Comparison of Flavor Profile Relationship of Soy Sauce under Different Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soy Sauce Samples
2.2. Chemicals
2.3. Electronic Nose Measurement
2.4. Electronic Tongue Measurement
2.5. The Isolation of Volatiles by SE-SAFE
2.6. GC-MS-O Analysis Conditions
2.7. Qualitative Analysis of Volatile Compounds
2.8. Quantitative Analysis of Odor-Active Compounds
2.9. Amino Acid Determination
2.10. Data Analysis
3. Results and Discussion
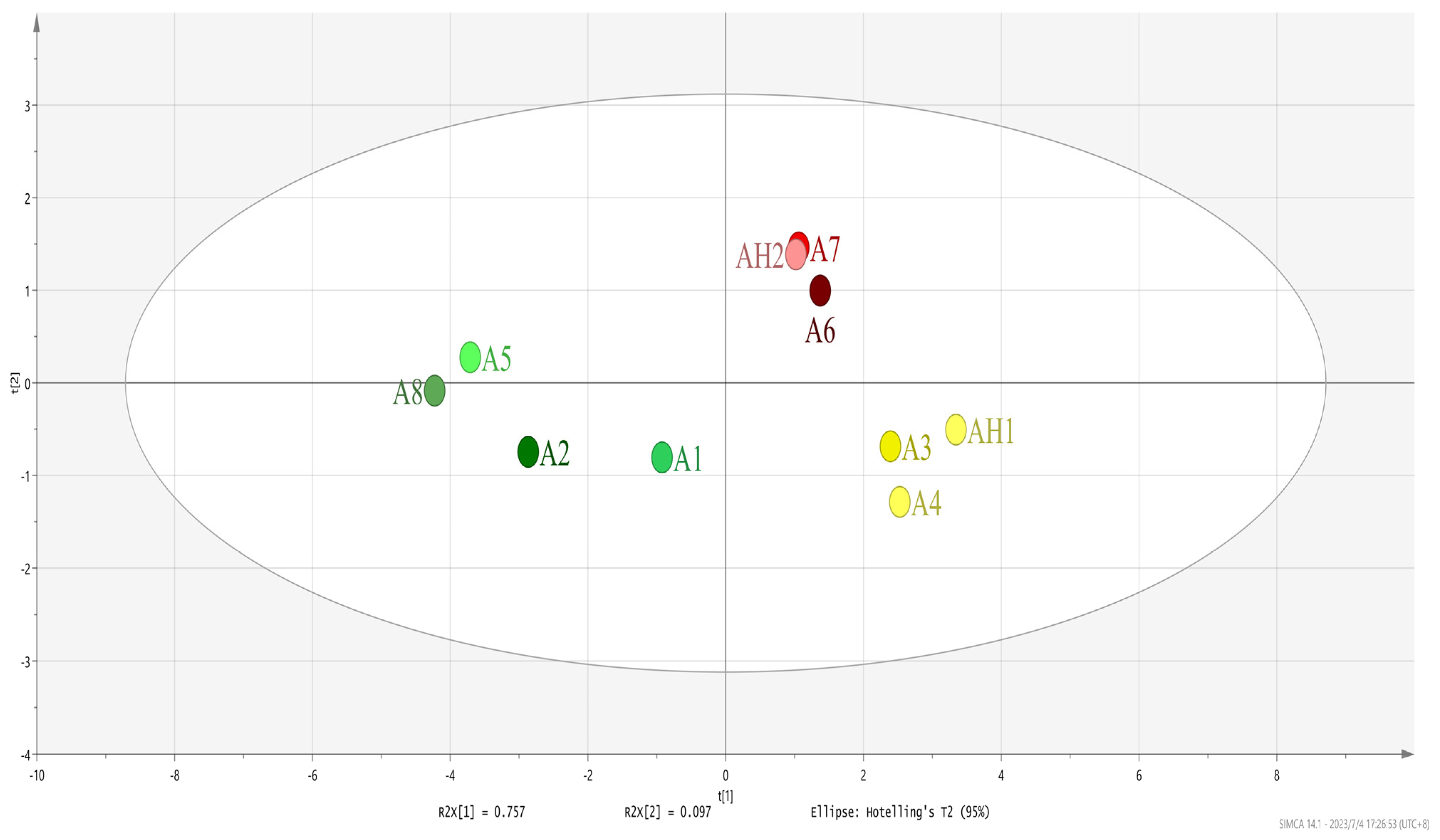
3.1. Electronic Nose Analysis
3.2. Electronic Tongue Analysis
3.3. Analysis of Odor-Active Compounds
3.3.1. Alcohols
3.3.2. Furans
3.3.3. Acids
3.3.4. Ketones
3.3.5. Phenols
3.3.6. Esters
3.3.7. Pyrazines
3.3.8. Aldehydes
3.4. Heatmap Analysis of Odor-Active Compounds in 10 SS
3.5. OAVs Analysis
3.6. Composition and Content Analysis of Amino Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, G.Z.; Feng, Y.Z.; Hadiatullah, H.; Zheng, F.P.; Yao, Y.P. Chemical Characteristics of Three Kinds of Japanese Soy Sauce Based on Electronic Senses and GC-MS Analyses. Front. Microbiol. 2020, 11, 579808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Z.; Li, J.J.; Zheng, F.P.; Yao, Y.J. The fermentation properties and microbial diversity of soy sauce fermented by germinated soybean. J. Sci. Food Agric. 2021, 101, 2920–2929. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Robert, D. Chemical and Sensory Characteristics of Soy Sauce: A Review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.L.; Guo, M.Y.; Meng, Q.; Song, H.L. Characterization of key odor-active compounds in high quality high-salt liquid-state soy sauce. J. Food. Compos. Anal. 2023, 117, 105148. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, L.L.; Zhang, Y.Y.; Sun, B.G.; Sun, Y.; Zhao, J. Evaluation of non-volatile taste components in commercial soy sauces. Int. J. Food Prop. 2018, 21, 1854–1866. [Google Scholar]
- Wang, W.C.; Zheng, Y.F.; Wang, S.C.; Kuo, C.Y.; Chien, H.J.; Hong, X.G.; Hsu, Y.M.; Lai, C.C. The identification of soy sauce adulterated with bean species and the origin using headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Food Chem. 2023, 404, 134638. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Y.; Wang, X.J.; Meng, Q.; Song, H.L. Analysis of key ddor compounds and changes in flavor characteristics of dark soy sauce during storage. Food Sci. Technol. 2021, 46, 259–266. [Google Scholar]
- Gharibzahedi, S.M.T.; Barba, F.J.; Zhou, J.; Wang, M.; Altintas, Z. Electronic Sensor Technologies in Monitoring Quality of Tea: A Review. Biosensors 2022, 12, 356. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.Y.; Wang, Y.; Kong, B.H.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2020, 140, 110764. [Google Scholar] [CrossRef]
- Guan, C.B.; Liu, T.T.; Li, Q.H.; Wang, D.W.; Zhang, Y.R. Analyzing the Effect of Baking on the Flavor of Defatted Tiger Nut Flour by E-Tongue, E-Nose and HS-SPME-GC-MS. Foods 2022, 11, 446. [Google Scholar] [CrossRef]
