Characteristics and Whole-Genome Analysis of Limosilactobacillus fermentum Phage LFP02
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Phages, and Culture Conditions
2.2. Bacteriophage Host Range
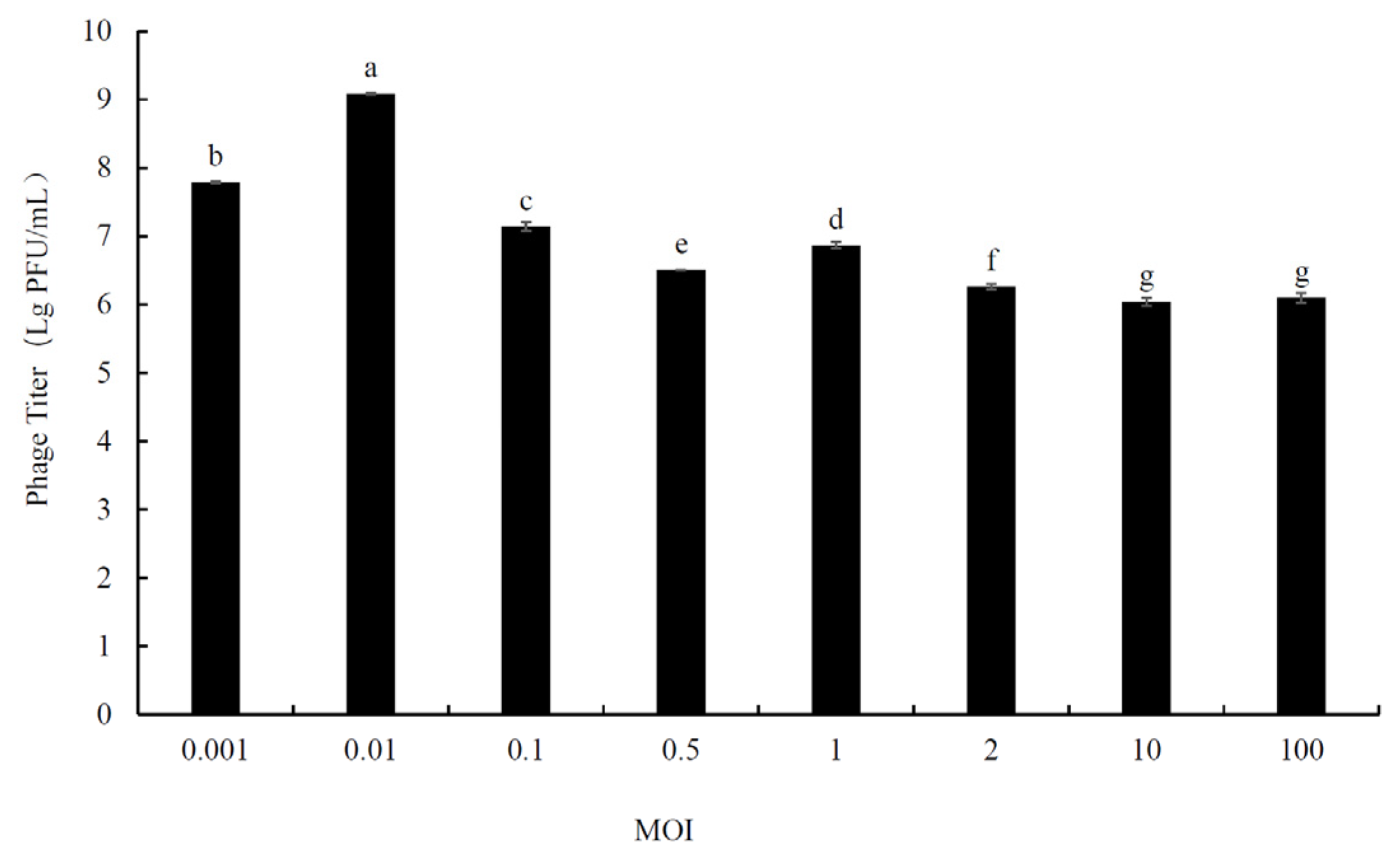
2.3. Determination of Optimal Multiplicity of Infection (MOI)
2.4. Infective Characteristic
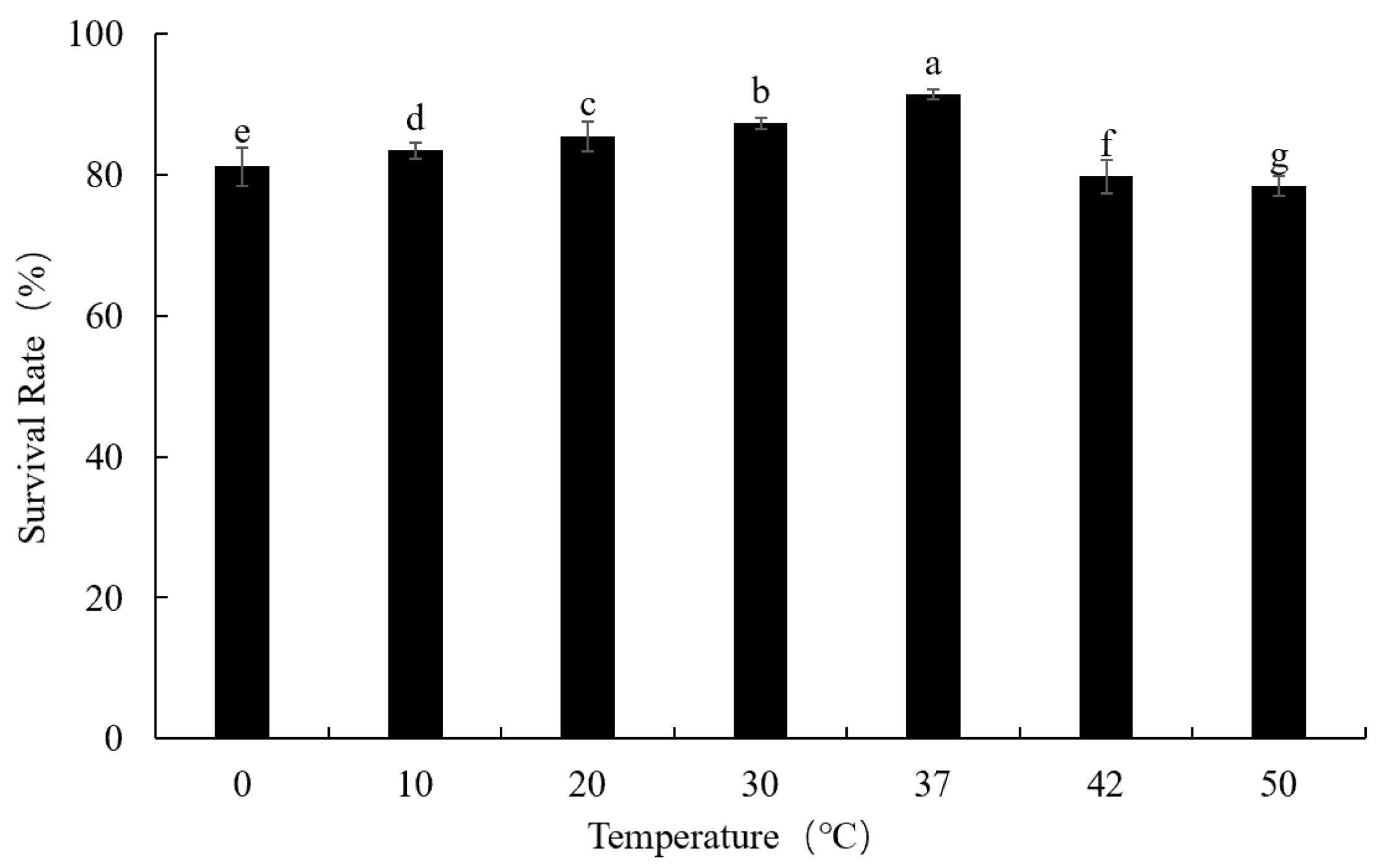
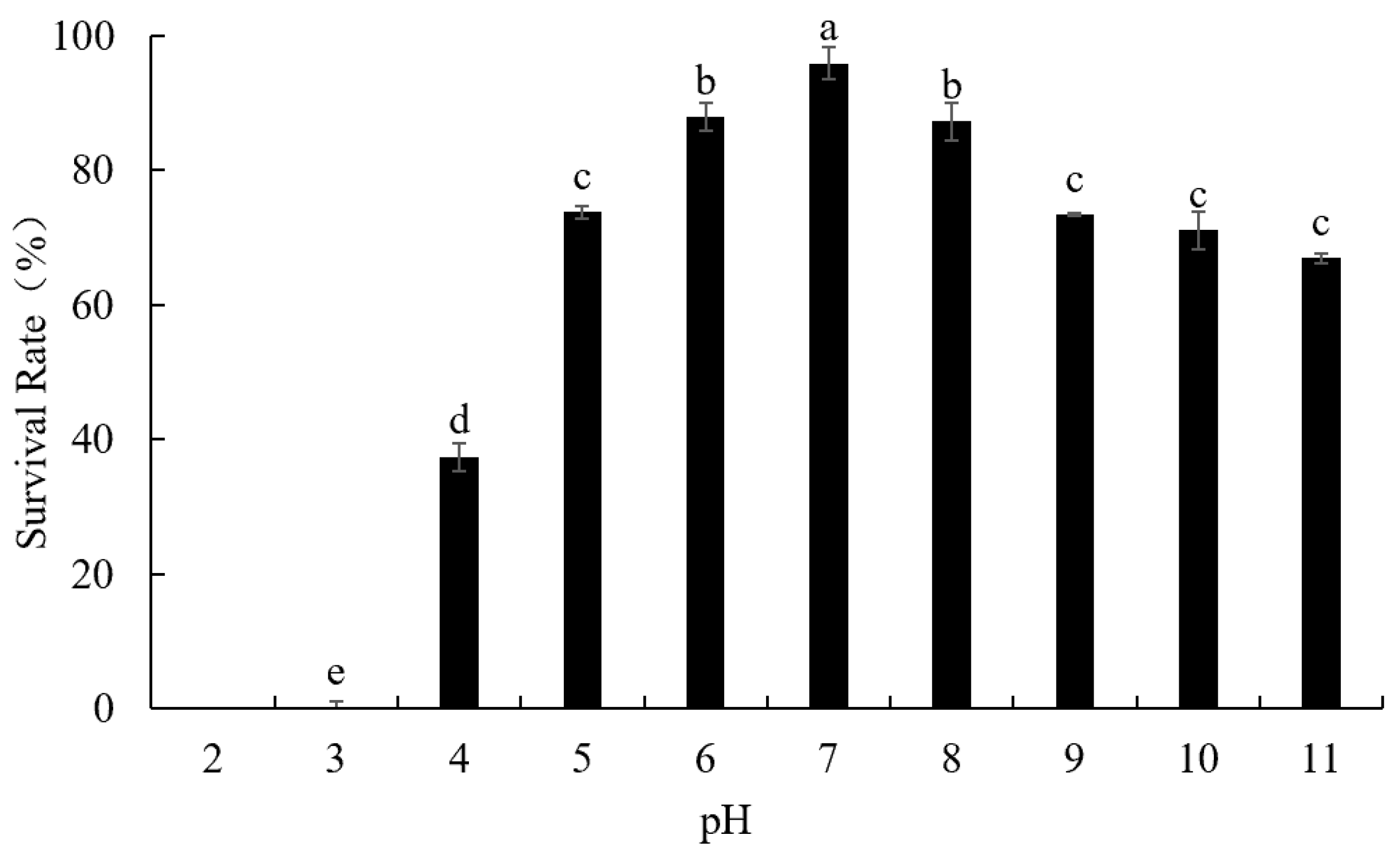
2.5. Environment Stress Tolerance
2.6. Factors Influencing Phage Adsorption
2.7. Genome Sequencing and Assembly
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phage Host Range
3.2. Determination of Optimal Multiplicity of Infection (MOI)
3.3. Infective Characteristic
3.4. Environmental Stress Tolerance
3.4.1. Thermal Stability
3.4.2. Phage LFP02 Stability at Different pH Values
3.5. Factors Influencing Phage Adsorption
3.5.1. Influence of Temperature on Phage Adsorption
3.5.2. Influence of pH on Phage Adsorption
3.5.3. Influence of Divalent Cations on Phage Adsorption
3.5.4. Influence of Cell Protein Synthesis on Phage Adsorption
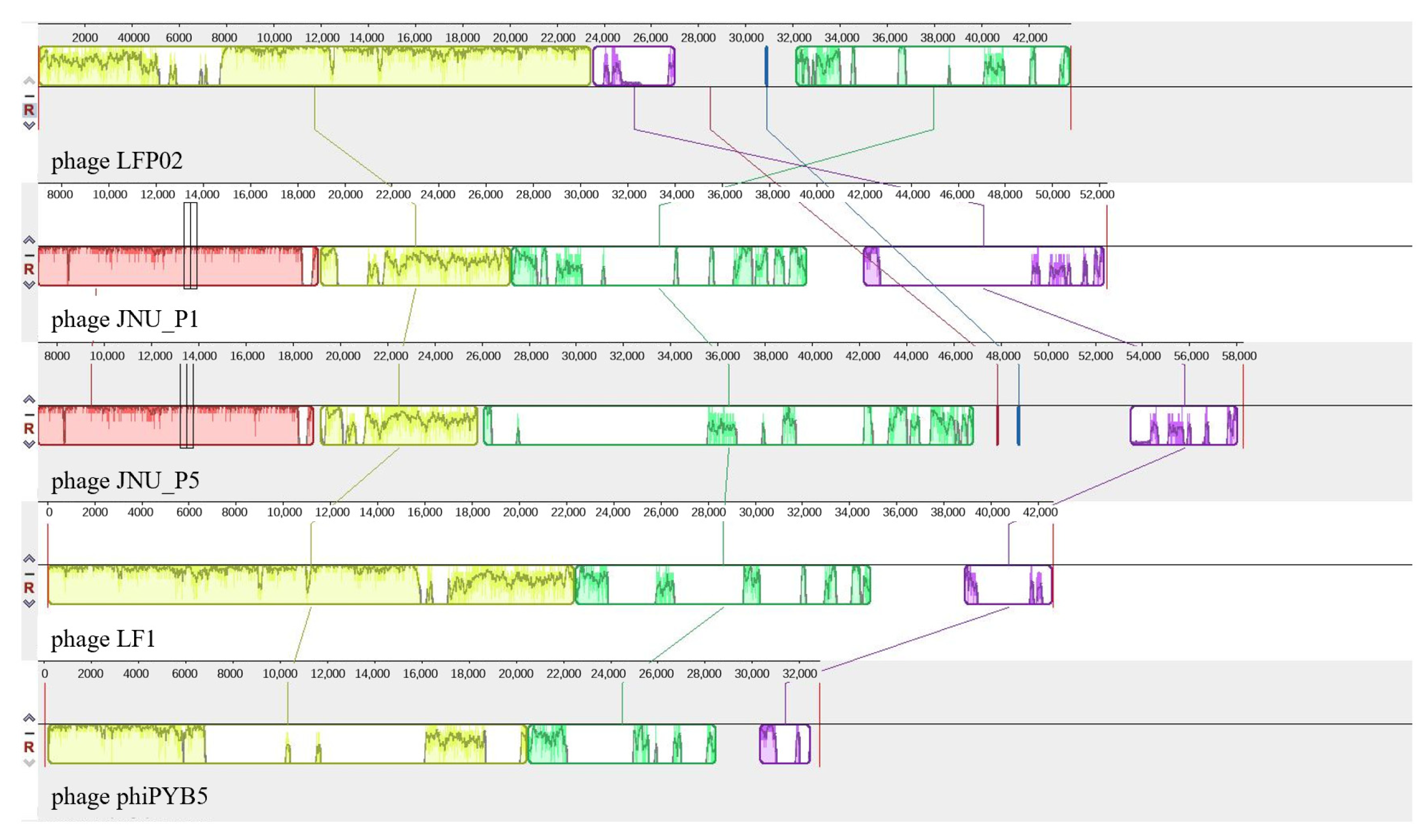
3.6. Genome Analysis
3.6.1. Genome Organization of Phage LFP02
3.6.2. Genomic Differences amongst L. fermentum Phages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3—An antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuschner, R.G.; Robinson, T.P.; Hugas, M.; Cocconcelli, P.S.; Richard-Forget, F.; Klein, G.; Licht, T.R.; Nguyen-The, C.; Querol, A.; Richardson, M.; et al. Qualified presumption of safety (QPS): A generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA). Trends Food Sci. Technol. 