The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential
Abstract
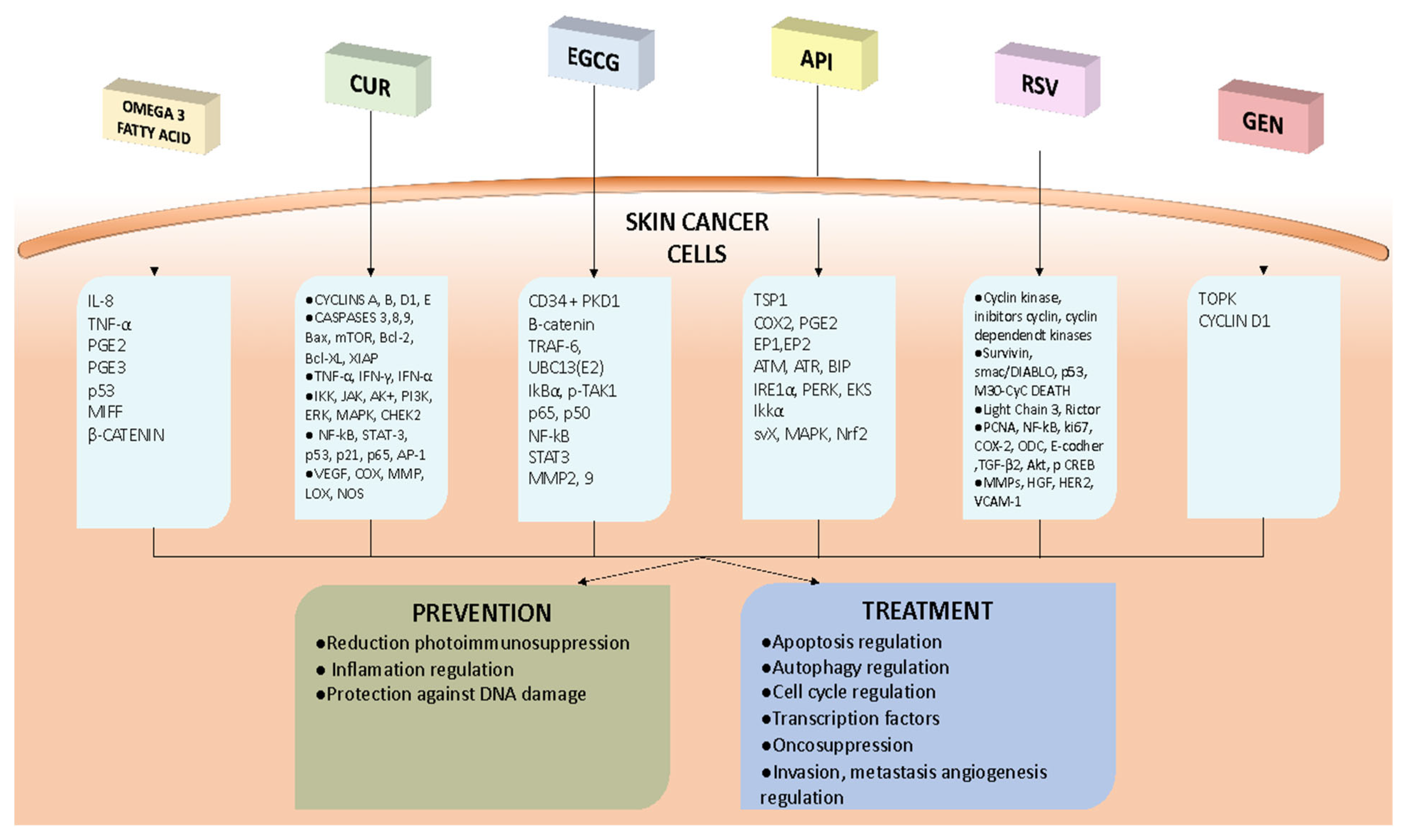
1. Introduction
- (1)
- clarify their mechanism of action;
- (2)
- and evaluate their possible use in the treatment and prevention of melanoma and NMSC.
2. Discussion
2.1. Fatty Acids and Skin Cancer
2.2. Dietary Polyphenols and Skin Cancer
2.2.1. Curcumin and Skin Cancer
2.2.2. Epigallocatechin Gallate and Skin Cancer
2.2.3. Apigenin and Skin Cancer
2.2.4. Resveratrol and Skin Cancer
2.2.5. Genistein and Skin Cancer
2.3. Limits of the Study
- The precise mechanism by which these substances act has not been established and several pathways have been proposed that seem to confirm this property.
- Due to the variability in the studies performed, succeeding in homogenizing the results is not simple.
- The wide variability in dosages, delivery systems, and combinations of compounds used in the different studies may create bias.