- Zhu, D.S.; Ren, X.J.; Wei, L.W.; Cao, X.H.; Ge, Y.H.; Liu, H.; Li, J.R. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Wang, X.J.; Guo, M.Y.; Song, H.L.; Meng, Q.; Guan, X.S. Characterization of key odor-active compounds in commercial high-salt liquid-state soy sauce by switchable GC/GC× GC–olfactometry–MS and sensory evaluation. Food Chem. 2021, 342, 128224. [Google Scholar] [CrossRef]
- Liang, R.; Huang, J.; Wu, X.M.; Fan, J.; Xu, Y.; Wu, C.D.; Jin, Y.; Zhou, R.Q. Investigating the differences of flavor profiles between two types of soy sauce by heat-treatment. Int. J. Food Prop. 2019, 22, 1998–2008. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Guo, T.; Lu, Y.L.; Hadiatullah, H.; Li, P.; Ding, K.L.; Zhao, G.Z. Effects of amino acid composition of yeast extract on the microbiota and aroma quality of fermented soy sauce. Food Chem. 2022, 393, 133289. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, B.G.; Mao, X.Y.; Chen, H.T.; Zhang, Y.Y. Flavor formation in frying process of green onion (Allium fistulosum L.) deep-fried oil. Food Res. Int. Food Res. Int. 2019, 121, 296–306. [Google Scholar] [CrossRef]
- Liang, L.; Duan, W.; Zhang, J.C.; Huang, Y.; Zhang, Y.Y.; Sun, B.G. Characterization and molecular docking study of taste peptides from chicken soup by sensory analysis combined with nano-LC-Q-TOF-MS/MS. Food Chem. 2022, 383, 132455. [Google Scholar] [CrossRef] [PubMed]
- Ayseli, M.T.; Kelebek, H.; Selli, S. Elucidation of aroma-active compounds and chlorogenic acids of Turkish coffee brewed from medium and dark roasted Coffea arabica beans. Food Chem. 2020, 338, 127821. [Google Scholar]
- Zhang, D.L.; He, Y.Y.; Cao, Y.F.; Ma, C.Y.; Li, H.J. Flavor improvement of fermented soy sauce by extrusion as soybean meal pretreatment. J. Food. Process Pers. 2017, 41, e13172. [Google Scholar] [CrossRef]
- Shu, W.J.; Lin-Xia, H.E.; Jiang, S.S.; Liu, S.L.; Guo, J.; Jian, L.U.; Gao, X.L. Study on the characteristics of taste compounds and taste of sweet soy sauce. Food Sci. Technol. 2015, 11, 54–58. [Google Scholar]
- Kim, M.J.; Son, H.J.; Kim, Y.; Misaka, T.; Rhyu, M.R. Umami–bitter interactions: The suppression of bitterness by umami peptides via human bitter taste receptor. Biochem. Biophys. Rep. 2015, 456, 586–590. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.T.; Zhang, Z.M.; Liu, Y.P.; Liu, B.S.; Sun, B.G. Investigations on the Key Odorants Contributing to the Aroma of Children Soy Sauce by Molecular Sensory Science Approaches. Foods 2021, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Studies on the key aroma compounds in raw (unheated) and heated Japanese soy sauce. J. Agric. Food Chem. 2013, 61, 3396–3402. [Google Scholar] [CrossRef]
- Xiang, H.; Yin, W.Y.; Cui, C. Effects of Different Concentrations of Solids during Fermentation on the Aroma Compounds of Soy Sauce Assessed by SPME-DSE. Modern Food Sci. Technol. 2016, 32, 259–267. [Google Scholar]
- Sluis, C.; Tramper, J.; Wijffels, R.H. Technology Enhancing and accelerating flavour formation by salt-tolerant yeasts in Japanese soy-sauce processes. Trends Food Sci. Technol. 2001, 12, 322–327. [Google Scholar] [CrossRef]