2010, 21, 425–435. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.; Abedon, S.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temper-ate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.-H.; Lin, X.B.; Zhang, S.; Tollenaar, S.L.; Özçam, M.; Dunphy, C.; Walter, J.; van Pijkeren, J.-P. Prophages in Lactobacillus reuteri are associated with fitness trade-offs but can in-crease competitiveness in the gut ecosystem. Appl. Environ. Microbiol. 2019, 86, e01922-19. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.; Sadiq, F.A.; Han, X.; Zhao, J.; Zhang, H.; Ross, R.P.; Lu, W.; Chen, W. Identification, characterization, and phylogenetic analysis of eight new inducible prophages in Lactobacillus. Virus Res. 2020, 286, 198003. [Google Scholar] [CrossRef]
- Sadiq, F.A.; He, G.; Sakandar, H.A.; Li, Y.; Ou, K. Lactococcus lactis phages from the perspective of their diversity, thermal and biocidal resistance. Int. Dairy J. 2018, 90, 28–38. [Google Scholar] [CrossRef]
- Marcó, M.B.; Reinheimer, J.; Quiberoni, A. Phage adsorption and lytic propagation in Lactobacillus plantarum: Could host cell starvation affect them? BMC Microbiol. 2015, 15, 273. [Google Scholar]
- Maske, B.L.; Pereira, G.V.d.M.; Vale, A.d.S.; Souza, D.S.M.; Lindner, J.D.D.; Soccol, C.R. Viruses in fermented foods: Are they good or bad? Two sides of the same coin. Food Microbiol. 2021, 98, 103794. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Ács, N.; Gambino, M.; Brøndsted, L. Bacteriophage Enumeration and Detection Methods. Front. Microbiol. 2020, 11, 594868. [Google Scholar] [CrossRef]