3. Conclusions
- Omega-3 fatty acids: these have been demonstrated to possess anti-tumor properties through the modulation of TNF-α, leading to a reduction in IL-8 expression and promoting a regulatory environment in the skin by reducing pro-inflammatory eicosanoids and increasing anti-inflammatory ones; oral ω-3 PUFAs appear to repeal photoimmunosuppression in human skin, giving support to their chemopreventive action; DHA inhibits melanoma cell growth, migration, and invasion, respectively, by increasing nuclear beta-catenin content and expression; to reduce exposure to environmental contaminants, it is recommended to consume fish in moderation and choose lower-mercury varieties (such as salmon, sardines, and trout).
- Curcumin: this plays an antitumor role in the skin by inhibiting cancer cell growth (inhibiting STAT3 and the G2/M checkpoint block,) and promoting apoptosis (modification of p53, reduction in Bcl-2, and increases in Bax, caspase-3, and -9) in both melanoma and NMSC; curcumin also appears to have a chemopreventive role in skin cancers and seems to have an adjuvant action in the photothermal therapy and sonodynamic therapy of melanoma by increasing ROS production; the challenge of the low bioavailability of curcumin can be overcome using liposomes, nanoparticles, and nanopattern films or by the use of analogs (e.g., DMC and DM-1); a combination of curcumin and tocopherol or siRNA seems to potentiate its antitumor action.
- Epigallocatechin gallate: this has shown potential in inhibiting skin tumors, inactivating β-catenin, and suppressing the TRAF6 E3 ubiquitin ligase activity in melanoma cells; the association of EGCG and metformin has a synergistic effect on melanoma cells; EGCG, encapsulated in different vesicular systems, also inhibits epidermoid carcinoma cell lines and reduces tumor sizes in mice.
- Apigenin: this could have a chemopreventive function, target TSP1, reduce the synthesis of COX-2, PGE2, EP1, and EP2, and restore the inhibition of autophagy in UVB-exposed human keratinocytes; apigenin could have a role in NMSC treatment, since it can regulate the mTOR pathway, inhibit IKKα, downregulate Srx, and reverse the hypermethylation of 15 CpG sites in the Nrf2 promoter; nanoparticles as carriers for apigenin represent a promising treatment for skin cancers, in particular NMSC.
- Resveratrol: this reduces cyclin D1 and increases cyclins A2, E1, Cdk1, and Cdk2, blocking tumor growth in melanoma; an important role is the promotion of apoptosis by resveratrol through the downregulation of Bcl-2 and FLIP; an inhibition of survivin by resveratrol appears to be implicated in the promotion of the apoptosis of both melanoma and NMSC cells; resveratrol could be a radiation sensitizer against melanoma; the use of nanostructured lipid carrier gels, solid lipid nanoparticles, and liposomes improves the release and bioavailability of resveratrol.
- Genistein: orobol (a metabolite of GEN) has been found to reduce the development of chronic, solar-simulated, light-induced skin cancer and to have therapeutic efficacy in the early stages of tumors by binding to TOPK, an oncogenic protein; a biphasic action of GEN on cyclin D1 expression was found to be associated with the suppression of cell proliferation and subsequent apoptosis in malignant melanoma cells; GEN has a low bioavailability when administered systemically, but NE formulations can improve the dermal delivery and efficacy of natural compounds such as GEN for skin photoprotection applications.
- Despite the limitations of the currently available data, the therapeutic role of these nutraceuticals seems clear. Further studies are needed to better understand how they can be exploited as an additional therapeutic tool.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bull, C.; Fenech, M. Genome-Health Nutrigenomics and Nutrigenetics: Nutritional Requirements or “nutriomes” for Chromosomal Stability and Telomere Maintenance at the Individual Level. Proc. Nutr. Soc. 2008, 67, 146–156. [Google Scholar] [CrossRef]
- Gordon, R. Skin Cancer: An Overview of Epidemiology and Risk Factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Vaccaro, F.; Irrera, N.; Borgia, F.; Li Pomi, F.; Squadrito, F.; Vaccaro, M. Wnt Signaling Pathways: From Inflammation to Non-Melanoma Skin Cancers. Int. J. Mol. Sci. 2023, 24, 1575. [Google Scholar] [CrossRef]