- Meng, Q.; Imamura, M.; Katayama, H.; Obata, A.; Sugawara, E. Key compounds contributing to the fruity aroma characterization in Japanese raw soy sauce. Biosci. Biotechnol. Biochem. 2017, 81, 1984–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunomura, N.; Sasaki, M.; Asao, Y.; Tokotsuka, T. Isolation and Identification of 4-Hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone, as a Flavor Component in Shoyu (Soy Sauce). Agric. Biol. Chem. 1976, 40, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Steinhaus, P.; Schieberle, P. Characterization of the Key Aroma Compounds in Soy Sauce Using Approaches of Molecular Sensory Science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, Y.; Nishimura, K.; Slaughter, J.C. Formation Mechanisms of Flavour-active Furanones, 4-Hydroxy-2, 5-dimethyl-3 (2 H)-furanone (HDMF) and 4-Hydorxy-2 (or 5)-ethyl-5 (or 2)-methyl-3 (2 H)-furanone (HEMF), in Mugi-miso, Fermented Soy-beans Paste with Barley-Koji. J. Inst. Brew. 1998, 93, 730–738. [Google Scholar]
- Wang, X.J.; Meng, Q.; Song, H.L. Characterization of odor-active compounds in high-salt liquid-state soy sauce after cooking. Food Chem. 2022, 373, 131460. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Seo, B.C.; Kim, Y.S. Volatile compounds in fermented and acid-hydrolyzed soy sauces. J. Food Sci. 2006, 71, C146–C156. [Google Scholar] [CrossRef]
- Choi, U.K.; Kim, M.H.; Kwon, O.; Lee, T.J. Biotechnology. Characterization of Aroma Components in Barley Bran Sauce Using Statistical Analysis. Food Sci. Biotechnol. 2007, 16, 23–28. [Google Scholar]
- Gao, L.H.; Liu, T.; An, X.J.; Zhang, J.L.; Ma, X.R.; Cui, J.M. Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC–MS combined with HS-SPME and discrimination with electronic nose. J. Food Sci. Technol. 2017, 54, 130–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieberle, P. The role of free amino acids present in yeast as precursors of the odorants 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine in wheat bread crust. Z. Fuer Lebensm. Unters. Und Forsch. 1990, 191, 206–209. [Google Scholar] [CrossRef]
- Pearl, I. Vanillin from Lignin Materials. Acs Sustain. Chem. Eng. 1942, 64, 1429–1431. [Google Scholar] [CrossRef]
- Ozkara, K.T.; Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. GC-MS-Olfactometric Differentiation of Aroma-Active Compounds in Turkish Heat-Treated Sausages by Application of Aroma Extract Dilution Analysis. Food Anal. Methods 2019, 12, 729–741. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Cui, C.; Zhao, H.F.; Gao, X.L.; Zhao, M.M.; Sun, W.Z. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int. J. Food Sci. Technol. 2013, 48, 609–619. [Google Scholar] [CrossRef]
- Wanakhachornkrai, P.; Lertsiri, S. Comparison of determination method for volatile compounds in Thai soy sauce. Food Chem. 2003, 83, 619–629. [Google Scholar] [CrossRef]
- Gao, X.L.; Cui, C.; Zhao, H.F.; Zhao, M.M.; Yang, L.; Ren, J.Y. Changes in Volatile Aroma Compounds of Traditional Chinese-type Soy Sauce During Moromi Fermentation and Heat Treatment. Food Sci. Biotechnol. 2010, 19, P889–P898. [Google Scholar] [CrossRef]
