Separation of α-Lactalbumin-Enriched Fractions from Caprine and Ovine Native Whey Concentrate by Combining Membrane and High-Pressure Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Native Concentrated Whey Preparation and High-Pressure Processing (HPP)
2.2. Chemicals and Standards
2.3. HPLC Proteins Quantification
2.4. Process Performance Parameters
2.5. Hydrophobicity Index
2.6. Total Free Thiol Groups Index
2.7. Statistics
3. Results and Discussion
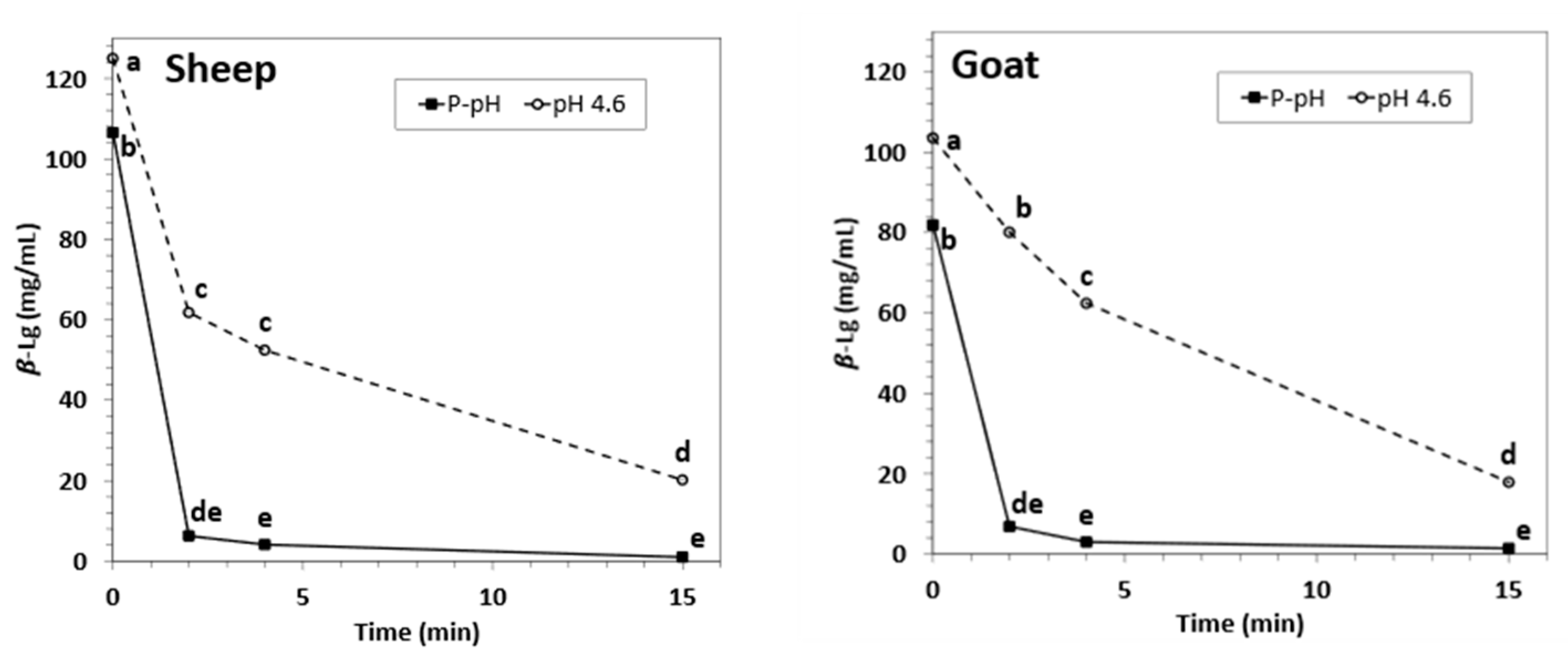
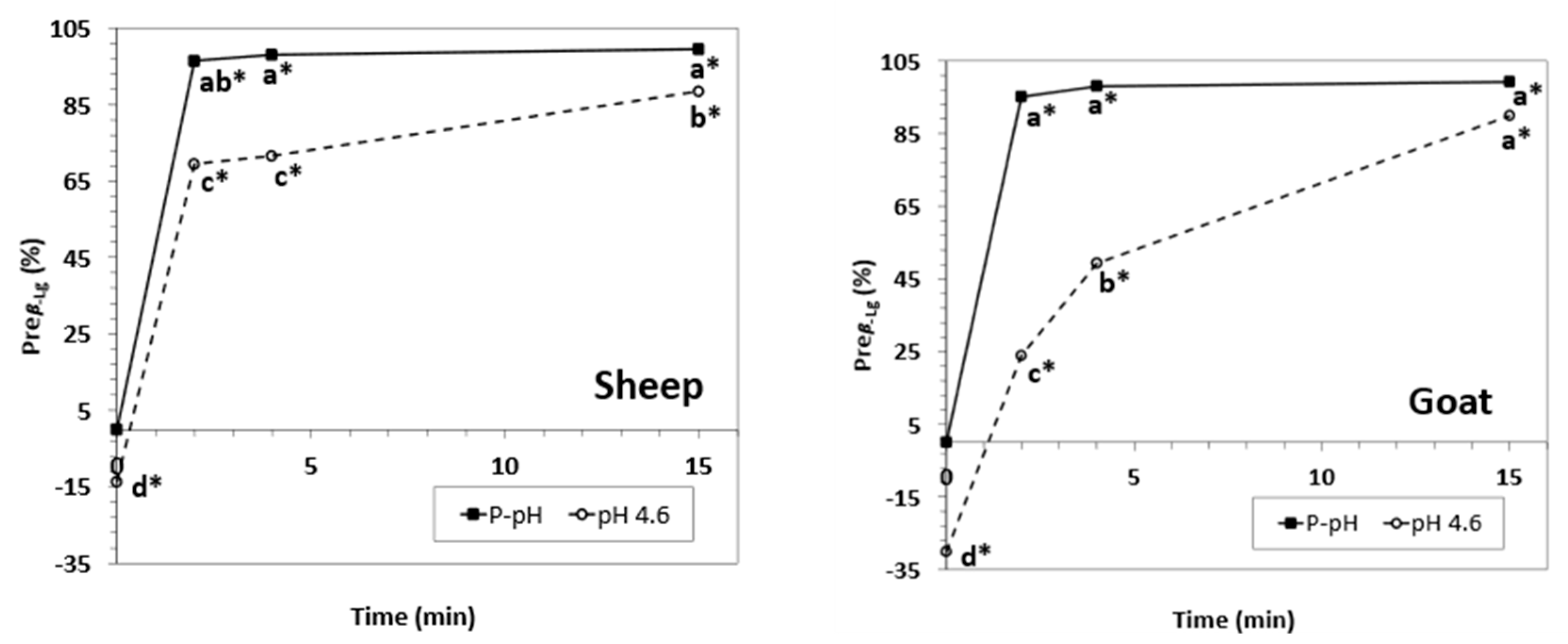
3.1. Main Process Performance Parameters
