Difference in Volatile Aroma Components of Stropharia rugosoannulata under Two Cultivated Environments Investigated by SPME-GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
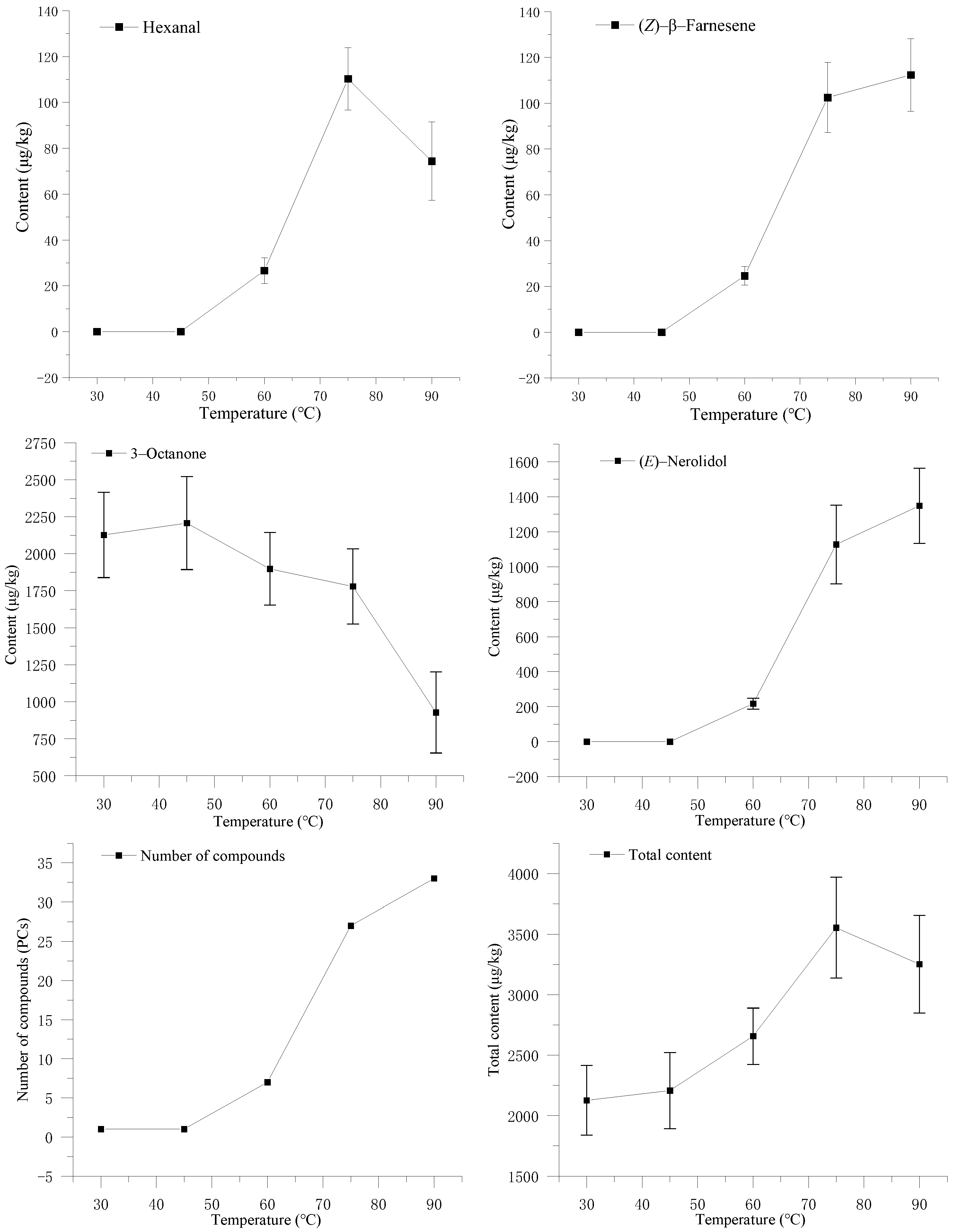
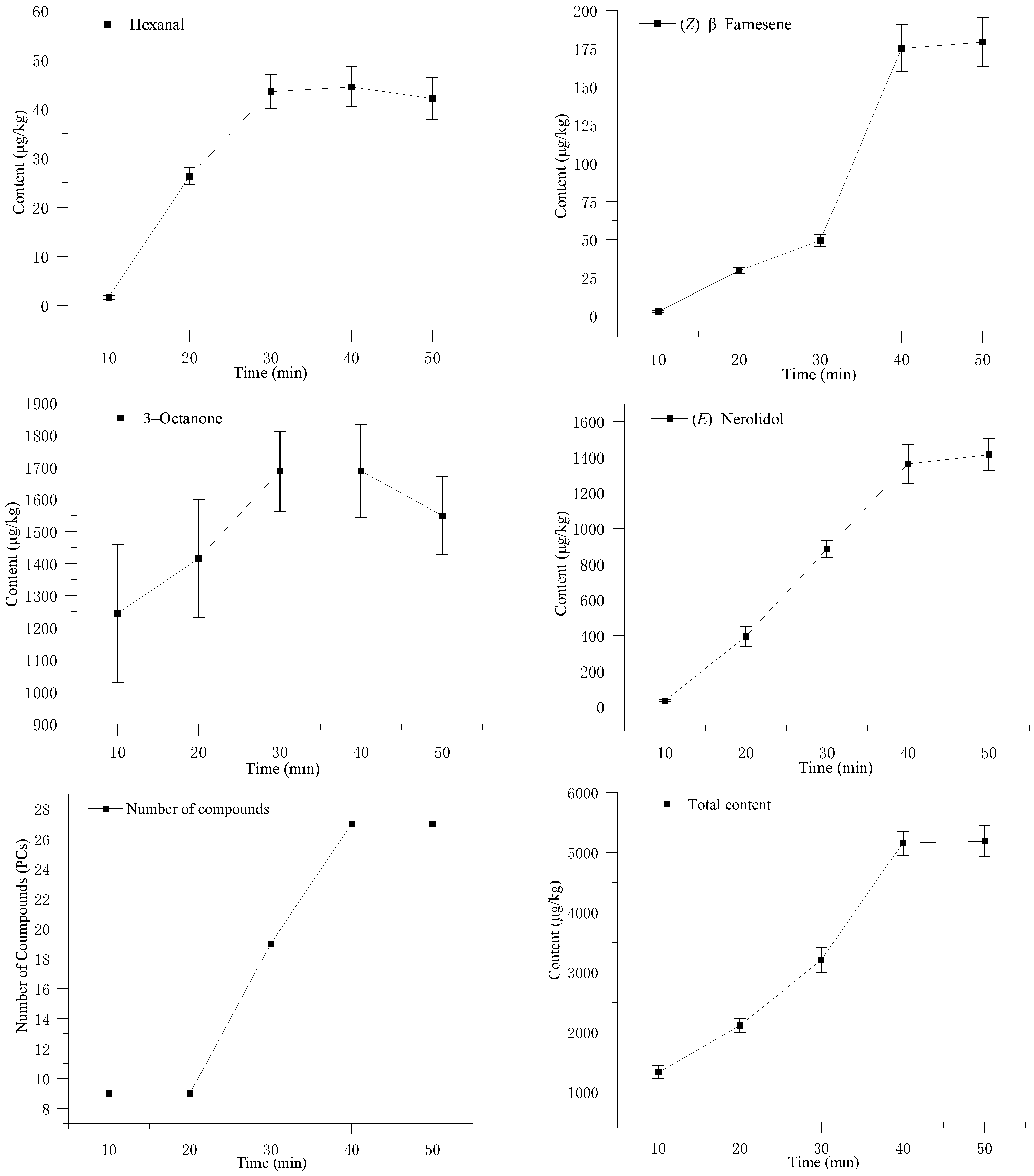
2.3. Optimization of the Adsorption Conditions of SPME
2.4. Determination of Volatile Aroma Compounds via SPME–GC–MS
- —Volatile compound’s relative content in the sample, μg/kg;
- —The external standard content in the sample, μg;
- —The peak area of the external standard, %;
- —The peak area of the target volatile compound, %;
2.5. Odor Activity Values for Hexanal, 3-Octanone, (E)-Nerolidol, (Z)-β-Farnesene, and 1-Hexanol in S. rugosoannulata
- —The odor activity values of the volatile compound (i);
- —The content of the volatile compound (i) in the sample, μg/kg;
- —The odor threshold of the volatile compound (i), μg/kg;
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of the Adsorption Temperature on Volatile Components of S. rugosoannulata
3.2. Effect of Adsorption Time on Volatile Components of S. rugosoannulata
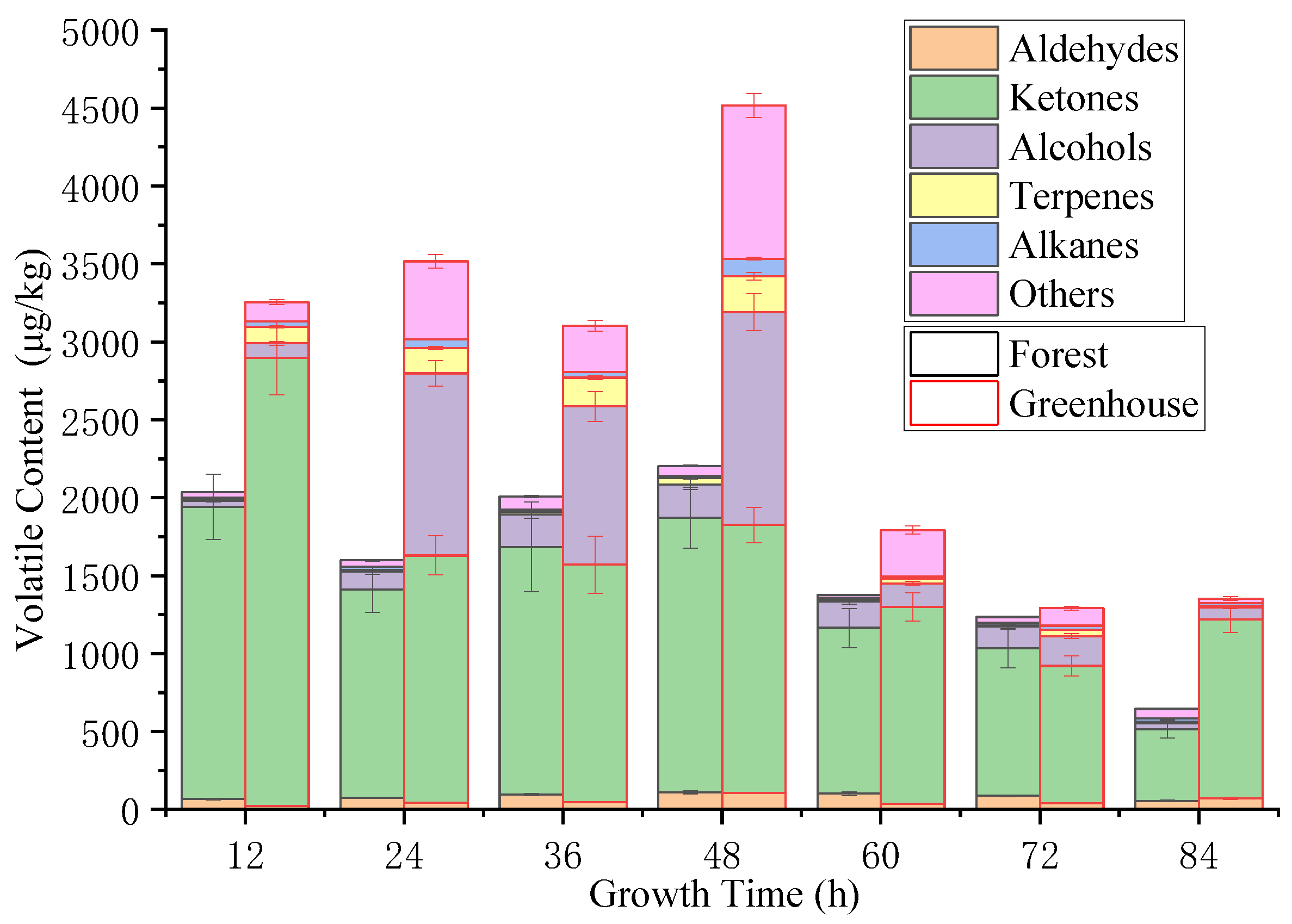
3.3. Volatile Substances Changes in S. rugosoannulata during Growth under a Forest and Greenhouse
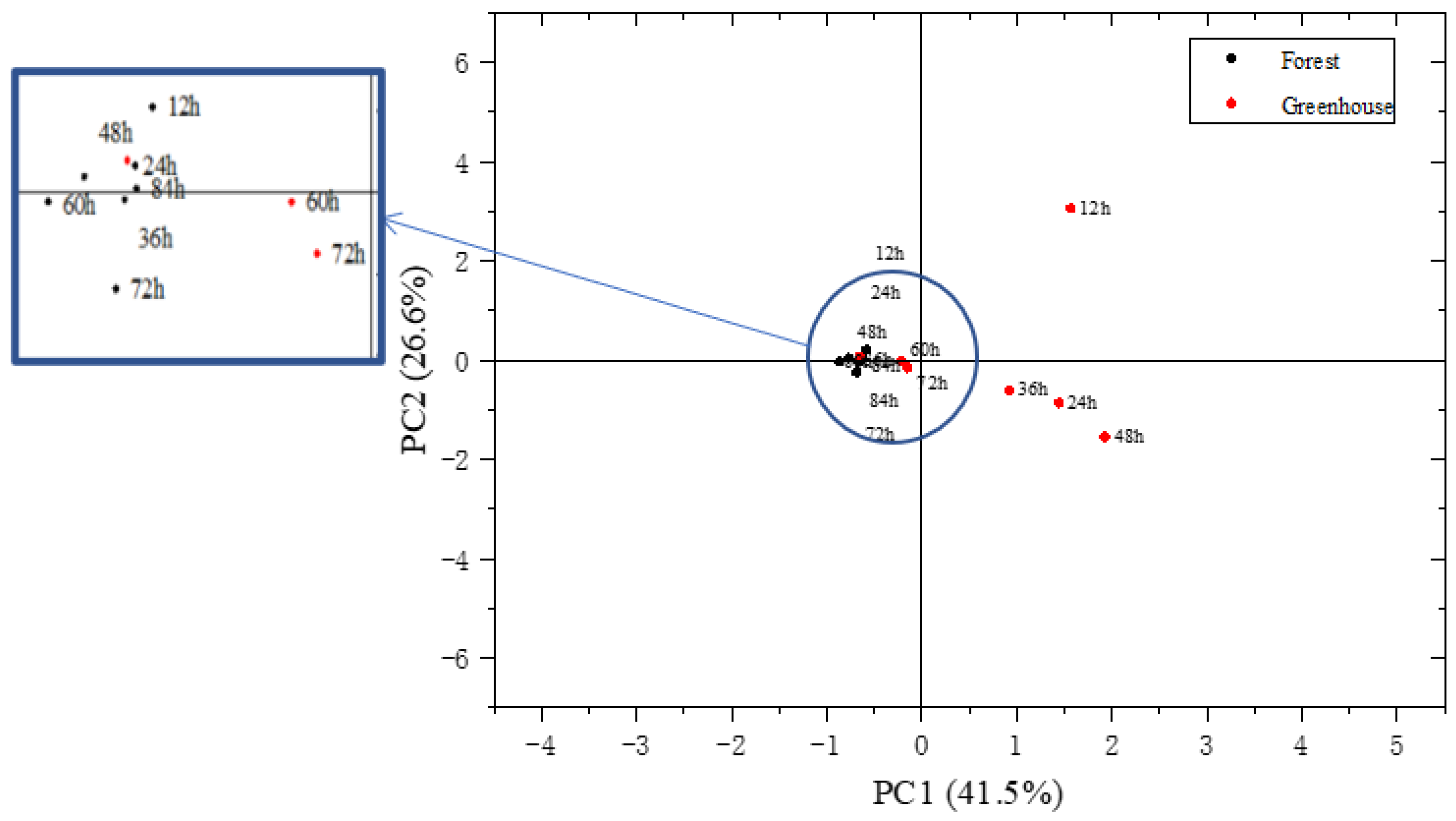
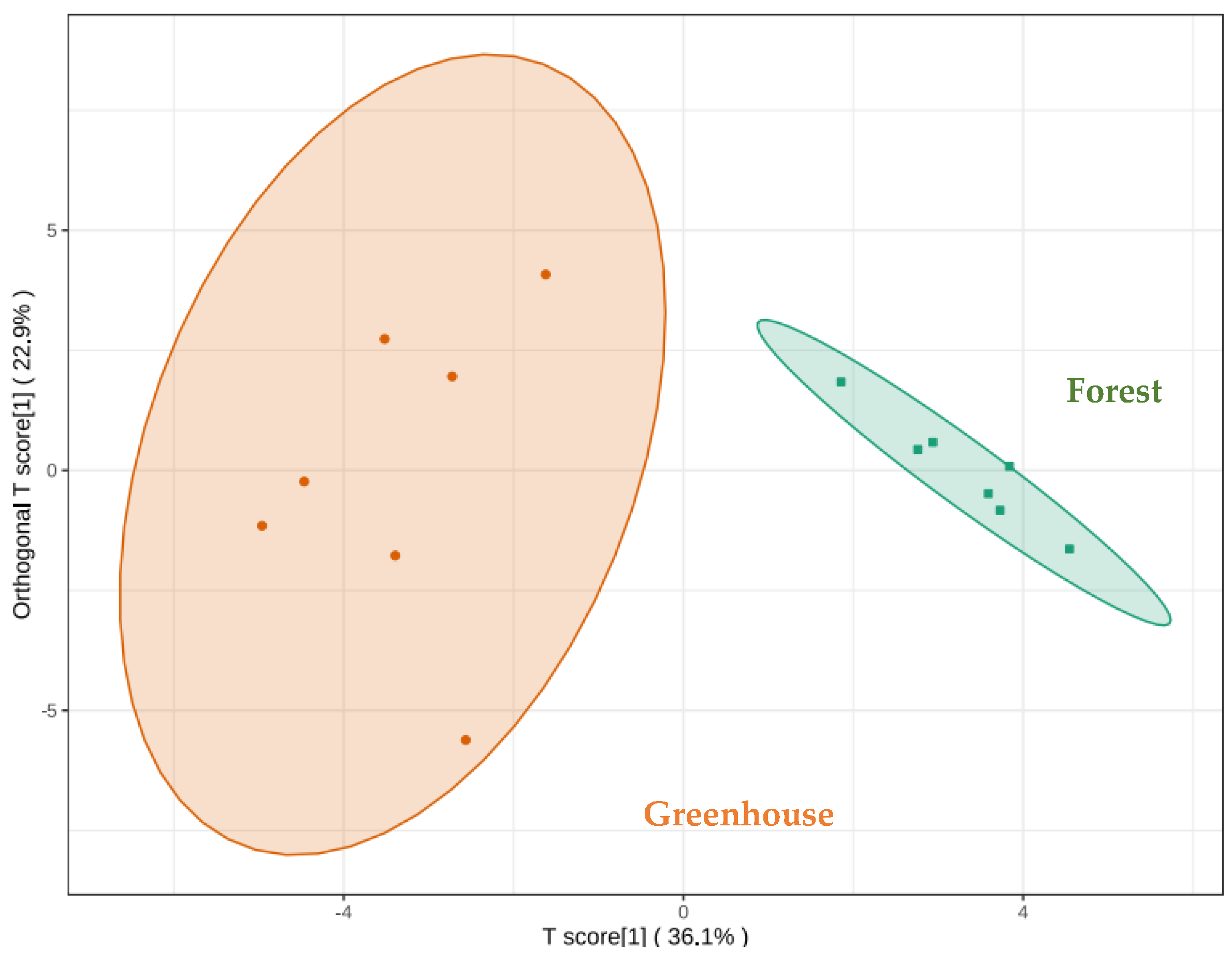
3.4. Aroma Difference Analysis of S. rugosoannulata
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, C.; Xin, M.; Su, K.; Guan, C.; Wang, D. Effects of Ultra-High Pressure Synergistic Enzymatic Hydrolysis on Flavor of Stropharia rugosoannulata. Foods 2023, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Zhang, Z.Q.; Wang, L.; Zhao, H.Q.; Jia, Y.H.; Ma, X.; Li, J.Z.; Wang, Y.; Ma, B.-J. Three-phase extraction of polysaccharide from Stropharia rugosoannulata: Process optimization, structural characterization and bioactivities. Front. Immunol. 2022, 13, 994706. [Google Scholar] [CrossRef]