- Atamer, Z.; Dietrich, J.; Müller-Merbach, M.; Neve, H.; Heller, K.J.; Hinrichs, J. Screening for and characterization of Lactococcus lactis bacteriophages with high thermal resistance. Int. Dairy J. 2009, 19, 228–235. [Google Scholar] [CrossRef]
- Lu, Z.; Breidt, F.; Fleming, H.; Altermann, E.; Klaenhammer, T. Isolation and characterization of a Lactobacillus plantarum bacteriophage, ΦJL-1, from a cucumber fermentation. Int. J. Food Microbiol. 2003, 84, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Xu, M.; Guo, S.; Yao, J.; Sakandar, H.A.; Guo, J.; Zhang, C.; Chen, X. Characterization of a novel Bacillus methylotrophicus phage BM-P1. Food Qual. Saf. 2023, 7, fyad016. [Google Scholar] [CrossRef]
- Briggiler Marcó, M.; Reinheimer, J.A.; Quiberoni, A. Phage adsorption to Lactobacillus plantarum: Influence of physiological and environmental factors. Int. J. Food Microbiol. 2010, 138, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.; Quiberoni, A.; Reinheimer, J. Phages of Lactobacillus casei/paracasei: Response to environmental factors and interaction with collection and commercial strains. J. Appl. Microbiol. 2006, 100, 334–342. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.H.; Lee, N.G.; Lee, J.S.; Yoon, S.S. Whole-genome sequencing and genomic analysis of a virulent bacteriophage infecting Bacillus cereus. Intervirology 2018, 61, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Bickhart, D.M.; Behsaz, B.; Gurevich, A.; Rayko, M.; Shin, S.B.; Kuhn, K.; Yuan, J.; Polevikov, E.; Smith, T.P.L.; et al. metaFlye: Scalable long-read metagenome assembly using repeat graphs. Nat. Methods 2020, 17, 1103–1110. [Google Scholar] [CrossRef]
