trans. Chin. Soc. Agric. Eng. 2017, 33, 275–283. [Google Scholar]
- Jones, O.G. Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogs. Curr. Opin. Food Sci. 2016, 7, 7–13. [Google Scholar] [CrossRef]
- Rehrah, D.; Ahmedna, M.; Goktepe, I.; Yu, J. Extrusion parameters and consumer acceptability of a peanut-based meat analogue. Int. J. Food Sci. Technol. 2009, 44, 2075–2084. [Google Scholar] [CrossRef]
- Yao, G.; Liu, K.; Hsieh, F. A New Method for Characterizing Fiber Formation in Meat Analogs during High-moisture Extrusion. J. Food Sci. 2006, 69, 303–307. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Las Heras, K.; Santos-Vizcaino, E.; Garrido, T.; Borja Gutierrez, F.; Aguirre, J.J.; de la Caba, K.; Guerrero, P.; Igartua, M.; Hernandez, R.M. Soy protein and chitin sponge-like scaffolds: From natural by-products to cell delivery systems for biomedical applications. Green Chem. 2020, 22, 3445–3460. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Ghalkhani, M.; Maleki, H. Directional Freeze-Casting: A Bioinspired Method to Assemble Multifunctional Aligned Porous Structures for Advanced Applications. Adv. Eng. Mater. 2020, 22, 2000033. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Wang, Z.; Gao, H.; Chen, S.; Cong, Y.; Yang, L.; Wen, S.; Cheng, D.; He, J.; et al. Radially Porous Nanocomposite Scaffolds with Enhanced Capability for Guiding Bone Regeneration In Vivo. Adv. Funct. Mater. 2022, 32, 2110931. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Leisk, G.G.; Lo, T.J.; Yucel, T.; Lu, Q.; Kaplan, D.L. Electrogelation for Protein Adhesives. Adv. Mater. 2010, 22, 711–715. [Google Scholar] [CrossRef]
- Kojic, N.; Panzer, M.J.; Leisk, G.G.; Raja, W.K.; Kojic, M.; Kaplan, D.L. Ion electrodiffusion governs silk electrogelation. Soft Matter 2012, 8, 6897–6905. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z. Curcumin-loaded silk fibroin e-gel scaffolds for wound healing applications. Mater. Technol. 2018, 33, 276–287. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Lee, K.; Nienow, A.W.; Thomas, R.J.; Smith, M.; Thomas, C.R. Expansion of human mesenchymal stem cells on microcarriers. Biotechnol. Lett. 2011, 33, 2325–2335. [Google Scholar] [CrossRef]
- Park, Y.; Chen, Y.; Ordovas, L.; Verfaillie, C.M. Hepatic differentiation of human embryonic stem cells on microcarriers. J. Biotechnol. 2014, 174, 39–48. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Rodrigues, C.A.V.; Gomes, A.R.; Vieira, S.F.; Badenes, S.M.; Diogo, M.M.; Cabral, J.M.S. Dissolvable Microcarriers Allow Scalable Expansion And Harvesting Of Human Induced Pluripotent Stem Cells Under Xeno-Free Conditions. Biotechnol. J. 2019, 14, 1800461. [Google Scholar] [CrossRef] [PubMed]
- Mccrary, M.W.; Bousalis, D.; Mobini, S.; Song, Y.H.; Schmidt, C.E.J.A.B. Decellularized Tissues as Platforms for In Vitro Modeling of Healthy and Diseased Tissues. Acta Biomater. 2020, 111, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cavid, A. Laterally positioned flap along with acellular dermal matrix graft in the management of maxillary localized recessions. Clin. Oral Investig. 2019, 23, 595–601. [Google Scholar]
- Hickey, R.J.; Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Customizing the Shape and Microenvironment Biochemistry of Biocompatible Macroscopic Plant-Derived Cellulose Scaffolds. ACS Biomater. Sci. Eng. 2018, 4, 3726–3736. [Google Scholar] [CrossRef]
- Joshi, V.; Kumar, S. Meat Analogues: Plant based alternatives to meat products—A review. Int. J. Food Ferm. Technol. 2016, 5, 107–119. [Google Scholar] [CrossRef]