- Pomi, F.L.; Borgia, F.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Role of HMGB1 in Cutaneous Melanoma: State of the Art. Int. J. Mol. Sci. 2022, 23, 9327. [Google Scholar] [CrossRef]
- Borgia, F.; Pomi, F.L.; Alessandrello, C.; Vaccaro, M.; Gangemi, S. Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases. J. Clin. Med. 2023, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Tonacci, A.; Bertino, L.; Casciaro, M.; Borgia, F.; Gangemi, S. The Role of Oxidative Stress in the Biology of Melanoma: A Systematic Review. Pathol. Res. Pract. 2019, 215, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Irrera, N.; Cutroneo, G.; Rizzo, G.; Vaccaro, F.; Anastasi, G.P.; Borgia, F.; Cannavò, S.P.; Altavilla, D.; Squadrito, F. Differential Expression of Nitric Oxide Synthase Isoforms NNOS and INOS in Patients with Non-Segmental Generalized Vitiligo. Int. J. Mol. Sci. 2017, 18, 2533. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Cicero, N.; Gangemi, S. Alopecia Areata: A Review of the Role of Oxidative Stress, Possible Biomarkers, and Potential Novel Therapeutic Approaches. Antioxidants 2023, 12, 135. [Google Scholar] [CrossRef]
- Borgia, F.; Custurone, P.; Peterle, L.; Pioggia, G.; Guarneri, F.; Gangemi, S. Involvement of MicroRNAs as a Response to Phototherapy and Photodynamic Therapy: A Literature Review. Antioxidants 2021, 10, 1310. [Google Scholar] [CrossRef]
- Borgia, F.; Pomi, F.L.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Gordon, L.G.; Leung, W.; Johns, R.; McNoe, B.; Lindsay, D.; Merollini, K.M.D.; Elliott, T.M.; Neale, R.E.; Olsen, C.M.; Pandeya, N.; et al. Estimated Healthcare Costs of Melanoma and Keratinocyte Skin Cancers in Australia and Aotearoa New Zealand in 2021. Int. J. Environ. Res. Public Health 2022, 19, 3178. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G. Nutraceuticals and Functional Foods: Introduction and Meaning. Nutrition 2000, 16, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Fumia, A.; Cicero, N.; Gitto, M.; Nicosia, N.; Alesci, A. Role of Nutraceuticals on Neurodegenerative Diseases: Neuroprotective and Immunomodulant Activity. Nat. Prod. Res. 2022, 36, 5916–5933. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Lauriano, E.R.; Fumia, A.; Irrera, N.; Mastrantonio, E.; Vaccaro, M.; Gangemi, S.; Santini, A.; Cicero, N.; Pergolizzi, S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules 2022, 27, 1953. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Custurone, P.; Irrera, N.; Borgia, F.; Vaccaro, F.; Squadrito, F.; Vaccaro, M. Vitiligo and Mental Health: Natural Compounds’ Usefulness. Antioxidants 2023, 12, 176. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an Anticancer Agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Pomi, F.L.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus Officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus Officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- Li, W.; Peng, C.; Zhaojie, L.; Wei, W. Chemopreventive and Therapeutic Properties of Anthocyanins in Breast Cancer: A Comprehensive Review. Nutr. Res. 2022, 107, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.-Y.; Fang, X. Fatty Acid Oxidation: An Emerging Facet of Metabolic Transformation in Cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 Fatty Acids in Obesity and Metabolic Syndrome: A Mechanistic Update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of Adipose Tissue Inflammation by Bioactive Food Compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Sohn, K.H.; Rhee, S.H.; Lee, S.W.; Hunt, J.D.; Hwang, D.H. Suppression of Tumor Cell Growth Both in Nude Mice and in Culture by N-3 Polyunsaturated Fatty Acids: Mediation through Cyclooxygenase-Independent Pathways. Cancer Res. 2001, 61, 1386–1391. [Google Scholar] [CrossRef]
- Anti, M.; Marra, G.; Armelao, F.; Bartoli, G.M.; Ficarelli, R.; Percesepe, A.; De Vitis, I.; Maria, G.; Sofo, L.; Rapaccini, G.L. Effect of Omega-3 Fatty Acids on Rectal Mucosal Cell Proliferation in Subjects at Risk for Colon Cancer. Gastroenterology 1992, 103, 883–891. [Google Scholar] [CrossRef]
- Storey, A.; McArdle, F.; Friedmann, P.S.; Jackson, M.J.; Rhodes, L.E. Eicosapentaenoic Acid and Docosahexaenoic Acid Reduce UVB- and TNF-Alpha-Induced IL-8 Secretion in Keratinocytes and UVB-Induced IL-8 in Fibroblasts. J. Investig. Dermatol. 2005, 124, 248–255. [Google Scholar] [CrossRef]
