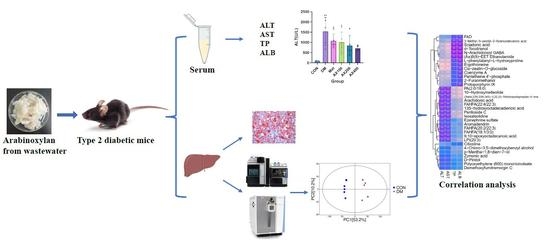
Effect of Arabinoxylan from Wastewater Generated during Vital Wheat Gluten Production on Liver Metabolism in Type 2 Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of AX
2.2. Animal Experiment
2.3. Sample Collection
2.4. Serum Liver Functional Indicators Analysis
2.5. Histological Analysis
2.6. Metabolomics Analysis of Liver Tissue
2.7. Statistical Analysis
3. Results
3.1. The Impact of AX on Liver Functional Indicators
3.2. The Effect of AX on Hepatic Tissue
3.3. The Effect of AX on Hepatic Metabolism
3.3.1. Multivariate Statistical Analysis
3.3.2. Screen and Identification of Potential Biomarkers
3.4. Metabolic Pathway Analysis
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Q.; Li, Y.; Zhao, R.; Tang, Z.; Li, J.; Chen, C.; Liu, X.; Hu, Y.; Wang, T.; Zhao, B. Therapeutic Mechanisms of Mulberry Leaves in Type 2 Diabetes Based on Metabolomics. Front. Pharmacol. 2022, 13, 954477. [Google Scholar] [CrossRef]
- Li, J.; Jia, S.; Yuan, C.; Yu, B.; Zhang, Z.; Zhao, M.; Liu, P.; Li, X.; Cui, B. Jerusalem Artichoke Inulin Supplementation Ameliorates Hepatic Lipid Metabolism in Type 2 Diabetes Mellitus Mice by Modulating the Gut Microbiota and Fecal Metabolome. Food Funct. 2022, 13, 11503–11517. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, D.; Zhou, X.; Song, J.; Yang, Z.; Shi, C.; Li, R.; Zhang, Y.; Zhang, J.; Yan, J.; et al. Study on the Mechanism of American Ginseng Extract for Treating Type 2 Diabetes Mellitus Based on Metabolomics. Front. Pharmacol. 2022, 13, 960050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, H.; Peng, W.; Yang, S.; Feng, Y.; Ouyang, H.; Zhu, W.; Liu, R. Efficacy and Mechanism of Pueraria Lobata and Pueraria Thomsonii Polysaccharides in the Treatment of Type 2 Diabetes. Nutrients 2022, 14, 3926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yan, Q.; Li, Y.; Liu, J.; Liu, H.; Jiang, Z. Effect of Konjac Mannan Oligosaccharides on Glucose Homeostasis via the Improvement of Insulin and Leptin Resistance In Vitro and In Vivo. Nutrients 2019, 11, 1705. [Google Scholar] [CrossRef]
- Ganesan, X. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary Fiber in the Prevention of Obesity and Obesity-Related Chronic Diseases: From Epidemiological Evidence to Potential Molecular Mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Yan, X.; Chao, X.; Zhang, W.; Yuan, C.; Zeng, Q. Anti-diabetic Effect of Banana Peel Dietary Fibers on Type 2 Diabetic Mellitus Mice Induced by Streptozotocin and High-sugar and High-fat Diet. J. Food Biochem. 2022, 46, e14275. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Liang, C.; Liang, G.; Lai, J.; Zhang, R.; Wang, Q.; Xiao, G. Physicochemical Properties of Dietary Fiber of Bergamot and Its Effect on Diabetic Mice. Front. Nutr. 2022, 9, 1040825. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Arabinoxylans and Human Health. Food Hydrocoll. 2014, 42, 239–243. [Google Scholar] [CrossRef]