- Li, X.; Cui, W.; Cui, Y.; Song, X.; Jia, L.; Zhang, J. Stropharia rugosoannulata acetylated polysaccharides alleviate NAFLD via Nrf2/JNK1/AMPK signaling pathways. Int. J. Biol. Macromol. 2022, 215, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Jing, B.N.; Li, X.; Hou, Y.Y.; Xie, X.Y.; Wang, Z.Y.; Liu, Y.Q.; Zhou, Y.; Chang, X.; Wang, W. Evaluation of nutritional in gredients, biologically active materials, and pharmacological activities of stropharia rugosoannulata grown under the bamboo forest and in the greenhouse. J. Food Qual. 2021, 2021, 5478227. [Google Scholar] [CrossRef]
- Zhang, W.W.; Tian, G.T.; Geng, X.R.; Zhao, Y.C.; Ng, T.B.; Zhao, L.Y.; Wang, H.X. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules 2014, 19, 19880–19891. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Li, W.; Wu, D.; Zhang, Z.; Chen, H.; Zhang, J.J.; Wang, C.G.; Wu, T.; Yang, Y. Characterization of novel umami-active peptides from Stropharia rugoso-annulata mushroom and in silico study on action mechanism. J. Food Compos. Anal. 2022, 110, 104530. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.C.; Wu, D.; Zhang, Z.; Yang, Y. Taste peptides derived from Stropharia rugosoannulata fermentation mycelium and molecular docking to the taste receptor T1R1/T1R3. Front. Nutr. 2022, 9, 960218. [Google Scholar] [CrossRef]
- Wu, J.; Kobori, H.; Kawaide, M.; Suzuki, T.; Choi, J.-H.; Yasuda, N.; Noguchi, K.; Matsumoto, T.; Hirai, H.; Kawagishi, H. Isolation of bioactive steroids from the Stropharia rugosoannulata mushroom and absolute configuration of strophasterol B. Biosci. Biotechnol. Biochem. 2013, 77, 1779–1781. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.-F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.T.; Huang, W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2020, 155, 883–889. [Google Scholar] [CrossRef]
- Wei, L.; Wang, W.; Hou, Y.Y.; Xie, X.Y.; Li, X.; Chen, F.; Wang, Z.Y.; Zhou, Y.; Li, F.; Jing, B.N. Chemical Composition, Antibacterial Test, and Antioxidant Activity of Essential Oils from Fresh and Dried Stropharia rugosoannulata. J. Chem. 2023, 2023, 6965755. [Google Scholar] [CrossRef]
- Jia, J. Evaluation of Germplasm Resources and Cultivation Techniques of Stropharia rugosoannulata. Master’s Thesis, Shaanxi University of Technology, Hanzhong, China, 14 June 2022. [Google Scholar] [CrossRef]