- Rhodes, L.E.; Shahbakhti, H.; Azurdia, R.M.; Moison, R.M.W.; Steenwinkel, M.-J.S.T.; Homburg, M.I.; Dean, M.P.; McArdle, F.; van Henegouwen, G.M.J.B.; Epe, B.; et al. Effect of Eicosapentaenoic Acid, an Omega-3 Polyunsaturated Fatty Acid, on UVR-Related Cancer Risk in Humans. An Assessment of Early Genotoxic Markers. Carcinogenesis 2003, 24, 919–925. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Massey, K.A.; Bennett, S.P.; Al-Aasswad, N.M.; Roshdy, K.; Gibbs, N.K.; Friedmann, P.S.; Nicolaou, A.; Rhodes, L.E. Randomized Controlled Trial of Oral Omega-3 PUFA in Solar-Simulated Radiation-Induced Suppression of Human Cutaneous Immune Responses. Am. J. Clin. Nutr. 2013, 97, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Rhodes, L.E.; Al-Aasswad, N.M.I.; Massey, K.A.; Nicolaou, A. Impact of EPA Ingestion on COX- and LOX-Mediated Eicosanoid Synthesis in Skin with and without a pro-Inflammatory UVR Challenge--Report of a Randomised Controlled Study in Humans. Mol. Nutr. Food Res. 2014, 58, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Gibbs, N.K.; Costello, P.; Bennett, S.P.; Massey, K.A.; Friedmann, P.S.; Nicolaou, A.; Rhodes, L.E. Effect of Oral Eicosapentaenoic Acid on Epidermal Langerhans Cell Numbers and PGD(2) Production in UVR-Exposed Human Skin: A Randomised Controlled Study. Exp. Dermatol. 2016, 25, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Zinzi, A.; Vasconcelos, R.O.; Fasano, E.; Riillo, M.G.; Celleno, L.; Trombino, S.; Cassano, R.; Calviello, G. Role of β-Catenin Signaling in the Anti-Invasive Effect of the Omega-3 Fatty Acid DHA in Human Melanoma Cells. J. Dermatol. Sci. 2016, 84, 149–159. [Google Scholar] [CrossRef]
- Miura, K.; Vail, A.; Chambers, D.; Hopkins, P.M.; Ferguson, L.; Grant, M.; Rhodes, L.E.; Green, A.C. Omega-3 Fatty Acid Supplement Skin Cancer Prophylaxis in Lung Transplant Recipients: A Randomized, Controlled Pilot Trial. J. Heart Lung Transplant. 2019, 38, 59–65. [Google Scholar] [CrossRef]
- Donat-Vargas, C.; Berglund, M.; Glynn, A.; Wolk, A.; Åkesson, A. Dietary Polychlorinated Biphenyls, Long-Chain n-3 Polyunsaturated Fatty Acids and Incidence of Malignant Melanoma. Eur. J. Cancer 2017, 72, 137–143. [Google Scholar] [CrossRef]
- Wallingford, S.C.; van As, J.A.; Hughes, M.C.; Ibiebele, T.I.; Green, A.C.; van der Pols, J.C. Intake of Omega-3 and Omega-6 Fatty Acids and Risk of Basal and Squamous Cell Carcinomas of the Skin: A Longitudinal Community-Based Study in Australian Adults. Nutr. Cancer 2012, 64, 982–990. [Google Scholar] [CrossRef]
- Li, Y.; Liao, L.M.; Sinha, R.; Zheng, T.; Vance, T.M.; Qureshi, A.A.; Cho, E. Fish Intake and Risk of Melanoma in the NIH-AARP Diet and Health Study. Cancer Causes Control 2022, 33, 921–928. [Google Scholar] [CrossRef]
- Oh, C.C.; Jin, A.-Z.; Yuan, J.-M.; Koh, W.-P. Fish Intake and Risk of Nonmelanoma Skin Cancer in a Chinese Population: The Singapore Chinese Health Study. Clin. Exp. Dermatol. 2020, 45, 461–463. [Google Scholar] [CrossRef]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Russo, G.L.; Tedesco, I.; Daglia, M.; Orhan, I.E.; Nabavi, S.F.; Bishayee, A.; Nagulapalli Venkata, K.C.; Abdollahi, M.; Hajheydari, Z. Curcumin and Melanoma: From Chemistry to Medicine. Nutr. Cancer 2018, 70, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Zhang, X.; Hazarika, P.; Aggarwal, B.B.; Duvic, M. Curcumin Selectively Induces Apoptosis in Cutaneous T-Cell Lymphoma Cell Lines and Patients’ PBMCs: Potential Role for STAT-3 and NF-KappaB Signaling. J. Investig. Dermatol. 2010, 130, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Hassanian, S.M.; Mohammadzadeh, E.; ShahidSales, S.; Maftouh, M.; Fayazbakhsh, H.; Khazaei, M.; Avan, A. Therapeutic Potential of Curcumin in Treatment of Pancreatic Cancer: Current Status and Future Perspectives. J. Cell. Biochem. 2017, 118, 1634–1638. [Google Scholar] [CrossRef]
- Malathi, S.; Pavithra, P.S.; Sridevi, S.; Verma, R.S. Fabrication of Nanopatterned PLGA Films of Curcumin and TPGS for Skin Cancer. Int. J. Pharm. 2020, 578, 119100. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Venuganti, V.V.K. Co-Delivery of Curcumin and STAT3 SiRNA Using Deformable Cationic Liposomes to Treat Skin Cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef]