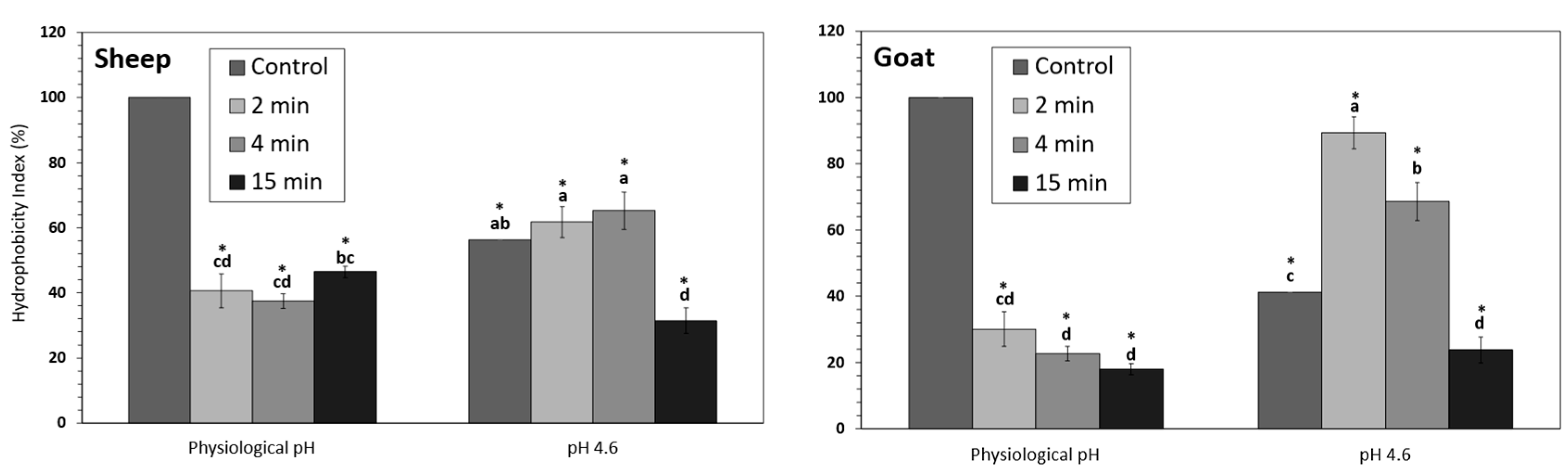
3.2. Hydrophobicity Index
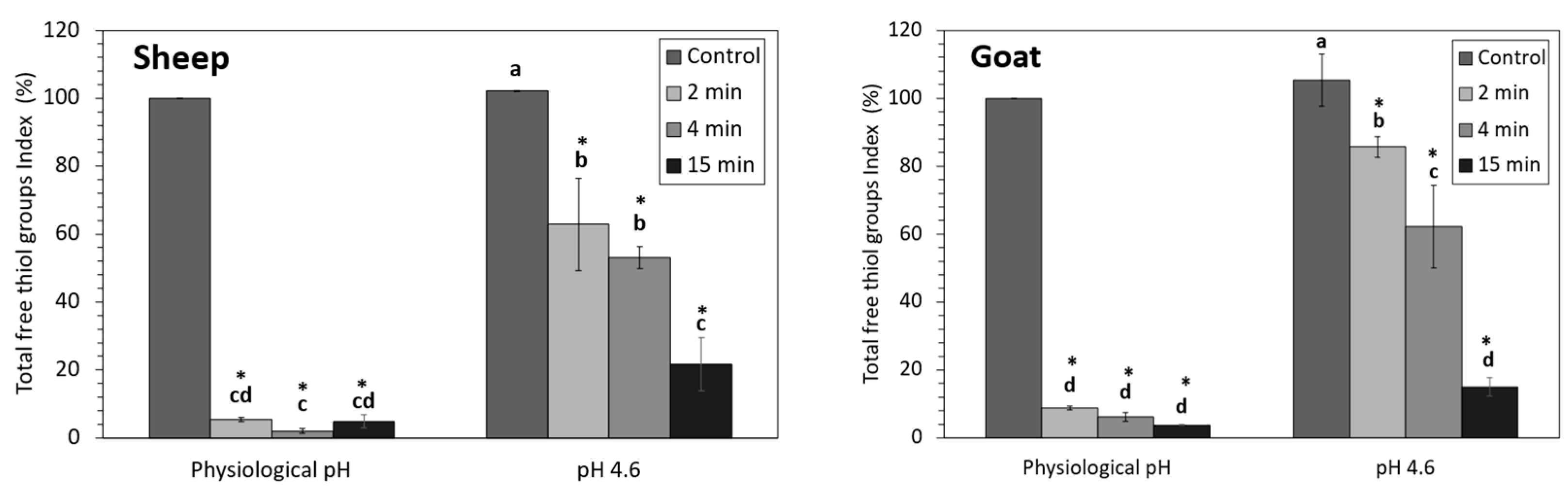
3.3. Total Free Thiol Groups Index
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. FAOSTAT Statistics Database. 2021. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 November 2022).