- Xue, M.D.; Guo, Z.Y.; Gu, X.Y.; Gao, H.L.; Weng, S.M.; Zhou, J.; Gu, D.G.; Lu, H.X.; Zhou, X.Q. Rare rather than abundant microbial communities drive the effects of long-term greenhouse cultivation on ecosystem functions in subtropical agricultural soils. Sci. Total Environ. 2020, 706, 139961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; Wu, F.Z. Land-use conversion from open field to greenhouse cultivation differently affected the diversities and assembly processes of soil abundant and rare fungal communities. Sci. Total Environ. 2021, 788, 147751. [Google Scholar] [CrossRef] [PubMed]
- Gong, S. The Influnce on Soil Nutrients and Microbe of Cultivation of Stropharia rugosoannulata in the Forest. Master’s Thesis, Shangdong Agriculture University, Tai’an, China, 2 June 2017. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkZIGkvqfmUZglMdu7fCR486pJf1xu9CaOLc2qzHaAA8hAu0HBQd294BdjcWPc9gAT&uniplatform=NZKPT (accessed on 18 May 2023).
- Lu, H.; Shang, X.D.; Wang, X.Y.; Wang, C.H.; Liu, J.H.; Chen, W.Z.; Wang, R.J.; Xu, N. Comparison and analysis of volatile components in different parts of fruiting body of Stropharia rugosoannulata. Mod. Food Sci. Technol. 2022, 38, 271–281. [Google Scholar] [CrossRef]
- Bao, C.L.; Guan, C.B.; Xin, M.H.; Teng, X.; Liu, T.T.; Wang, D.W. Effect of roasting on volatile flavor compounds of Stropharia rugosoannulata analyzed by headspace-solid phase microextraction-gas chromatography-mass spectrometry combined with electronic nose. Food Sci. 2022, 43, 226–233. [Google Scholar] [CrossRef]
- Li, J.L.; Yang, Y.; Li, W.; Chen, Y.C.; Liu, X.F. Aroma change and its relationship with key enzymatic reactions in drying process of Stropharia rugosoannulata. J. Food Sci. Technol. 2023, 41, 30–42. [Google Scholar] [CrossRef]
- Maggi, F.; Bílek, T.; Lucarini, D.; Papa, F.; Sagratini, G.; Vittori, S. Melittis melissophyllum L. subsp. melissophyllum (Lamiaceae) from central Italy: A new source of a mushroom-like flavor. Food Chem. 2009, 113, 216–221. [Google Scholar] [CrossRef]