- Wang, S.; Tamura, T.; Kyouno, N.; Liu, X.F.; Zhang, H.; Akiyama, Y.; Chen, J.Y. Effect of volatile compounds on the quality of Japanese fermented soy sauce. LWT 2019, 111, 594–601. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Baek, H.H.; Kim, H.J. Solid Phase Microextraction-Gas Chromatography-Olfactometry of Soy Sauce Based on Sample Dilution Analysis. Food Sci. Biotechnol. 1999, 47, 1616–1618. [Google Scholar]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]



| Nos. | Sensor Names | General Description |
|---|---|---|
| 1 | W1C | Aromatic components |
| 2 | W5S | Sensitive to nitrogen oxides |
| 3 | W3C | Ammonia, sensitive to aromatic compounds |
| 4 | W6S | Mainly hydrogen (selective) |
| 5 | W5C | Alkanes, aromatic constituents |
| 6 | W1S | Sensitive to methane |
| 7 | W1W | Sensitive to sulfur compounds |
| 8 | W2S | Sensitive to alcohol compounds |
| 9 | W2W | Aromatic components, sensitive to sulfur organic compounds |
| 10 | W3S | Sensitive to alkane components |
| Nos. a | Compounds | RI b | CAS | Odor Description c | Identification d | |
|---|---|---|---|---|---|---|
| DB-WAX | HP-5MS | |||||
| 1 | Isobutanol | 1099 | ND e | 78-83-1 | sour | O, MS, RI, S |
| 2 | 1-Butanol | 1149 | ND | 71-36-3 | malty, balsam | O, MS, RI, S |
| 3 | 3-Methyl-1-butanol | 1213 | 745 | 123-51-3 | malty | O, MS, RI, S |
| 4 | Methylpyrazine | 1277 | 833 | 109-08-0 | roasted, nutty | O, MS, RI, S |
| 5 | Acetoin | 1294 | 723 | 513-86-0 | creamy | O, MS, RI, S |
| 6 | Acetol | 1309 | 704 | 116-09-6 | sweet | O, MS, RI, S |
| 7 | 2,5-Dimethylpyrazine | 1335 | 917 | 123-32-0 | roasted | O, MS, RI, S |
| 8 | 2,6-Dimethylpyrazine | 1341 | 917 | 108-50-9 | roasted, nutty | O, MS, RI, S |
| 9 | 2-Acetyl-1-pyrrolidine | 1344 | ND | 85213-22-5 | rice, popcorn | O, MS, RI |
| 10 | Ethyl lactate | 1347 | 826 | 97-64-3 | fruity, buttery | O, MS, RI, S |
| 11 | 1-Hydroxy-2-butanone | 1382 | 777 | 5077-67-8 | sweet, coffee | O, MS, RI, S |
| 12 | 2-Ethyl-5-methylpyrazine | 1396 | 1003 | 13360-64-0 | roasted, coffee | O, MS, RI, S |
| 13 | 2,3,5-Trimethylpyrazine | 1417 | 1007 | 14667-55-1 | nutty, peanut | O, MS, RI, S |
| 14 | Acetic acid | 1441 | ND | 64-19-7 | sour | O, MS, RI, S |
| 15 | Methional | 1461 | 912 | 3268-49-3 | cooked potato-like | O, MS, RI, S |
| 16 | Propionic acid | 1537 | 734 | 79-09-4 | cheesy | O, MS, RI, S |
| 17 | Isobutyric acid | 1569 | ND | 79-31-2 | sour | O, MS, RI, S |
| 18 | Phenylacetaldehyde | 1650 | 1050 | 122-78-1 | honey, sweet | O, MS, RI, S |
| 19 | γ-Butyrolactone | 1643 | 923 | 96-48-0 | creamy, caramel | O, MS, RI, S |
| 20 | Furfuryl alcohol | 1660 | 964 | 98-00-0 | burnt | O, MS, RI, S |
| 21 | 3-Methylbutanoic acid | 1670 | 869 | 503-74-2 | sweaty, cheese | O, MS, RI, S |
| 22 | Methionol | 1724 | 985 | 505-10-2 | cooked potato-like | O, MS, RI, S |
| 23 | 3-Methyl-2(5H)-furanone | 1729 | ND | 22122-36-7 | roasted | O, MS, RI, S |
| 24 | 2(5H)-Furanone | 1766 | ND | 497-23-4 | buttery | O, MS, RI, S |
| 25 | 4-Methylpentanoic acid | 1809 | 960 | 646-07-1 | cheese | O, MS, RI, S |
| 26 | Methyl cyclopentenolone | 1835 | ND | 80-71-7 | caramel | O, MS, RI, S |
| 27 | Guaiacol | 1865 | 1095 | 90-05-1 | smoky | O, MS, RI, S |
| 28 | Phenylethyl alcohol | 1919 | 1124 | 60-12-8 | floral, rose | O, MS, RI, S |