- Wu, J.; Lu, W.-Y.; Cui, L.-L. Inhibitory Effect of Curcumin on Invasion of Skin Squamous Cell Carcinoma A431 Cells. Asian Pac. J. Cancer Prev. 2015, 16, 2813–2818. [Google Scholar] [CrossRef]
- Ghazaeian, M.; Khorsandi, K.; Hosseinzadeh, R.; Naderi, A.; Abrahamse, H. Curcumin-Silica Nanocomplex Preparation, Hemoglobin and DNA Interaction and Photocytotoxicity against Melanoma Cancer Cells. J. Biomol. Struct. Dyn. 2021, 39, 6606–6616. [Google Scholar] [CrossRef]
- Phillips, J.; Moore-Medlin, T.; Sonavane, K.; Ekshyyan, O.; McLarty, J.; Nathan, C.-A.O. Curcumin Inhibits UV Radiation-Induced Skin Cancer in SKH-1 Mice. Otolaryngol. Head Neck Surg. 2013, 148, 797–803. [Google Scholar] [CrossRef]
- Tremmel, L.; Rho, O.; Slaga, T.J.; DiGiovanni, J. Inhibition of Skin Tumor Promotion by TPA Using a Combination of Topically Applied Ursolic Acid and Curcumin. Mol. Carcinog. 2019, 58, 185–195. [Google Scholar] [CrossRef]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR Triggered Liposome Gold Nanoparticles Entrapping Curcumin as in Situ Adjuvant for Photothermal Treatment of Skin Cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Soratijahromi, E.; Vais, R.D.; Sattarahmady, N. Phototherapy and Sonotherapy of Melanoma Cancer Cells Using Nanoparticles of Selenium-Polyethylene Glycol-Curcumin as a Dual-Mode Sensitizer. J. Biomed. Phys. Eng. 2020, 10, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, P.; Yang, H.; Ge, Y.; Xin, Y. Effects of Demethoxycurcumin on the Viability and Apoptosis of Skin Cancer Cells. Mol. Med. Rep. 2017, 16, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.-J.; Jiang, G.; Li, L.-T.; Zheng, J.-N. Curcumin Induces Apoptosis through Mitochondrial Pathway and Caspases Activation in Human Melanoma Cells. Mol. Biol. Rep. 2015, 42, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Tian, Y.; Mei, Z.; Guo, G. Inhibition of Autophagy Enhances Curcumin United Light Irradiation-Induced Oxidative Stress and Tumor Growth Suppression in Human Melanoma Cells. Sci. Rep. 2016, 6, 31383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin Induces Autophagy, Inhibits Proliferation and Invasion by Downregulating AKT/MTOR Signaling Pathway in Human Melanoma Cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M. Curcumin Induce DNA Damage and Apoptosis through Generation of Reactive Oxygen Species and Reducing Mitochondrial Membrane Potential in Melanoma Cancer Cells. Cell. Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Rong, X.; Moore-Medlin, T.; Ekshyyan, O.; Abreo, F.; Gu, X.; Nathan, C.-A.O. Photopreventive Effect and Mechanism of AZD4547 and Curcumin C3 Complex on UVB-Induced Epidermal Hyperplasia. Cancer Prev. Res. 2016, 9, 296–304. [Google Scholar] [CrossRef]
- Faião-Flores, F.; Suarez, J.A.Q.; Soto-Cerrato, V.; Espona-Fiedler, M.; Pérez-Tomás, R.; Maria, D.A. Bcl-2 Family Proteins and Cytoskeleton Changes Involved in DM-1 Cytotoxic Effect on Melanoma Cells. Tumour Biol. 2013, 34, 1235–1243. [Google Scholar] [CrossRef]
- Kim, E.J.; Lewis, D.J.; Dabaja, B.S.; Duvic, M. Curcumin for the Treatment of Tumor-Stage Mycosis Fungoides. Dermatol. Ther. 2017, 30, e12511. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective Toxicity of Catechin-a Natural Flavonoid towards Bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-Gallate (EGCG): Chemical and Biomedical Perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and Anti-Carcinogenic Activities of Tea Polyphenols. Arch. Toxicol. 2009, 83, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Sang, S.; Cheng, K.-H.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Peracetylated (-)-Epigallocatechin-3-Gallate (AcEGCG) Potently Prevents Skin Carcinogenesis by Suppressing the PKD1-Dependent Signaling Pathway in CD34+ Skin Stem Cells and Skin Tumors. Carcinogenesis 2013, 34, 1315–1322. [Google Scholar] [CrossRef]
- Sarkar, N.; Sinha, D. Epigallocatechin-3-Gallate Partially Restored Redox Homeostasis in Arsenite-Stressed Keratinocytes. J. Appl. Toxicol. 2018, 38, 1071–1080. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Scharadin, T.M.; Han, B.; Xu, W.; Eckert, R.L. The Bmi-1 Helix-Turn and Ring Finger Domains Are Required for Bmi-1 Antagonism of (-)-Epigallocatechin-3-Gallate Suppression of Skin Cancer Cell Survival. Cell. Signal. 2015, 27, 1336–1344. [Google Scholar] [CrossRef]