- Shun, B.G.; Chen, H.T. The Technology of Food Flavoring, 3rd ed.; Chemical Industry Press: Beijing, China, 2017; pp. 28–39. [Google Scholar]
- Sun, L.B.; Zhang, Z.Y.; Xin, G.; Sun, B.X.; Bao, X.J.; Wei, Y.Y.; Zhao, X.M.; Xu, H.R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- He, Q.H.; Fang, R.; Zhang, Y.Q.; Xie, Y.K.; Tong, X.Q.; Yang, S.Z. The correlation of yield of various Stropharia rugosoannulata cultivated strains with forest environmental factors. Mycosystema 2022, 41, 759–768. [Google Scholar] [CrossRef]
- Li, X.M.; Li, H.J.; Xiao, X.; Liao, L.; Chen, R.; Xie, Z.H.; He, Z.F. Study on the change of volatile flavor substances during the processing of deep fried and battered mushroom stalks of Stropharia rugosoannulata. Food Ferment. Ind. 2022. [Google Scholar] [CrossRef]
- Khalilzadeh, M.-A.; Tajbakhsh, M.; Rineh, A. Study of the essential oils composition of leaves and flowers of two subspecies Phlomis herba-Venti (Pungens and Lenkoranica) from Iran. J. Essent. Oil Res. 2008, 20, 46–48. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Z.X.; Cui, Z.Y.; Qi, Q.S.; Hou, J. Progress and perspectives for microbial production of farnesene. Bioresour. Technol. 2022, 347, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lü, S.; Dong, J.; Li, H.; Zhang, Y.; Xu, D.; Wu, Y.; Mo, H. Analysis of nutritional components and volatile flavor compounds in common edible fungi. Sci. Technol. Food Ind. 2023. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef] [PubMed]

| Compound | CAS# | Formula | Relative Content (μg/kg) | Forest | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 84 h | ||||
| Aldehyde | Hexanal | 66-25-1 | C6H12O | 37.8 ± 3.3 c | 45.4 ± 3.1 b | 61.4 ± 4.6 a | 51.7 ± 5.6 ab | 51.8 ± 4.5 ab | 38.1 ± 2.0 c | 30.1 ± 2.3 d |
| Benzaldehyde | 100-52-7 | C7H6O | 8.5 ± 2.5 cd | 11.5 ± 2.8 bc | 11.6 ± 1.9 bc | 20.0 ± 2.8 a | 11.3 ± 3.6 bc | 16.2 ± 2.5 ab | 5.9 ± 1.3 d | |
| (E)-2-Octenal | 2548-87-0 | C8H14O | 8.0 ± 0.9 b | 3.4 ± 0.3 d | 7.6 ± 0.6 b | 12.1 ± 1.4 a | 12.7 ± 1.3 a | 8.5 ± 0.7 b | 5.5 ± 0. 5 c | |
| Benzeneacetaldehyde | 122-78-1 | C8H8O | 2.7 ± 0.4 c | 2.6 ± 0.7 c | 0.8 ± 0.2 e | 10.6 ± 1.5 b | 14.6 ± 0.8 a | 3.4 ± 0.9 c | 1.9 ± 0.3 d | |