- Sajdakowska, M.; Gębski, J.; Gutkowska, K. Directions of changes in the health values of dairy products in the opinion of consumers. Nutrients 2021, 13, 1945. [Google Scholar] [CrossRef]
- López-Aliaga, I.; Díaz-Castro, J.; Alférez, M.J.M.; Barrionuevo, M.; Campos, M.S. A review of the nutritional and health aspects of goat milk in cases of intestinal resection. Dairy Sci. Technol. 2010, 90, 611–622. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk from Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Park, Y.W. Hypo-allergenic and therapeutic significance of goat milk. Small Rumin. Res. 1994, 14, 151–159. [Google Scholar] [CrossRef]
- Maduko, C.O.; Park, Y.W. Production of Infant Formula Analogs by Membrane Fractionation of Caprine Milk: Effect of Temperature Treatment on Membrane Performance. Food Sci. Nutr. 2011, 2, 1097–1104. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Ramos, M.; Gómez-Ruiz, J.Á. Bioactive components of ovine and caprine cheese whey. Small Rumin. Res. 2011, 101, 196–204. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, R.; Zhu, Z.; Shi, L. LC-Q-Orbitrap HRMS-based proteomics reveals potential nutritional function of goat whey fraction. J. Funct. Foods 2021, 82, 104502. [Google Scholar] [CrossRef]
- Ruprichová, L.; Králová, M.; Borkovcová, I.; Vorlová, L.; Bedáňová, I. Determination of whey proteins in different types of milk. Acta Vet. Brno 2014, 83, 67–72. [Google Scholar] [CrossRef]
- Natale, M.; Bisson, C.; Monti, G.; Peltran, A. Cow ’s milk allergens identification by two-dimensional immunoblotting and mass spectrometry cow’s milk allergens identification by two-dimensional immunoblotting and mass spectrometry. Mol. Nutr. Food Res. 2004, 48, 363–369. [Google Scholar] [CrossRef]
- Kurpiewska, K.; Biela, A.; Loch, J.I.; Lipowska, J.; Siuda, M. Towards understanding the effect of high pressure on food protein allergenicity: B-lactoglobulin structural studies. Food Chem. 2019, 270, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Moloney, C.; Regan, J.O.; Kelly, A.L.; Mahony, J.A.O. Chemical composition, protein profile and physicochemical properties of whey protein concentrate ingredients enriched in a-lactalbumin. J. Food Compos. Anal. 2020, 92, 103546. [Google Scholar] [CrossRef]
- Holland, B.; Kackmar, J.; Corredig, M. Short communication: Isolation of a whey fraction rich in a-lactalbumin from skim milk using tangential flow ultrafiltration. J. Dairy Sci. 2012, 95, 5604–5607. [Google Scholar] [CrossRef]
- Carter, B.; Dimarzo, L.; Pranata, J.; Barbano, D.M.; Drake, M. Determination of the efficiency of removal of whey protein from sweet whey with ceramic microfiltration membranes. J. Dairy Sci. 2021, 104, 7534–7543. [Google Scholar] [CrossRef]
- Macedo, A.; Azedo, D.; Duarte, E.; Pereira, C. Valorization of goat cheese whey through an integrated process of ultrafiltration and nanofiltration. Membranes 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.M.; Giraldo, G.I.; Romero, C.M. An improved method for isolation of b-lactoglobulin. Int. Dairy J. 2008, 18, 55–63. [Google Scholar] [CrossRef]
- Haller, N.; Kulozik, U. Continuous centrifugal separation of selectively precipitated a-lactalbumin. Int. Dairy J. 2020, 101, 104566. [Google Scholar] [CrossRef]
- El-Zahar, K.; Sitohy, M.; Dalgalarrondo, M.; Choiset, Y.; Métro, F.; Haertlé, T.; Chobert, J.M. Purification and physicochemical characterization of ovine β-lactoglobulin and α-lactalbumin. Mol. Nutr. Food Res. 2004, 48, 177–183. [Google Scholar] [CrossRef]
- Abbas, Z.H.; Awada, J.M.; Alreda, E.M.; Mahdi, A.J. Isolation and purification of lactoferrin protein from goats’ colostrum and its determination using high pressure liquid chromatography and study its inhibitory effect on some pathogenic bacteria. Biochem. Cell. Arch. 2019, 19, 2555–2560. [Google Scholar] [CrossRef]
- Fuciños, C.; Fuciños, P.; Estévez, N.; Pastrana, L.M.; Vicente, A.A.; Luisa, M. One-step chromatographic method to purify α-lactalbumin from whey for nanotube synthesis purposes. Food Chem. 2019, 275, 480–488. [Google Scholar] [CrossRef]