- Singh, T.; Katiyar, S.K. Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Induces Toxicity in Human Skin Cancer Cells by Targeting β-Catenin Signaling. Toxicol. Appl. Pharmacol. 2013, 273, 418–424. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-Gallate(EGCG) Suppresses Melanoma Cell Growth and Metastasis by Targeting TRAF6 Activity. Oncotarget 2016, 7, 79557–79571. [Google Scholar] [CrossRef]
- Xu, A.; Lee, J.; Zhao, Y.; Wang, Y.; Li, X.; Xu, P. Potential Effect of EGCG on the Anti-Tumor Efficacy of Metformin in Melanoma Cells. J. Zhejiang Univ. Sci. B 2021, 22, 548–562. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Modulation of MMP-2 and -9 Secretion by Cytokines, Inducers and Inhibitors in Human Melanoma A-2058 Cells. Oncol. Rep. 2017, 37, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-Epigallocatechin-3-Gallate Vesicular Systems for Prevention and Treatment of Skin Cancer: A Comprehensive Experimental Study with Preclinical Investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (-)-Epigallocatechin Gallate (EGCG)-Nanoethosomes as a Transdermal Delivery System for Docetaxel to Treat Implanted Human Melanoma Cell Tumors in Mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef]
- Mitrica, R.; Dumitru, I.; Ruta, L.L.; Ofiteru, A.M.; Farcasanu, I.C. The Dual Action of Epigallocatechin Gallate (EGCG), the Main Constituent of Green Tea, against the Deleterious Effects of Visible Light and Singlet Oxygen-Generating Conditions as Seen in Yeast Cells. Molecules 2012, 17, 10355–10369. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin Promotes Apoptosis, Inhibits Invasion and Induces Cell Cycle Arrest of T24 Human Bladder Cancer Cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Li, S.; Wang, X.; Liang, Z.; Xu, X.; Xu, X.; Hu, Z.; Lin, Y.; Chen, H.; et al. Apigenin Inhibits Migration and Invasion via Modulation of Epithelial Mesenchymal Transition in Prostate Cancer. Mol. Med. Rep. 2015, 11, 1004–1008. [Google Scholar] [CrossRef]
- Zhu, H.; Jin, H.; Pi, J.; Bai, H.; Yang, F.; Wu, C.; Jiang, J.; Cai, J. Apigenin Induced Apoptosis in Esophageal Carcinoma Cells by Destruction Membrane Structures. Scanning 2016, 38, 322–328. [Google Scholar] [CrossRef]
- Tong, X.; Mirzoeva, S.; Veliceasa, D.; Bridgeman, B.B.; Fitchev, P.; Cornwell, M.L.; Crawford, S.E.; Pelling, J.C.; Volpert, O. V Chemopreventive Apigenin Controls UVB-Induced Cutaneous Proliferation and Angiogenesis through HuR and Thrombospondin-1. Oncotarget 2014, 5, 11413–11427. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930–942. [Google Scholar] [CrossRef]
- Kiraly, A.J.; Soliman, E.; Jenkins, A.; Van Dross, R.T. Apigenin Inhibits COX-2, PGE2, and EP1 and Also Initiates Terminal Differentiation in the Epidermis of Tumor Bearing Mice. Prostaglandins Leukot Essent Fat. Acids 2016, 104, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kubik, J.; Waszak, Ł.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Adamczuk, K.; Michalczuk, M.; Korga-Plewko, A.; Józefczyk, A. Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea Castriferrei Borbás & Waisb Genus of Centaurea L. Molecules 2022, 27, 7537. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X. Inhibition of MTOR by Apigenin in UVB-Irradiated Keratinocytes: A New Implication of Skin Cancer Prevention. Cell. Signal. 2016, 28, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Xu, S.; Chen, H.; Chen, X.; Gu, H. Apigenin Restores Impairment of Autophagy and Downregulation of Unfolded Protein Response Regulatory Proteins in Keratinocytes Exposed to Ultraviolet B Radiation. J. Photochem. Photobiol. B 2019, 194, 84–95. [Google Scholar] [CrossRef] [PubMed]
- García-García, V.A.; Alameda, J.P.; Page, A.; Mérida-García, A.; Navarro, M.; Tejero, A.; Paramio, J.M.; García-Fernández, R.A.; Casanova, M.L. IKKα Induces Epithelial-Mesenchymal Changes in Mouse Skin Carcinoma Cells That Can Be Partially Reversed by Apigenin. Int. J. Mol. Sci. 2022, 23, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Zhang, Z.; Yin, M.; Chen, X.; Zhao, S.; Wu, L. Apigenin Induced Apoptosis by Downregulating Sulfiredoxin Expression in Cutaneous Squamous Cell Carcinoma. Oxid. Med. Cell. Longev. 2022, 2022, 8172866. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Su, Z.-Y.; Kong, A.-N.T. Apigenin Reactivates Nrf2 Anti-Oxidative Stress Signaling in Mouse Skin Epidermal JB6 P + Cells through Epigenetics Modifications. AAPS J. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Waheed, A.; Zameer, S.; Sultana, N.; Ali, A.; Aqil, M.; Sultana, Y.; Iqbal, Z. Engineering of QbD Driven and Ultrasonically Shaped Lyotropic Liquid Crystalline Nanoparticles for Apigenin in the Management of Skin Cancer. Eur. J. Pharm. Biopharm. 2022, 180, 269–280. [Google Scholar] [CrossRef]