| Nonanal | 124-19-6 | C9H18O | 6.4 ± 1.7 b | 5.7 ± 1.2 b | 6.7 ± 1.3 b | 5.6 ± 1.1 b | 5.8 ± 1.0 b | 12.0 ± 1.1 a | 7.0 ± 0.9 b | |
| Decanal | 112-31-2 | C10H20O | 1.5 ± 0.4 c | 1.6 ± 0.3 c | 3.2 ± 0.4 b | 3.3 ± 0.4 b | 3.1 ± 0.5 b | 5.5 ± 0.9 a | 2.8 ± 0.3 b | |
| (E,E)-2,4-Decadienal | 25152-84-5 | C10H16O | 0.4 ± 0.4 c | 3.2 ± 0.7 a | 2.6 ± 0.6 ab | 3.1 ± 0.8 a | 0.9 ± 0.4 c | 1.8 ± 0.4 b | 0.6 ± 0.1 c | |
| (E,E)-2,4-Nonadienal | 5910-87-2 | C9H14O | 1.3 ± 0.3 b | 1.8 ± 0.3 ab | 1.4 ± 0.2 b | 2.8 ± 0.6 a | 1.8 ± 0.5 ab | 1.6 ± 0.3 ab | 1.2 ± 0.2 b | |
| Ketone | 3-Octanone | 106-68-3 | C8H16O | 1855.1 ± 208.7 a | 1312.2 ± 143.7 b | 1560.4 ± 284.3 ab | 1732.1 ± 192.7 a | 1036.1 ± 122.5 bc | 928.4 ± 124.7 c | 456.9 ± 55.9 d |
| 2-Undecanone | 112-12-9 | C11H22O | 19.6 ± 2.2 c | 23.4 ± 3.0 bc | 28.4 ± 3.4 ab | 30.4 ± 2.3 a | 26.2 ± 3.4 ab | 19.0 ± 1.8 c | 4.1 ± 0.8 d | |
| Alcohols | (E)-Nerolidol | 7212-44-4 | C15H26O | 37.9 ± 6.2 h | 113.9 ± 15.3 e | 209.7 ± 26.9 c | 212.7 ± 32.4 c | 169.1 ± 18.6 cd | 89.0 ± 10.3 f | - |
| 1-Hexanol | 111-27-3 | C6H14O | 0.9 ± 0.1 cd | 0.99 ± 0.1 c | 1.0 ± 0.1 c | 0.8 ± 0.1 d | 0.7 ± 0.1 d | 49.8 ± 7.3 a | 38.0 ± 6.1 b | |
| Terpenes | (Z)-β-Farnesene | 28973-97-9 | C15H24 | 2.8 ± 0.4 d | 3.7 ± 0.4 c | 6.6 ± 0.7 b | 14.4 ± 3.1 a | 7.4 ± 2.1 b | 2.6 ± 0.5 d | 3.9 ± 0.8 cd |
| β-Elemene | 515-13-9 | C15H24 | - | 2.9 ± 1.1 b | 3.0 ± 0.9 b | 9.6 ± 1.7 a | 2.0 ± 0.9 b | 0.2 ± 0.1 c | 0.4 ± 0.1 c | |
| Thujopsene | 470-40-6 | C15H24 | 2.6 ± 0.2 d | 2.7 ± 0.2 d | 4.8 ± 0.5 b | 9.6 ± 1.3 a | 3.1 ± 0.2 c | 3.2 ± 0.3 c | 3.5 ± 0.3 c | |
| α-Cubebene | 17699-14-8 | C15H24 | 1.4 ± 0.5 bc | 0.4 ± 0.6 c | 2.1 ± 0.6 b | 6.6 ± 1.1 a | 1.8 ± 0.5 b | 0.5 ± 0.1 c | 0.4 ± 0.2 c | |
| Other | Methoxy-phenyl-oxime | 1000222-86-6 | C8H9NO2 | 31.8 ± 2.2 c | 23.6 ± 2.7 d | 77.3 ± 5.1 a | 57.6 ± 4.3 b | 16.2 ± 1.8 e | 31.8 ± 2.9 c | 55.1 ± 4.8 b |
| 2-Methyl-naphthalene | 91-57-6 | C11H10 | 2.1 ± 0.4 c | 17.1 ± 1.2 a | 5.4 ± 0.9 b | 5.4 ± 0.9 b | 2.2 ± 0.4 c | 5.5 ± 0.7 b | 5.5 ± 0.6 b | |
| Alkanes | Tetradecane | 629-59-4 | C14H30 | 3.8 ± 0.2 c | 5.3 ± 0.5 b | 3.9 ± 0.3 c | 6.5 ± 0.7 a | 2.5 ± 0.4 d | 3.9 ± 0.2 c | 4.5 ± 0.6 bc |