- Konrad, G.; Kleinschmidt, T. A new method for isolation of native a-lactalbumin from sweet whey. Int. Dairy J. 2008, 18, 47–54. [Google Scholar] [CrossRef]
- Cheison, S.C.; Leeb, E.; Toro-Sierra, J.; Kulozik, U. Influence of hydrolysis temperature and pH on the selective hydrolysis of whey proteins by trypsin and potential recovery of native alpha-lactalbumin. Int. Dairy J. 2011, 21, 166–171. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, P.; Jaimni, S.; Kehinde, B.A.; Kaur, S. Recent developments in purification techniques and industrial applications for whey valorization: A review. Chem. Eng. Commun. 2020, 207, 123–138. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Carrascosa, A.V.; Olano, A. The Effects of High Pressure on Whey Protein Denaturation and Cheese-Making Properties of Raw Milk. J. Dairy Sci. 1996, 79, 929–936. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. High pressure-induced denaturation of α-lactalbumin and β-lactoglobulin in bovine milk and whey: A possible mechanism. J. Dairy Res. 2004, 71, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of Heat and High Hydrostatic Pressure Treatments on Disulfide Bonding Interchanges among the Proteins in Skim Milk. J. Agric. Food Chem. 2006, 54, 3409–3420. [Google Scholar] [CrossRef]
- Felipe, X.; Capellas, M.; Law, A.J.R. Comparison of the Effects of High-Pressure Treatments and Heat Pasteurization on the Whey Proteins in Goat’s Milk. J. Agric. Food Chem. 1997, 45, 627–631. [Google Scholar] [CrossRef]
- Nassar, K.S.; Zhang, S.; Lu, J.; Pang, X.; Ragab, E.S.; Yue, Y.; Lv, J. Combined effects of high-pressure treatment and storage temperature on the physicochemical properties of caprine milk. Int. Dairy J. 2019, 96, 66–72. [Google Scholar] [CrossRef]
- Kiełczewska, K.; Dąbrowska, A.; Jankowska, A.; Wachowska, M.; Kowalik, J. The Effect of High-Pressure Treatment and Skimming on Caprine Milk Proteins. Appl. Sci. 2021, 11, 5982. [Google Scholar] [CrossRef]
- Huppertz, T.; Kelly, A.; Fox, P.F. High pressure-induced changes in ovine milk. 2. Effects on casein micelles and whey proteins. Milchwissenschaft 2006, 61, 394–397. [Google Scholar]
- Moatsou, G.; Katsaros, G.; Bakopanos, C.; Kandarakis, I.; Taoukis, P.; Politis, I. Effect of high-pressure treatment at various temperatures on activity of indigenous proteolytic enzymes and denaturation of whey proteins in ovine milk. Int. Dairy J. 2008, 18, 1119–1125. [Google Scholar] [CrossRef]
- Sakkas, L.; Tzevdou, M.; Zoidou, E.; Gkotzia, E.; Karvounis, A.; Samara, A.; Taoukis, P.; Moatsou, G. Yoghurt-type gels from skim sheep milk base enriched with whey protein concentrate hydrolysates and processed by heating or high hydrostatic pressure. Foods 2019, 8, 342. [Google Scholar] [CrossRef]
- Wazed, M.A.; Farid, M. Hypoallergenic and Low-Protein Ready-to-Feed (RTF) Infant Formula by High Pressure Pasteurization: A Novel Product. Foods 2019, 8, 408. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Suwal, S.; Touhami, S.; Chamberland, J.; Pouliot, Y.; Doyen, A. Production of highly purified fractions of α-lactalbumin and β-lactoglobulin from cheese whey using high hydrostatic pressure. J. Dairy Sci. 2020, 103, 7939–7950. [Google Scholar] [CrossRef]
- Romo, M.; Castellari, M.; Fartdinov, D.; Felipe, X. Separation of α-Lactalbumin Enriched Fraction from Bovine Native Whey-Concentrate by Combining Membrane and High-Pressure Processing. Foods 2023, 12, 480. [Google Scholar] [CrossRef]
- ISO 1211:2010|IDF 1:2010; Milk—Determination of Fat Content—Gravimetric Method (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 8968-3:2004|IDF 20-3:2004; Milk—Determination of Nitrogen Content—Part 3: Block-Digestion Method (Semi-Micro Rapid Routine Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 2920:2004|IDF 58:2004; Whey Cheese—Determination of Dry Matter (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- BOE-A-1977-16116. Orden de 31 de Enero de 1977 por la que se Establecen los Métodos Oficiales de Análisis de Aceites y Grasas, Cereales y Derivados, Productos Lácteos y Productos Derivados de la Uva. Available online: https://www.boe.es/eli/es/o/1977/01/31/(1) (accessed on 20 December 2022).