- Dell’Anna, M.L.; Maresca, V.; Briganti, S.; Camera, E.; Falchi, M.; Picardo, M. Mitochondrial Impairment in Peripheral Blood Mononuclear Cells during the Active Phase of Vitiligo. J. Investig. Dermatol. 2001, 117, 908–913. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Strategic Formulation of Apigenin-Loaded PLGA Nanoparticles for Intracellular Trafficking, DNA Targeting and Improved Therapeutic Effects in Skin Melanoma in Vitro. Toxicol. Lett. 2013, 223, 124–138. [Google Scholar] [CrossRef]
- Jangdey, M.S.; Gupta, A.; Saraf, S. Fabrication, in-Vitro Characterization, and Enhanced in-Vivo Evaluation of Carbopol-Based Nanoemulsion Gel of Apigenin for UV-Induced Skin Carcinoma. Drug Deliv. 2017, 24, 1026–1036. [Google Scholar] [CrossRef]
- Jangdey, M.S.; Gupta, A.; Saraf, S.; Saraf, S. Development and Optimization of Apigenin-Loaded Transfersomal System for Skin Cancer Delivery: In Vitro Evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Jangdey, M.S.; Kaur, C.D.; Saraf, S. Efficacy of Concanavalin-A Conjugated Nanotransfersomal Gel of Apigenin for Enhanced Targeted Delivery of UV Induced Skin Malignant Melanoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef]
- Aziz, S.W.; Aziz, M.H. Protective Molecular Mechanisms of Resveratrol in UVR-Induced Skin Carcinogenesis. Photodermatol Photoimmunol. Photomed. 2018, 34, 35–41. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J.H. Potential of Resveratrol in Anticancer and Anti-Inflammatory Therapy. Nutr. Rev. 2008, 66, 445–454. [Google Scholar] [CrossRef]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Shankar, S.; Nall, D.; Tang, S.-N.; Meeker, D.; Passarini, J.; Sharma, J.; Srivastava, R.K. Resveratrol Inhibits Pancreatic Cancer Stem Cell Characteristics in Human and KrasG12D Transgenic Mice by Inhibiting Pluripotency Maintaining Factors and Epithelial-Mesenchymal Transition. PLoS ONE 2011, 6, e16530. [Google Scholar] [CrossRef]
- Yi, C.-O.; Jeon, B.T.; Shin, H.J.; Jeong, E.A.; Chang, K.C.; Lee, J.E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Resveratrol Activates AMPK and Suppresses LPS-Induced NF-ΚB-Dependent COX-2 Activation in RAW 264.7 Macrophage Cells. Anat. Cell Biol. 2011, 44, 194–203. [Google Scholar] [CrossRef]
- Kim, C.-W.; Hwang, K.-A.; Choi, K.-C. Anti-Metastatic Potential of Resveratrol and Its Metabolites by the Inhibition of Epithelial-Mesenchymal Transition, Migration, and Invasion of Malignant Cancer Cells. Phytomedicine 2016, 23, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; D’Amico, M.; De Luca, A.; Conforti, F.L.; Pezzi, V.; De Amicis, F. Resveratrol, Epigallocatechin Gallate and Curcumin for Cancer Therapy: Challenges from Their Pro-Apoptotic Properties. Life 2023, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical Nanostructured Lipid Carrier Gel of Quercetin and Resveratrol: Formulation, Optimization, in Vitro and Ex Vivo Study for the Treatment of Skin Cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of Quercetin and Resveratrol Co-Incorporated in Liposomes against Inflammatory/Oxidative Response Associated with Skin Cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Caldas, A.R.; Catita, J.; Machado, R.; Ribeiro, A.; Cerqueira, F.; Horta, B.; Medeiros, R.; Lúcio, M.; Lopes, C.M. Omega-3- and Resveratrol-Loaded Lipid Nanosystems for Potential Use as Topical Formulations in Autoimmune, Inflammatory, and Cancerous Skin Diseases. Pharmaceutics 2021, 13, 1202. [Google Scholar] [CrossRef]
- Bano, S.; Ahmed, F.; Khan, F.; Chaudhary, S.C.; Samim, M. Enhancement of the Cancer Inhibitory Effect of the Bioactive Food Component Resveratrol by Nanoparticle Based Delivery. Food Funct. 2020, 11, 3213–3226. [Google Scholar] [CrossRef]