| Heptadecane | 629-78-7 | C17H36 | 4.4 ± 0.3 d | 6.3 ± 0.6 b | 5.2 ± 0.4 c | 4.1 ± 0.4 d | 4.1 ± 0.9 cd | 7.0 ± 0.2 b | 8.9 ± 1.0 a | |
| Hexadecane | 544-76-3 | C16H34 | 3.3 ± 0.3 b | 5.1 ± 0.6 a | 2.7 ± 0.2 c | 2.7 ± 0.1 c | 1.7 ± 0.5 d | 2.8 ± 0.3 c | 4.1 ± 0.4 ab | |
| Pentadecane | 629-62-9 | C15H32 | 2.6 ± 0.2 c | 5.5 ± 0.4 a | 2.5 ± 0.4 c | 2.3 ± 0.3 c | 2.3 ± 0.9 c | 3.5 ± 0.3 b | 4.7 ± 0.9 ab | |
| Compound | CAS# | Formula | Relative Content (μg/kg) | Greenhouse | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 84 h | ||||
| Aldehyde | Hexanal | 66-25-1 | C6H12O | 12.4 ± 3.1 d | 17.6 ± 2.2 d | 28.2 ± 3.0 c | 38.9 ± 4.2 b | 17.5 ± 2.1 d | 26.9 ± 3.5 c | 54.5 ± 2.0 a |
| Benzaldehyde | 100-52-7 | C7H6O | 4.9 ± 1.7 cd | 6.7 ± 1.1 bc | 10.2 ± 3.4 b | 38.3 ± 11.5 a | 6.4 ± 2.3 bc | 0.4 ± 0.3 e | 2.2 ± 1.1 d | |
| Nonanal | 124-19-6 | C9H18O | 3.9 ± 0.7 c | 15.1 ± 2.2 b | 4.3 ± 0.9 c | 23.3 ± 3.9 a | 4.5 ± 0.7 c | 5.2 ± 0.6 c | 4.5 ± 0.9 c | |
| Decanal | 112-31-2 | C10H20O | - | 4.9 ± 0.7 a | 4.1 ± 0.7 a | 3.7 ± 0.7 a | 4.2 ± 0.3 a | 4.3 ± 1.1 a | 2.4 ± 0.6 b | |
| Benzeneacetaldehyde | 122-78-1 | C8H8O | - | - | - | 1.5 ± 0.8 b | 3.9 ± 1.1 a | 1.1 ± 0.6 b | 1.7 ± 0.3 b | |
| (E)-2-Octenal | 2548-87-0 | C8H14O | - | - | - | - | - | 1.5 ± 0.3 b | 6.1 ± 0.5 a | |
| Ketone | 3-Octanone | 106-68-3 | C8H16O | 2861.0 ± 234.7 a | 1568.7 ± 124.5 bc | 1500.0 ± 181.5 bc | 1688.4 ± 108.8 b | 1252.0 ± 88.5 cd | 872.7 ± 62.5 d | 1140.3 ± 83.6 d |
| 2-Undecanone | 112-12-9 | C11H22O | 15.5 ± 2.8 c | 16.6 ± 1.9 c | 24.5 ± 1.7 b | 31.7 ± 4.7 a | 10.7 ± 2.7 d | 9.1 ± 1.3 de | 7.1 ± 0.7 e | |
| Alcohols | (E)-Nerolidol | 7212-44-4 | C15H26O | 62.0 ± 5.8 d | 1165.3 ± 83.0 ab | 1012.7 ± 125.7 b | 1362.6 ± 188.7 a | 149.9 ± 21.5 c | 185.1 ± 16.8 c | 14.5 ± 4.1 e |
| 1-Hexanol | 111-27-3 | C6H14O | 1.1 ± 0.2 e | 2.1 ± 0.3 d | 2.0 ± 0.2 d | 3.4 ± 0.3 c | 1.8 ± 0.3 d | 6.3 ± 0.7 b | 63.9 ± 6.5 a | |
| Globulol | 489-41-8 | C15H26O | 29.1 ± 5.1 a | - | - | - | - | - | - | |
| Cedrol | 77-53-2 | C15H26O | 1.1 ± 0.3 a | 1.4 ± 0.2 a | - | - | - | - | - | |