| 29 | Maltol | 1977 | 1128 | 118-71-8 | sweet | O, MS, RI, S |
| 30 | 4-ethyl-2-methoxyphenol | 2034 | 1286 | 2785-89-9 | smoky | O, MS, RI, S |
| 31 | HDMF | 2038 | 1086 | 85554-61-6 | caramel | O, MS, RI, S |
| 32 | HEMF | 2072 | ND | 27538-09-6 | caramel | O, MS, RI, S |
| 33 | 4-Ethylphenol | 2175 | ND | 123-07-9 | smoky | O, MS, RI, S |
| 34 | Phenylacetic acid | 2579 | 1263 | 103-82-2 | honey | O, MS, RI, S |
| 35 | Ethyl vanillate | 2633 | 1597 | 617-05-0 | burnt | O, MS, RI, S |
| Nos. | Compounds | f a | Ions (m/z) b | Concentrations (Mean ± Standard Deviation, μg/L) c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | AH1 | A5 | A6 | A7 | A8 | AH2 | ||||
| 1 | Isobutanol | 2.31 | 74 | 707 ± 6.73c | 741 ± 21.3b | 759 ± 6.27b | 790 ± 5.47a | 742 ± 5.39c | 770 ± 130a | 871 ± 20.3a | 639 ± 51b | 636 ± 18.6b | 838 ± 14.5a |
| 2 | 1-Butanol | 0.68 | 56 | 5269 ± 109b | 5268 ± 250b | 5473 ± 99.1b | 5479 ± 101b | 5316 ± 436a | 5198 ± 59.3d | 6581 ± 20.5a | 5783 ± 68c | 5217 ± 35.5d | 6008 ± 105b |
| 3 | 3-Methyl-1-butanol | 0.94 | 70 | 6596 ± 63.8c | 6551 ± 184c | 6832 ± 61b | 6947 ± 43.7b | 7276 ± 18.1a | 7075 ± 1382a | 8163 ± 159a | 7625 ± 317a | 5677 ± 130b | 7480 ± 93.1a |
| 6 | Acetol | 1.69 | 74 | 3556 ± 8.61d | 3794 ± 74.1c | 3905 ± 26b | 3915 ± 24.8b | 4168 ± 27.4a | 4059 ± 530b | 4562 ± 29.9a | 4508 ± 176a | 3594 ± 68.7c | 4569 ± 60.7a |
| 28 | Phenylethyl alcohol | 0.70 | 122 | 4550 ± 9.6b | 4601 ± 48.4b | 4586 ± 49.1b | 4617 ± 38.2b | 4802 ± 27.1a | 4715 ± 836c | 5533 ± 69.1ab | 5776 ± 191a | 4226 ± 15.9c | 4871 ± 32.5bc |
| 20 | Furfuryl alcohol | 1.03 | 98 | 4593 ± 13.1a | 4562 ± 85.7a | 4726 ± 35b | 4765 ± 28.8b | 5120 ± 13.6a | 4896 ± 996b | 5700 ± 55ab | 6112 ± 162a | 3931 ± 42.6c | 5628 ± 59.1ab |
| 22 | Methionol | 0.73 | 106 | 7062 ± 34.6c | 6970 ± 158.3c | 7242 ± 39.6b | 7281 ± 28.3b | 7688 ± 19.1a | 7620 ± 1317b | 8635 ± 57.9b | 9689 ± 176a | 6423 ± 25.3c | 8039 ± 155b |
| 11 | 1-Hydroxy-2-butanone | 1.41 | 57 | 4657 ± 36.3d | 4846 ± 25.7c | 5134 ± 20.7a | 5002 ± 24.7b | 5121 ± 26.2a | 5456 ± 511c | 5920 ± 55.7ab | 6304 ± 90.1a | 5360 ± 32.9c | 5525 ± 48.9bc |
| 26 | Methyl cyclopentenolone | 0.67 | 112 | 485 ± 1.19c | 485 ± 6.93c | 502 ± 2.06b | 504 ± 4b | 532 ± 4.26a | 511 ± 1.85e | 591 ± 4.05c | 710 ± 5.54a | 575 ± 0.87d | 598 ± 2.86b |
| 9 | 2-Acetyl-1-pyrrolidine | 1.00 | 83 | 67.0 ± 0.82c | 58.0 ± 4.01d | 84.0 ± 1.09b | 96.0 ± 0.63a | 83.0 ± 0.86b | 73.7 ± 9.95c | 83.3 ± 0.39b | 91.5 ± 1.99a | 40.4 ± 1.04d | 75.7 ± 1.45bc |
| 29 | Maltol | 0.95 | 126 | 7984 ± 50.5d | 8080 ± 56.2cd | 8181 ± 56.2bc | 8270 ± 60.9ab | 8363 ± 96.2a | 8382 ± 1057c | 9552 ± 109b | 10,597 ± 258a | 8066 ± 49.4c | 8899 ± 71.7bc |
| 5 | Acetoin | 1.70 | 88 | 1396 ± 8.08d | 1484 ± 15.2c | 1553 ± 12.4b | 1547 ± 6.28b | 1577 ± 11.5a | 1607 ± 139c | 1754 ± 4.85b | 1766 ± 40.4b | 1547 ± 16.8c | 1722 ± 18.6b |
| 35 | Ethyl vanillate | 0.78 | 91 | 127 ± 0.81c | 77 ± 0.34e | 148 ± 1.94a | 140 ± 3.1b | 111 ± 0.61d | 156 ± 2.59d | 178 ± 2.87c | 187 ± 2.79b | 194 ± 1.57a | 153 ± 2.3d |
| 10 | Ethyl lactate | 1.52 | 75 | 3054 ± 32.9b | 2950 ± 69.7c | 3123 ± 26.3ab | 3123 ± 39.2ab | 3192 ± 31.2a | 2736 ± 31.3d | 3754 ± 0b | 3997 ± 34.4a | 2767 ± 16.5d | 3284 ± 34.7c |