- Palliyage, G.H.; Hussein, N.; Mimlitz, M.; Weeder, C.; Alnasser, M.H.A.; Singh, S.; Ekpenyong, A.; Tiwari, A.K.; Chauhan, H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021, 38, 851–871. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable Liposomes as Multidrug Carrier of Resveratrol and 5-Fluorouracil for Their Topical Delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef]
- Junco, J.J.; Mancha-Ramirez, A.; Malik, G.; Wei, S.-J.; Kim, D.J.; Liang, H.; Slaga, T.J. Ursolic Acid and Resveratrol Synergize with Chloroquine to Reduce Melanoma Cell Viability. Melanoma Res. 2015, 25, 103–112. [Google Scholar] [CrossRef]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular Analysis of Differential Antiproliferative Activity of Resveratrol, Epsilon Viniferin and Labruscol on Melanoma Cells and Normal Dermal Cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef]
- Fang, Y.; Bradley, M.J.; Cook, K.M.; Herrick, E.J.; Nicholl, M.B. A Potential Role for Resveratrol as a Radiation Sensitizer for Melanoma Treatment. J. Surg. Res. 2013, 183, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tang, L.; Chen, W.; Su, J.; Li, F.; Chen, X.; Wu, L. Resveratrol-Induced Apoptosis Is Associated with Regulating the MiR-492/CD147 Pathway in Malignant Melanoma Cells. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, W.; Liao, M.; Zhu, Y.; Liu, H.; Hao, C.; Liu, G.; Zhang, G.; Feng, H.; Ning, X.; et al. The Inhibition of Resveratrol to Human Skin Squamous Cell Carcinoma A431 Xenografts in Nude Mice. Fitoterapia 2013, 86, 84–91. [Google Scholar] [CrossRef]
- Habibie; Yokoyama, S.; Abdelhamed, S.; Awale, S.; Sakurai, H.; Hayakawa, Y.; Saiki, I. Survivin Suppression through STAT3/β-Catenin Is Essential for Resveratrol-Induced Melanoma Apoptosis. Int. J. Oncol. 2014, 45, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Barua, K.; Buseman, G.; Murphy, P.A. Soy Isoflavone Analysis: Quality Control and a New Internal Standard. Am. J. Clin. Nutr. 1998, 68, 1474S–1479S. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-Targeted Therapy of Cancer by Genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Pathak, M.A. Inhibition of Ultraviolet-Induced Formation of Reactive Oxygen Species, Lipid Peroxidation, Erythema and Skin Photosensitization by Polypodium Leucotomos. Photodermatol. Photoimmunol. Photomed. 1996, 12, 45–56. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.-E.; Zhang, T.; Shin, S.H.; Kim, B.-G.; Li, J.; Ma, X.; Lee, K.W.; Dong, Z. Orobol, 3′-Hydroxy-Genistein, Suppresses the Development and Regrowth of Cutaneous SCC. Biochem. Pharmacol. 2023, 209, 115415. [Google Scholar] [CrossRef]
- Aprilliantina, Y.S.; Novita, H.D.; Sadono, E.G.; Aldina, R. Protective Effect of Genistein on Cyclin D1 Expression in Malignant Ocular Melanoma Cells. Med. Arch. 2021, 75, 180–183. [Google Scholar] [CrossRef]
- Motlekar, N.; Khan, M.A.; Youan, B.-B.C. Preparation and Characterization of Genistein Containing Poly(Ethylene Glycol) Microparticles. J. Appl. Polym. Sci. 2006, 101, 2070–2078. [Google Scholar] [CrossRef]
- Brownlow, B.; Nagaraj, V.J.; Nayel, A.; Joshi, M.; Elbayoumi, T. Development and In Vitro Evaluation of Vitamin E-Enriched Nanoemulsion Vehicles Loaded with Genistein for Chemoprevention Against UVB-Induced Skin Damage. J. Pharm. Sci. 2015, 104, 3510–3523. [Google Scholar] [CrossRef] [PubMed]
- De Zampieri, A.L.T.C.; Ferreira, F.S.; Resende, E.C.; Gaeti, M.P.N.; Diniz, D.G.A.; Taveira, S.F.; Lima, E.M. Biodegradable Polymeric Nanocapsules Based on Poly(DL-Lactide) for Genistein Topical Delivery: Obtention, Characterization and Skin Permeation Studies. J. Biomed. Nanotechnol. 2013, 9, 527–534. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. https://doi.org/10.3390/foods12132629
Peterle L, Sanfilippo S, Borgia F, Li Pomi F, Vadalà R, Costa R, Cicero N, Gangemi S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods. 2023; 12(13):2629. https://doi.org/10.3390/foods12132629
Chicago/Turabian StylePeterle, Lucia, Serena Sanfilippo, Francesco Borgia, Federica Li Pomi, Rossella Vadalà, Rosaria Costa, Nicola Cicero, and Sebastiano Gangemi. 2023. "The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential" Foods 12, no. 13: 2629. https://doi.org/10.3390/foods12132629
APA StylePeterle, L., Sanfilippo, S., Borgia, F., Li Pomi, F., Vadalà, R., Costa, R., Cicero, N., & Gangemi, S. (2023). The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods, 12(13), 2629. https://doi.org/10.3390/foods12132629