| Terpenes | (Z)-β-Farnesene | 28973-97-9 | C15H24 | 14.8 ± 2.1 d | 100.0 ± 11.7 b | 122.5 ± 14.1 ab | 146.0 ± 22.0 a | 20.3 ± 3.3 c | 23.9 ± 3.8 c | 5.2 ± 1.2 e |
| β-Bisabolene | 495-61-4 | C15H24 | 0.1 ± 0.0 e | 17.6 ± 1.9 b | 19.3 ± 1.6 b | 27.2 ± 3.6 a | 3.4 ± 0.5 d | 5.8 ± 0.8 c | - | |
| Thujopsene | 470-40-6 | C15H24 | 10.1 ± 0.8 b | 6.8 ± 0.8 c | 4.8 ± 0.6 d | 20.1 ± 3.8 a | 1.8 ± 0.3 f | 3.2 ± 0.6 e | 2.8 ± 0.4 e | |
| Calamenene | 483-77-2 | C15H22 | 14.0 ± 1.2 a | 6.8 ± 0.6 c | 9.6 ± 1.3 b | 11.7 ± 1.0 b | - | 1.4 ± 0.3 d | 0.6 ± 0.1 e | |
| δ—Cadinene | 483-76-1 | C15H24 | 18.5 ± 0.4 a | 3.6 ± 0.7 c | 2.8 ± 0.7 c | 7.5 ± 1.3 b | 1.5 ± 0.2 d | 1.4 ± 0.3 d | 0.8 ± 0.2 d | |
| α-Cubebene | 17699-14-8 | C15H24 | 17.8 ± 3.0 a | 4.8 ± 1.2 c | 11.4 ± 1.9 b | 6.8 ± 1.3 c | 1.6 ± 0.6 d | 1.5 ± 0.7 d | - | |
| α-Curcumene | 644-30-4 | C15H22 | 0.4 ± 0.2 e | 5.1 ± 0.8 ab | 4.2 ± 0.6 b | 5.8 ± 0.4 a | 1.5 ± 0.1 d | 2.3 ± 0.4 c | - | |
| α-Bergamotene | 17699-05-7 | C15H24 | 2.4 ± 0.8 b | 3.6 ± 0.7 ab | 3.4 ± 0.4 ab | 4.1 ± 0.6 a | 1.1 ± 0.4 c | 2.0 ± 0.7 bc | - | |
| β-Cubebene | 13744-15-5 | C15H24 | 11.29 ± 1.56 a | - | - | - | - | - | - | |
| 1,2,3,5,6,7,8,8a-Octahydro-1-methyl-6-methylene-4-(1-methylethyl)naphthalene | 150320-52-8 | C15H24 | 4.8 ± 1.8 a | - | - | - | - | - | - | |
| α-Muurolene | 31983-22-9 | C15H24 | 4.5 ± 0.7 b | 14.8 ± 1.6 a | 5.9 ± 0.8 b | - | - | - | 0.7 ± 0.3 c | |
| Copaene | 3856-25-5 | C15H24 | 2.7 ± 0.5 a | - | - | - | - | - | - | |
| Seychellene | 20085-93-2 | C15H24 | 1.4 ± 0.2 a | - | - | - | - | - | - | |
| Cedrene | 11028-42-5 | C15H24 | 1.3 ± 0.5 a | - | - | - | - | - | - | |
| β-Elemene | 515-13-9 | C15H24 | 0.9 ± 0.2 a | - | - | - | - | - | - | |
| Other | Methoxy-phenyl-oxime | 1000222-86-6 | C8H9NO2 | 77.3 ± 10.0 f | 401.1 ± 33.3 b | 222.2 ± 25.6 d | 918.4 ± 70.0 a | 289.1 ± 24.7 c | 106.3 ± 7.8 e | 24.4 ± 6.6 g |
| 2-Methyl-naphthalene | 91-57-6 | C11H10 | 38.1 ± 3.0 c | 83.4 ± 5.9 a | 65.6 ± 7.3 b | 60.4 ± 7.2 b | 5.2 ± 0.7 c | 4.0 ± 3.0 c | 5.0 ± 4.5 c | |
| 2,3-Dimethylnaphthalene | 581-40-8 | C12H22 | 9.0 ± 2.1 b | 15.9 ± 3.7 a | 7.5 ± 1.9 b | 4.4 ± 0.7 c | 3.2 ± 0.6 c | 2.0 ± 0.5 d | - | |