| 19 | γ-Butyrolactone | 1.40 | 86 | 1832 ± 5.88c | 1826 ± 8.21c | 1932 ± 9.62a | 1888 ± 1.77b | 1946 ± 18.5a | 2054 ± 184c | 2241 ± 10.7b | 2526 ± 37.3a | 2069 ± 17.5c | 2128 ± 11bc |
| 15 | Methional | 0.90 | 104 | 415 ± 9.37e | 585 ± 12.4a | 465 ± 16.4c | 439 ± 14d | 516 ± 8.05b | 444 ± 66.9c | 511 ± 13.5b | 589 ± 26.6a | 328 ± 8.53d | 536 ± 15.3ab |
| 18 | Phenylacetaldehyde | 0.27 | 91 | 1307 ± 9.04d | 2288 ± 33.7a | 1412 ± 17.3c | 1216 ± 22.2e | 1574 ± 43.1b | 1197 ± 223b | 1411 ± 24.7a | 1589 ± 65.3a | 747 ± 8c | 1537 ± 99a |
| 27 | Guaiacol | 0.60 | 124 | 211 ± 0.73d | 218 ± 0.96c | 221 ± 0.85b | 211 ± 1.48d | 237 ± 1.55a | 244 ± 19.3c | 264 ± 0.25b | 287 ± 1.46a | 276 ± 1.67ab | 285 ± 1.37a |
| 33 | 4-Ethylphenol | 3.58 | 107 | 255 ± 1.34c | 240 ± 2.52d | 259 ± 1.82bc | 260 ± 3.21b | 268 ± 1.69a | 267 ± 2.47e | 307 ± 0b | 493 ± 0.28a | 300 ± 0.7c | 292 ± 2.54d |
| 30 | 4-ethyl-2-methoxyphenol | 0.33 | 137 | 4512 ± 9.89d | 4542 ± 28.8cd | 4629 ± 19.4b | 4559 ± 27.8c | 4681 ± 13.5a | 4934 ± 585c | 5492 ± 20.2b | 5892 ± 39.7b | 5026 ± 45.5c | 4967 ± 12.6c |
| 34 | Phenylacetic acid | 0.62 | 91 | 2888 ± 45.6c | 2167 ± 18.8e | 3576 ± 56a | 3421 ± 46.7b | 2780 ± 85.8d | 3693 ± 163d | 3831 ± 63.8cd | 4518 ± 72.3a | 4219 ± 51.9b | 3866 ± 31.3c |
| 14 | Acetic acid | 1.36 | 60 | 8838 ± 55.5e | 9576 ± 260d | 10,286 ± 41.4c | 10,507 ± 45.5b | 10,335 ± 76.3a | 10,782 ± 1796b | 12,026 ± 161b | 9321 ± 313c | 8813 ± 140c | 12,284 ± 200b |
| 17 | Isobutyric acid | 1.18 | 73 | 522 ± 5.07d | 544 ± 4.96c | 595 ± 13.2a | 571 ± 6.27b | 597 ± 6.05a | 638 ± 58.5b | 685 ± 2.65ab | 707 ± 5.52a | 636 ± 9.59b | 663 ± 9ab |
| 21 | 3-Methylbutanoic acid | 1.16 | 60 | 860 ± 6.37d | 929 ± 4.18b | 956 ± 3.99a | 908 ± 7.89c | 947 ± 3.79a | 992 ± 134c | 1113 ± 4.75ab | 1155 ± 13.4a | 949 ± 11.3c | 1035 ± 3.86bc |
| 16 | Propionic acid | 0.76 | 74 | 2249 ± 79.3c | 2424 ± 94.2b | 2603 ± 105a | 2533 ± 106ab | 2617 ± 93.2a | 2661 ± 17.8c | 2882 ± 73.9b | 2874 ± 64.4b | 2931 ± 54.3b | 3071 ± 7.84a |
| 25 | 4-Methylpentanoic acid | 0.90 | 74 | 389 ± 0.76c | 381 ± 1.28d | 408 ± 2.03b | 405 ± 3.61b | 416 ± 1.71a | 409 ± 3.44c | 478 ± 1.09b | 555 ± 3.62a | 427 ± 46.4c | 471 ± 5.21b |
| 4 | Methylpyrazine | 0.44 | 94 | 430 ± 2.47d | 454 ± 5.03b | 473 ± 1.86a | 443 ± 3.99c | 470 ± 2a | 458 ± 5.12e | 550 ± 2.65b | 600 ± 4.55a | 540 ± 3.93c | 515 ± 5.79d |
| 8 | 2,6-Dimethylpyrazine | 0.42 | 108 | 1077 ± 5.63b | 1096 ± 62.9b | 1166 ± 4.5a | 1083 ± 15.7b | 1175 ± 6.09a | 1135 ± 16.4e | 1366 ± 9.55b | 1426 ± 7.43a | 1292 ± 2.08d | 1338 ± 16.85c |
| 7 | 2,5-Dimethylpyrazine | 1.07 | 108 | 337 ± 1.07ab | 316 ± 30.3b | 353 ± 1.65a | 344 ± 1.5a | 356 ± 2.67a | 334 ± 2.67d | 386 ± 32.4c | 463 ± 1.28b | 356 ± 5.64d | 393 ± 1.13c |
| 12 | 2-Ethyl-5-methylpyrazine | 0.96 | 121 | 138 ± 0.68d | 144 ± 1.35c | 148 ± 0.21b | 139 ± 1.84d | 151 ± 0.46a | 147 ± 2.22c | 174 ± 4.17b | 185 ± 0.86a | 178 ± 1.88b | 175 ± 1.88b |
| 13 | 2,3,5-Trimethylpyrazine | 0.37 | 122 | 555 ± 3.64c | 573 ± 5.67b | 592 ± 2.08a | 556 ± 6.75c | 588 ± 1.23a | 574 ± 7.37d | 698 ± 2.23b | 741 ± 3.18a | 659 ± 6c | 653 ± 7.73c |