- Fraser, R.Z.; Shitut, M.; Agrawal, P.; Mendes, O.; Klapholz, S. Safety Evaluation of Soy Leghemoglobin Protein Preparation Derived From Pichia pastoris, Intended for Use as a Flavor Catalyst in Plant-Based Meat. Int. J. Toxicol. 2018, 37, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Mireşan, V.; Răducu, C.; Ihuţ, A.; Uiuiu, P.; Pop, D.; Neacşu, A.; Cenariu, M.; Groza, I. Can Cultured Meat Be an Alternative to Farm Animal Production for a Sustainable and Healthier Lifestyle? Front. Nutr. 2021, 8, 749298. [Google Scholar] [CrossRef]
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef]
- Treich, N. Cultured Meat: Promises and Challenges. Environ. Resour. Econ. 2021, 79, 33–61. [Google Scholar] [CrossRef] [PubMed]


| Types of Scaffold Biomaterials | Advantages | Disadvantages |
|---|---|---|
| Animal-derived biomaterials | Excellent biocompatibility Promoting cell growth Nutrition rich | Relatively high production costs Environmental burden Unfriendly animal welfare |
| Plant-derived biomaterials | Healthy Sustainable Favorable nutritional value Natural 3D structure Relatively low cost Safety | Inferior scalability Poor tissue applicability |
| Synthetic polymer biomaterials | Multiple structures Excellent mechanical features | Potential toxicity Low nutritional value Slow degradation rate |
| Scaffold Technologies | Biomaterials | Cultured Cells | Innovative Points in Cultivating Meat | References |
|---|---|---|---|---|
| 3D printing | Plant protein, edible polysaccharides (seaweed salts, gelatin) | Bovine satellite cells, adipose-derived stem cells | Printing out ideal thickness and striped cultured meat products | [46,47] |
| Electrospinning | Gelatin, TG enzyme | Rabbit skeletal muscle cells, bovine aortic smooth muscle cells | Cultivated meat with certain fiber filaments and anisotropic structure | [48] |
| Extrusion | Soy protein | C2C12 muscle cells | Porous structure and texture similar to traditional meat | [13] |
| Directional freezing | Wheat gluten | BSCs | Anisotropic structure and mimic natural muscle fiber tissue | [39] |
| Electric field | Soy protein, polysaccharide | No cells involved | Anisotropic structure similar to traditional meat | [49] |
| Cell microcarriers | Chitosan, collagen, gelatin | C2C12, BSCs, rabbit smooth muscle cells, pig skeletal muscle cells | Similar sensory characteristics and nutritional value to traditional meat | [50,51,52] |
| Plant tissue decellularization | Natural plant tissue (spinach, broccoli floret) | BSCs | Natural plant vessels provide directional arrangement of cultured meat | [53,54] |
| Cell sheet engineering | Chitosan, cellulose | C2C12, 3T3-L1 | Cultivated meat with a multi-layer thickness and rich nutrition | [55,56] |
| Types of Meat | Advantages | Disadvantages |
|---|---|---|
| Cultured meat | Environmental resource efficiency Health and sustainability Non-animal protein utilization | Expensive production process Unsatisfied food sensory Lack of policies and regulations Challenge of large-scale production |
| Livestock meat | High nutritional value Better energy and mood Easy consumer acceptance | Resource unsustainability Health risks Potentially ethical issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zou, L.; Liu, W.; Chen, X. An Overview of Recent Progress in Engineering Three-Dimensional Scaffolds for Cultured Meat Production. Foods 2023, 12, 2614. https://doi.org/10.3390/foods12132614
Wang Y, Zou L, Liu W, Chen X. An Overview of Recent Progress in Engineering Three-Dimensional Scaffolds for Cultured Meat Production. Foods. 2023; 12(13):2614. https://doi.org/10.3390/foods12132614
Chicago/Turabian StyleWang, Yuan, Liqiang Zou, Wei Liu, and Xing Chen. 2023. "An Overview of Recent Progress in Engineering Three-Dimensional Scaffolds for Cultured Meat Production" Foods 12, no. 13: 2614. https://doi.org/10.3390/foods12132614
APA StyleWang, Y., Zou, L., Liu, W., & Chen, X. (2023). An Overview of Recent Progress in Engineering Three-Dimensional Scaffolds for Cultured Meat Production. Foods, 12(13), 2614. https://doi.org/10.3390/foods12132614