- Steen, L.; Glorieux, S.; Goemaere, O.; Brijs, K.; Paelinck, H.; Foubert, I.; Fraeye, I. Functional Properties of Pork Liver Proteins Fractions. Food Bioproc. Technol. 2016, 9, 970–980. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Parke, J.W. Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chem. 2003, 83, 409–416. [Google Scholar] [CrossRef]
- Pásztor-Huszár, K. Protein Changes of Various Types of Milk as Affected by High Hydrostatic Pressure. Ph.D. Thesis, Corvinus University of Budapest, Budapest, Hungary, 2008. [Google Scholar]
- Hinrichs, J.; Rademacher, B. High pressure thermal denaturation kinetics of whey proteins. J. Dairy Res. 2004, 71, 480–488. [Google Scholar] [CrossRef]
- López-Fandiño, R.; De La Fuente, M.A.; Ramos, M.; Olano, A. Distribution of minerals and proteins between the soluble and colloidal phases of pressurized milks from different species. J. Dairy Res. 1998, 65, 69–78. [Google Scholar] [CrossRef]
- Tanford, C.; Bunville, L.G.; Nozaki, Y. The reversible transformation of b-lactoglobulin at pH 7.5. J. Am. Chem. Soc. 1959, 81, 4032–4036. [Google Scholar] [CrossRef]
- Loch, J.I.; Molenda, M.; Kopeć, M.; Swiątek, S.; Lewiński, K. Structure of two crystal forms of sheep β-lactoglobulin with EF-loop in closed conformation. Biopolymers 2014, 101, 886–894. [Google Scholar] [CrossRef]
- Loch, J.I.; Bonarek, P.; Polit, A.; Świątek, S.; Czub, M.; Ludwikowska, M.; Lewiński, K. Conformational variability of goat β-lactoglobulin: Crystallographic and thermodynamic studies. Int. J. Biol. Macromol. 2015, 72, 1283–1291. [Google Scholar] [CrossRef]
- Loch, J.I.; Bonarek, P.; Lewinéski, K. Conformational flexibility and ligand binding properties of ovine β-lactoglobulin. Acta Biochim. Pol. 2019, 66, 577–589. [Google Scholar] [CrossRef]
- Gottschalk, M.; Nilsson, H.; Roos, H.; Halle, B. Protein self-association in solution: The bovine beta-lactoglobulin dimer and octamer. Protein Sci. 2003, 12, 2404–2411. [Google Scholar] [CrossRef]
- Pintado, M.E.; Malcata, F.X. Studies on genetic variants of α-lactalbumin and β-lactoglobulin from milk of native Portuguese ovine and caprine breeds. Int. J. Food Sci. Technol. 1999, 34, 245–252. [Google Scholar] [CrossRef]
- Amigo, L.; Recio, I.; Ramos, M. Genetic polymorphism of ovine milk proteins: Its influence on technological properties of milk—A review. Int. Dairy J. 2000, 10, 135–149. [Google Scholar] [CrossRef]
- Pena, R.; Sánchez, A.; Folch, J. Characterization of genetic polymorphism in the goat β-lactoglobulin gene. J. Dairy Res. 2000, 67, 217–224. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Ha, M.; Bekhit, A.E.; McConnell, M.; Mason, S.; Carne, A. Fractionation of whey proteins from red deer (Cervus elaphus) milk and comparison with whey proteins from cow, sheep and goat milks. Small Rumin. Res. 2014, 120, 125–134. [Google Scholar] [CrossRef]
- Hahn, R.; Schulz, P.M.; Schaupp, C.; Jungbauer, A. Bovine whey fractionation based on cation-exchange chromatography. J. Chromatogr. A 1998, 795, 277–287. [Google Scholar] [CrossRef]