| 31 | HDMF | 0.97 | 128 | 3485 ± 33.3e | 3380 ± 25.9d | 3641 ± 11.5c | 3736 ± 26.7b | 4118 ± 22.8a | 3853 ± 405d | 4256 ± 33.1c | 4955 ± 99.8b | 3708 ± 32.1d | 5689 ± 77.6a |
| 32 | HEMF | 1.00 | 142 | 28,756 ± 164bc | 25,929 ± 105d | 30,006 ± 125ab | 30,807 ± 227a | 33,389 ± 226c | 31,348 ± 2681c | 34,165 ± 65.3b | 39,729 ± 382a | 30,307 ± 214c | 31,688 ± 257c |
| 24 | 2(5H)-Furanone | 0.99 | 84 | 559 ± 25.9bc | 546 ± 17c | 620 ± 26.6a | 596 ± 26.4ab | 601 ± 18.8ab | 671 ± 34.9c | 713 ± 46.8bc | 786 ± 24.6a | 713 ± 28.5bc | 766 ± 40.2ab |
| 23 | 3-Methyl-2(5H)-furanone | 1.42 | 98 | 243 ± 1.36b | 236 ± 3.16c | 255 ± 0.71a | 245 ± 0.94b | 253 ± 1.57a | 275 ± 18c | 294 ± 1.87b | 337 ± 3.18a | 290 ± 1.95bc | 275 ± 1.26c |
| Nos. | Compounds | Threshold a (μg/kg) | OAVs b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | AH1 | A5 | A6 | A7 | A8 | AH2 | |||
| 1 | Isobutanol | 6505 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 6 | Acetol | 10,000 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 2 | 1-Butanol | 459 | 12 | 12 | 12 | 12 | 12 | 11 | 14 | 13 | 11 | 13 |
| 28 | Phenylethyl alcohol | 564 | 8 | 8 | 8 | 8 | 9 | 8 | 10 | 10 | 7 | 9 |
| 20 | Furfuryl alcohol | 4501 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 22 | Methionol | 123 | 57 | 57 | 59 | 59 | 62 | 62 | 70 | 79 | 52 | 65 |
| 3 | 3-Methyl-1-butanol | 4 | 1649 | 1638 | 1708 | 1737 | 1819 | 1769 | 2041 | 1906 | 1419 | 1870 |
| 11 | 1-Hydroxy-2-butanone | - c | - | - | - | - | - | - | - | - | - | - |
| 26 | Methyl cyclopentenolone | 300 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 9 | 2-Acetyl-1-pyrrolidine | 0.12 | 560 | 484 | 704 | 796 | 688 | 614 | 695 | 762 | 336 | 631 |
| 29 | Maltol | 1240 | 6 | 7 | 7 | 7 | 7 | 7 | 8 | 9 | 7 | 7 |
| 5 | Acetoin | 14 | 100 | 106 | 111 | 110 | 113 | 115 | 125 | 126 | 110 | 123 |
| 35 | Ethyl vanillate | - | - | - | - | - | - | - | - | - | - | - |
| 10 | Ethyl lactate | 50,000 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 19 | γ-Butyrolactone | 1000 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 |
| 15 | Methional | 0.45 | 923 | 1299 | 1033 | 977 | 1147 | 986 | 1136 | 1308 | 729 | 1192 |
| 18 | Phenylacetaldehyde | 6.3 | 208 | 363 | 224 | 193 | 250 | 190 | 224 | 252 | 119 | 244 |
| 27 | Guaiacol | 1.6 | 132 | 136 | 138 | 132 | 148 | 153 | 165 | 179 | 173 | 178 |
| 33 | 4-Ethylphenol | 21 | 12 | 11 | 12 | 12 | 13 | 13 | 15 | 24 | 14 | 14 |
| 30 | 4-ethyl-2-methoxyphenol | 89 | 51 | 51 | 52 | 51 | 53 | 55 | 62 | 66 | 56 | 56 |
| 34 | Phenylacetic acid | 12,000 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 14 | Acetic acid | 99,000 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 17 | Isobutyric acid | 6551 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 21 | 3-Methylbutanoic acid | 490 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 16 | Propionic acid | 2190 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25 | 4-Methylpentanoic acid | 810 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 4 | Methylpyrazine | 30,000 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 8 | 2,6-Dimethylpyrazine | 718 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 7 | 2,5-Dimethylpyrazine | 1750 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 12 | 2-Ethyl-5-methylpyrazine | 40 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 5 | 4 | 4 |