- McNair, K.; Aziz, R.K.; Pusch, G.D.; Overbeek, R.; Dutilh, B.E.; Edwards, R. Phage Genome Annotation Using the RAST Pipeline. Bacteriophages 2017, 1681, 231–238. [Google Scholar] [CrossRef]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Forde, A.; Fitzgerald, G.F. Bacteriophage defence systems in lactic acid bacteria. Antonie van Leeuwenhoek 1999, 76, 89–113. [Google Scholar] [CrossRef]
- Capra, M.L.; Quiberoni, A.D.L.; Ackermann, H.W.; Moineau, S.; Reinheimer, J.A. Characterization of a new virulent phage (MLC-A) of Lactobacillus paracasei. J. Dairy Sci. 2006, 89, 2414–2423. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Hou, Q.; Kwok, L.; Yu, Z.; Zheng, Y.; Sun, Z.; Zhang, H. Bacterial microbiota compositions of naturally fermented milk are shaped by both geographic origin and sample type. J. Dairy Sci. 2016, 99, 7832–7841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göller, P.C.; Elsener, T.; Lorgé, D.; Radulovic, N.; Bernardi, V.; Naumann, A.; Amri, N.; Khatchatourova, E.; Coutinho, F.H.; Loessner, M.J.; et al. Multi-species host range of staphylococcal phages isolated from wastewater. Nat. Commun. 2021, 12, 6965. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Grewal, R.K.; Roy, S. Modeling bacteria—Phage interactions and its implications for phage Therap. Adv. Appl. Microbiol. 2018, 103, 103–141. [Google Scholar] [PubMed]