- Garcia, A.; Steiniger, J.; Reich, S.; Weickert, M.; Harsch, I.; Machowetz, A.; Mohlig, M.; Spranger, J.; Rudovich, N.; Meuser, F.; et al. Arabinoxylan Fibre Consumption Improved Glucose Metabolism, but Did Not Affect Serum Adipokines in Subjects with Impaired Glucose Tolerance. Horm. Metab. Res. 2006, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and Immunomodulatory Activity of Arabinoxylans: A Major Constituent of Wheat Bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan Structural Characteristics, Interaction with Gut Microbiota and Potential Health Functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Vargas-Albores, F.; Fierro-Islas, J.M.; Gollas-Galván, T.; Magdaleno-Moncayo, D.; Rascon-Chu, A.; Martínez-Porchas, M.; Lago-Lestón, A. Arabinoxylans and Gelled Arabinoxylans Used as Anti-Obesogenic Agents Could Protect the Stability of Intestinal Microbiota of Rats Consuming High-Fat Diets. Int. J. Food Sci. Nutr. 2020, 71, 74–83. [Google Scholar] [CrossRef]
- Fadel, A.; Plunkett, A.; Li, W.; Tessu Gyamfi, V.E.; Nyaranga, R.R.; Fadel, F.; Dakak, S.; Ranneh, Y.; Salmon, Y.; Ashworth, J.J. Modulation of Innate and Adaptive Immune Responses by Arabinoxylans. J. Food Biochem. 2018, 42, e12473. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Gao, H.; Fan, L.; Long, Z.; Nie, S. Arabinoxylan Attenuates Type 2 Diabetes by Improvement of Carbohydrate, Lipid, and Amino Acid Metabolism. Mol. Nutr. Food Res. 2018, 62, 1800222. [Google Scholar] [CrossRef]
- Nie, Q.; Xing, M.; Chen, H.; Hu, J.; Nie, S. Metabolomics and Lipidomics Profiling Reveals Hypocholesterolemic and Hypolipidemic Effects of Arabinoxylan on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 10614–10623. [Google Scholar] [CrossRef]
- Nie, Q. Arabinoxylan Ameliorates Type 2 Diabetes by Regulating the Gut Microbiota and Metabolites. Food Chem. 2022, 371, 131106. [Google Scholar]
- Shen, X.; Wang, L.; Zhou, N.; Gai, S.; Liu, X.; Zhang, S. Beneficial Effects of Combination Therapy of Phloretin and Metformin in Streptozotocin-Induced Diabetic Rats and Improved Insulin Sensitivity in Vitro. Food Funct. 2020, 11, 392–403. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Nie, Q.; Huang, X.; Nie, S. Dendrobium Officinale Polysaccharide Ameliorates the Liver Metabolism Disorders of Type II Diabetic Rats. Int. J. Biol. Macromol. 2020, 164, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.M.; Sanap, A.P.; Bhonde, R.R. Treat Liver to Beat Diabetes. Med. Hypotheses 2020, 144, 110034. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, Y.; Jiang, X.; Li, D.; Qin, W.; Zhang, Q.; Lin, D.; Liu, Y.; Tan, C.; Huang, Z.; et al. Arabinoxylan Activates Lipid Catabolism and Alleviates Liver Damage in Rats Induced by High-Fat Diet: Arabinoxylan Activates Lipid Catabolism. J. Sci. Food Agric. 2018, 98, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Marcotuli, I.; Colasuonno, P.; Hsieh, Y.S.Y.; Fincher, G.B.; Gadaleta, A. Non-Starch Polysaccharides in Durum Wheat: A Review. Int. J. Mol. Sci. 2020, 21, 2933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ren, H.; Zhong, C. Economical Production of Vitamin K2 Using Wheat Starch Wastewater. J. Clean. Prod. 2020, 270, 122486. [Google Scholar] [CrossRef]
- Li, P.; Zhao, F.; Wei, X.; Tao, X.; Ding, F. Biological Modification of Pentosans in Wheat B Starch Wastewater and Preparation of a Composite Film. BMC Biotechnol. 2022, 22, 4. [Google Scholar] [CrossRef]