| 13 | 2,3,5-Trimethylpyrazine | 350 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 31 | HDMF | 22 | 156 | 152 | 163 | 168 | 185 | 173 | 191 | 222 | 166 | 255 |
| 32 | HEMF | 1.15 | 250,06 | 22,547 | 26,092 | 26,789 | 24,413 | 27,259 | 29,709 | 34,547 | 26,354 | 27,555 |
| 24 | 2(5H)-Furanone | 714 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 23 | 3-Methyl-2(5H)-furanone | - | - | - | - | - | - | - | - | - | - | - |
| Amino Acid | Concentrations (mg/mL) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | AH1 | A5 | A6 | A7 | A8 | AH2 | |
| Aspartic acid | 7.77 | 7.90 | 8.03 | 8.26 | 8.09 | 7.85 | 8.27 | 7.95 | 7.53 | 7.98 |
| Glycine | 3.11 | 3.16 | 3.20 | 3.26 | 3.22 | 3.09 | 3.29 | 3.17 | 3.00 | 3.15 |
| Alanine | 3.53 | 3.52 | 3.63 | 3.64 | 3.65 | 3.28 | 3.76 | 3.59 | 3.39 | 3.58 |
| Threonine | 2.67 | 2.64 | 2.75 | 2.77 | 2.79 | 2.70 | 2.81 | 2.72 | 2.58 | 2.71 |
| Serine | 3.59 | 3.71 | 3.73 | 3.88 | 3.76 | 3.64 | 3.84 | 3.66 | 3.50 | 3.74 |
| Proline | 4.03 | 4.06 | 4.18 | 4.30 | 4.24 | 4.12 | 4.32 | 4.14 | 3.91 | 4.15 |
| Valine | 4.49 | 4.06 | 4.49 | 4.13 | 4.55 | 4.27 | 4.60 | 4.67 | 4.27 | 4.15 |
| Isoleucine | 3.56 | 3.59 | 3.65 | 3.70 | 3.68 | 3.55 | 3.76 | 3.64 | 3.43 | 3.61 |
| Leucine | 5.24 | 5.27 | 5.39 | 5.49 | 5.45 | 5.28 | 5.56 | 5.37 | 5.08 | 5.33 |
| Phenylalanine | 3.81 | 3.38 | 3.79 | 3.57 | 3.79 | 3.76 | 3.94 | 3.96 | 3.51 | 3.53 |
| Histidine | 1.67 | 1.64 | 1.67 | 1.71 | 1.62 | 1.65 | 1.71 | 1.67 | 1.57 | 1.61 |
| Tyrosine | 0.87 | 0.91 | 0.90 | 0.94 | 0.91 | 0.88 | 0.94 | 0.89 | 0.85 | 0.90 |
| Lysine | 3.98 | 3.99 | 4.09 | 4.19 | 4.13 | 4.04 | 4.21 | 4.05 | 3.83 | 4.05 |
| Arginine | 1.40 | 1.40 | 1.44 | 1.47 | 1.43 | 1.42 | 1.47 | 1.43 | 1.35 | 1.38 |
| Cystine | 1.09 | 0.85 | 1.02 | 0.78 | 1.06 | 0.05 | 1.11 | 1.27 | 0.98 | 0.80 |
| Methionine | 1.05 | 1.26 | 1.05 | 1.12 | 1.03 | 0.89 | 1.07 | 1.06 | 0.98 | 1.01 |
| 51.8 | 51.3 | 53.0 | 53.2 | 53.4 | 50.5 | 54.7 | 53.2 | 49.8 | 51.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Liang, M.; Zhang, Z.; Wu, Y.; Liu, Y. Comparison of Flavor Profile Relationship of Soy Sauce under Different Storage Conditions. Foods 2023, 12, 2707. https://doi.org/10.3390/foods12142707
Wang R, Liang M, Zhang Z, Wu Y, Liu Y. Comparison of Flavor Profile Relationship of Soy Sauce under Different Storage Conditions. Foods. 2023; 12(14):2707. https://doi.org/10.3390/foods12142707
Chicago/Turabian StyleWang, Rui, Miao Liang, Zhimin Zhang, Yajian Wu, and Yuping Liu. 2023. "Comparison of Flavor Profile Relationship of Soy Sauce under Different Storage Conditions" Foods 12, no. 14: 2707. https://doi.org/10.3390/foods12142707
APA StyleWang, R., Liang, M., Zhang, Z., Wu, Y., & Liu, Y. (2023). Comparison of Flavor Profile Relationship of Soy Sauce under Different Storage Conditions. Foods, 12(14), 2707. https://doi.org/10.3390/foods12142707