- Foschino, R.; Venturelli, E.; Picozzi, C. Isolation and Characterization of a Virulent Lactobacillus sanfranciscensis Bacteriophage and Its Impact on Microbial Population in Sourdough. Curr. Microbiol. 2005, 51, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, T.; Quan, R.; Jiang, X.; Tong, G.; Wei, X.; Lin, M. Biological characteristics and genomic analysis of a novel Vibrio parahaemolyticus phage phiTY18 isolated from the coastal water of Xiamen China. Front. Cell. Infect. Microbiol. 2022, 12, 1035364. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, M.; Mahony, J.; Lugli, G.A.; Ventura, M.; Neve, H.; Franz, C.M.A.P.; Noben, J.-P.; O’sullivan, T.; van Sinderen, D. Isolation and Characterization of Lactobacillus brevis Phages. Viruses 2019, 11, 393. [Google Scholar] [CrossRef] [Green Version]
- Mercanti, D.J.; Ackermann, H.W.; Quiberoni, A. Characterization of two temperate Lactobacillus paracasei bacteriophages: Morphology, kinetics and adsorption. Intervirology 2015, 58, 49–56. [Google Scholar] [CrossRef]
- De Antoni, G.; Zago, M.; Vasek, O.; Giraffa, G.; Carminati, D.; Marcó, M.B.; Reinheimer, J.; Suárez, V. Lactobacillus plantarum bacteriophages isolated from Kefir grains: Phenotyp-ic and molecular characterization. J. Dairy Res. 2010, 77, 7–12. [Google Scholar] [CrossRef]
- Sunthornthummas, S.; Doi, K.; Rangsiruji, A.; Krajangsung, S.; Sarawaneeyaruk, S.; Pringsulaka, O. Isolation and characterization of spontaneous phage-resistant mutants of Lactobacillus paracasei. Food Control 2019, 99, 114–123. [Google Scholar] [CrossRef]