| Alkanes | Heptadecane | 629-78-7 | C17H36 | 3.7 ± 0.7 d | 15.5 ± 0.0 b | 8.7 ± 0.8 c | 39.8 ± 3.1 a | 5.6 ± 1.2 d | 8.6 ± 1.2 c | 8.7 ± 0.7 c |
| Pentadecane | 629-62-9 | C15H32 | 15.3 ± 1.7 b | 17.3 ± 1.7 b | 15.5 ± 2.2 b | 33.6 ± 2.8 a | 3.9 ± 0.5 d | 6.8 ± 1.7 c | 3.5 ± 0.4 d | |
| Hexadecane | 544-76-3 | C16H34 | - | 22.0 ± 2.0 a | 13.6 ± 1.5 b | 21.8 ± 1.8 a | - | 5.4 ± 2.7 c | 3.7 ± 0.3 c | |
| Tetradecane | 629-59-4 | C14H30 | 16.1 ± 1.3 a | - | - | 17.4 ± 2.2 a | 3.9 ± 0.3 b | 4.1 ± 1.2 b | - | |
| Main Volatile Compounds | Content (μg/kg) | * Odor Threshold [19] (μg/kg) | OAV | ||
|---|---|---|---|---|---|
| Forest | Greenhouse | Forest | Greenhouse | ||
| Hexanal | 51.7 | 38.9 | 4.5 | 11.5 | 8.6 |
| 3-Octanone | 1732.1 | 1688.4 | 28.0 | 61.9 | 60.3 |
| 2-Undecanone | 30.4 | 31.7 | 7.0 | 4.3 | 4.5 |
| 1-Hexanol | 0.8 | 3.4 | 2500.0 | 0.0 | 0.0 |
| (E)-Nerolidol | 212.7 | 1362.6 | 300.0 | 0.7 | 4.5 |
| (Z)-β-Farnesene | 14.4 | 146.0 | 450.0 | 0.0 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, D.; Wu, Y.; Tong, X.; Qin, Y.; Wang, L. Difference in Volatile Aroma Components of Stropharia rugosoannulata under Two Cultivated Environments Investigated by SPME-GC-MS. Foods 2023, 12, 2656. https://doi.org/10.3390/foods12142656
Wang Y, Wu D, Wu Y, Tong X, Qin Y, Wang L. Difference in Volatile Aroma Components of Stropharia rugosoannulata under Two Cultivated Environments Investigated by SPME-GC-MS. Foods. 2023; 12(14):2656. https://doi.org/10.3390/foods12142656
Chicago/Turabian StyleWang, Yanbin, Dan Wu, Yingqi Wu, Xiaoqing Tong, Yuchuan Qin, and Liling Wang. 2023. "Difference in Volatile Aroma Components of Stropharia rugosoannulata under Two Cultivated Environments Investigated by SPME-GC-MS" Foods 12, no. 14: 2656. https://doi.org/10.3390/foods12142656
APA StyleWang, Y., Wu, D., Wu, Y., Tong, X., Qin, Y., & Wang, L. (2023). Difference in Volatile Aroma Components of Stropharia rugosoannulata under Two Cultivated Environments Investigated by SPME-GC-MS. Foods, 12(14), 2656. https://doi.org/10.3390/foods12142656