- Nassar, K.S.; Lu, J.; Pang, X.; Ragab, E.S.; Yue, Y.; Obaroakpo, U.J.; Gebreyowhans, S.; Hussain, N.; Bayou, Y.; Zhang, S.; et al. The functionality of micellar casein produced from retentate caprine milk treated by HP. J. Food Eng. 2021, 288, 110144. [Google Scholar] [CrossRef]
- Altuner, E.M.; Alpas, H.; Erdem, Y.K.; Bozoglu, F. Effect of high hydrostatic pressure on physicochemical and biochemical properties of milk. Eur. Food Res. Technol. 2006, 222, 392–396. [Google Scholar] [CrossRef]
- Baier, D.; Purschke, B.; Schmitt, C.; Rawel, H.M.; Knorr, D. Effect of high pressure—Low temperature treatments on structural characteristics of whey proteins and micellar caseins. Food Chem. 2015, 187, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Torio, M.A.O.; Itoh, T.; Garcia, R.N.; Maruyama, N.; Utsumi, S.; Tecson-Mendoza, E.M. Introduction of sulfhydryl groups and disulfide linkage to mungbean 8Sa globulin and effects on physicochemical and functional properties. Food Res. Int. 2012, 45, 277–282. [Google Scholar] [CrossRef]
- Zhong, J.Z.; Liu, W.; Liu, C.M.; Wang, Q.H.; Li, T.; Tu, Z.C.; Luo, S.J.; Cai, X.F.; Xu, Y.J. Aggregation and conformational changes of bovine b-lactoglobulin subjected to dynamic high-pressure microfluidization in relation to antigenicity. J. Dairy Sci. 2012, 95, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, T.; Li, M.; Munkh-Amgalan, G.; Qayum, A.; Bilawal, A.; Jiang, Z. Consequences of dynamic high-pressure homoge-nization pretreatment on the physicochemical and functional characteristics of citric acid-treated whey protein isolate. LWT 2021, 136, 110303. [Google Scholar] [CrossRef]





| Component | Goat Whey | Sheep Whey |
|---|---|---|
| Ash (% w/w) | 0.60 ± 0.03 | 0.63 ± 0.04 |
| Total dry matter (% w/w) | 16.65 ± 2.26 | 20.60 ± 3.02 |
| Lactose * (% w/w) | 4.57 ± 0.33 | 4.00 ± 0.56 |
| Fat (% w/w) | 0.04 ± 0.02 | 0.01 ± 0.01 |
| Protein (NT × 6.38) (%) | 11.44 ± 1.79 | 15.96 ± 2.12 |
| Physiological pH (PpH) | 6.69 ± 0.05 | 6.74 ± 0.04 |
| Concentration factor + | 27.28 | 33.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romo, M.; Castellari, M.; Bou, R.; Gou, P.; Felipe, X. Separation of α-Lactalbumin-Enriched Fractions from Caprine and Ovine Native Whey Concentrate by Combining Membrane and High-Pressure Processing. Foods 2023, 12, 2688. https://doi.org/10.3390/foods12142688
Romo M, Castellari M, Bou R, Gou P, Felipe X. Separation of α-Lactalbumin-Enriched Fractions from Caprine and Ovine Native Whey Concentrate by Combining Membrane and High-Pressure Processing. Foods. 2023; 12(14):2688. https://doi.org/10.3390/foods12142688
Chicago/Turabian StyleRomo, María, Massimo Castellari, Ricard Bou, Pere Gou, and Xavier Felipe. 2023. "Separation of α-Lactalbumin-Enriched Fractions from Caprine and Ovine Native Whey Concentrate by Combining Membrane and High-Pressure Processing" Foods 12, no. 14: 2688. https://doi.org/10.3390/foods12142688
APA StyleRomo, M., Castellari, M., Bou, R., Gou, P., & Felipe, X. (2023). Separation of α-Lactalbumin-Enriched Fractions from Caprine and Ovine Native Whey Concentrate by Combining Membrane and High-Pressure Processing. Foods, 12(14), 2688. https://doi.org/10.3390/foods12142688