- Bai, Z.; Huang, X.; Wu, G.; Zhou, Y.; Deng, X.; Yang, J.; Yin, J.; Nie, S. Hepatic Metabolism-Related Effects of Polysaccharides from Red Kidney Bean and Small Black Soybean on Type 2 Diabetes. Food Chem. 2023, 403, 134334. [Google Scholar] [CrossRef]
- Pan, R.; Lou, J.; Wei, L. Significant Effects of Ganoderma Lucidum Polysaccharide on Lipid Metabolism in Diabetes May Be Associated with the Activation of the FAM3C-HSF1-CAM Signaling Pathway. Exp. Med. 2021, 22, 820. [Google Scholar] [CrossRef]
- Ma, T.; Liu, T.; Xie, P.; Jiang, S.; Yi, W.; Dai, P.; Guo, X. UPLC-MS-Based Urine Nontargeted Metabolic Profiling Identifies Dysregulation of Pantothenate and CoA Biosynthesis Pathway in Diabetic Kidney Disease. Life Sci. 2020, 258, 118160. [Google Scholar] [CrossRef]
- Ismail, N.; Kureishy, N.; Church, S.J.; Scholefield, M.; Unwin, R.D.; Xu, J.; Patassini, S.; Cooper, G.J.S. Vitamin B5 (d-Pantothenic Acid) Localizes in Myelinated Structures of the Rat Brain: Potential Role for Cerebral Vitamin B5 Stores in Local Myelin Homeostasis. Biochem. Biophys. Res. Commun. 2020, 522, 220–225. [Google Scholar] [CrossRef]
- Shurubor, Y.; D’Aurelio, M.; Clark-Matott, J.; Isakova, E.; Deryabina, Y.; Beal, M.; Cooper, A.; Krasnikov, B. Determination of Coenzyme A and Acetyl-Coenzyme A in Biological Samples Using HPLC with UV Detection. Molecules 2017, 22, 1388. [Google Scholar] [CrossRef] [PubMed]
- Dudzinska, W.; Lubkowska, A.; Dolegowska, B.; Safranow, K.; Jakubowska, K. Adenine, Guanine and Pyridine Nucleotides in Blood during Physical Exercise and Restitution in Healthy Subjects. Eur. J. Appl. Physiol. 2010, 110, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of Uric Acid Metabolism and Excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Varadaiah, Y.G.C.; Sivanesan, S.; Nayak, S.B.; Thirumalarao, K.R. Purine Metabolites Can Indicate Diabetes Progression. Arch. Physiol. Biochem. 2022, 128, 87–91. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Li, X.; Geng, M.; Zhang, Y.; Lan, H.; Li, J.; Qi, C.; Bai, Z.; Huang, J. Effect of Arabinoxylan from Wastewater Generated during Vital Wheat Gluten Production on Liver Metabolism in Type 2 Diabetic Mice. Foods 2023, 12, 2640. https://doi.org/10.3390/foods12142640
Luo D, Li X, Geng M, Zhang Y, Lan H, Li J, Qi C, Bai Z, Huang J. Effect of Arabinoxylan from Wastewater Generated during Vital Wheat Gluten Production on Liver Metabolism in Type 2 Diabetic Mice. Foods. 2023; 12(14):2640. https://doi.org/10.3390/foods12142640
Chicago/Turabian StyleLuo, Denglin, Xingguo Li, Mengyuan Geng, Yunhui Zhang, Honglin Lan, Jiale Li, Caili Qi, Zhouya Bai, and Jihong Huang. 2023. "Effect of Arabinoxylan from Wastewater Generated during Vital Wheat Gluten Production on Liver Metabolism in Type 2 Diabetic Mice" Foods 12, no. 14: 2640. https://doi.org/10.3390/foods12142640
APA StyleLuo, D., Li, X., Geng, M., Zhang, Y., Lan, H., Li, J., Qi, C., Bai, Z., & Huang, J. (2023). Effect of Arabinoxylan from Wastewater Generated during Vital Wheat Gluten Production on Liver Metabolism in Type 2 Diabetic Mice. Foods, 12(14), 2640. https://doi.org/10.3390/foods12142640