- Müller-Merbach, M.; Neve, H.; Hinrichs, J. Kinetics of the thermal inactivation of the Lactococcus lactis bacteriophage P008. J. Dairy Res. 2005, 72, 281–286. [Google Scholar] [CrossRef]
- Coffey, B.; Rivas, L.; Duffy, G.; Coffey, A.; Ross, R.P.; McAuliffe, O. Assessment of Escherichia coli O157:H7-specific bacteriophages e11/2 and e4/1c in model broth and hide environments. Int. J. Food Microbiol. 2011, 147, 188–194. [Google Scholar] [CrossRef]
- Sunthornthummas, S.; Doi, K.; Rangsiruji, A.; Sarawaneeyaruk, S.; Pringsulaka, O. Isolation and characterization of Lactobacillus paracasei LPC and phage ΦT25 from fermented milk. Food Control 2017, 73, 1353–1361. [Google Scholar] [CrossRef]
- Ruan, C.; Niu, X.; Xiong, G.; Chen, G.; Wu, H.; Ma, Z.; Zhu, K.; Liu, Y. Phenotypic and genotypic characterization of the new Bacillus Cereus phage SWEP1. Arch. Virol. 2021, 166, 3183–3188. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P.; Rodríguez, A. Environmental pH is a key modulator of Staphylococcus aureus biofilm development under predation by the virulent phage phiIPLA-RODI. ISME J. 2021, 15, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, B.W.; Wang, H.; Schwab, K.; Jacangelo, J. Selected Mechanistic Aspects of Viral Inactivation by Peracetic Acid. Environ. Sci. Technol. 2021, 55, 16120–16129. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiberoni, A.; Guglielmotti, D.; Binetti, A.; Reinheimer, J. Characterization of three Lactobacillus delbrueckii subsp. bulgaricus phages and the physicochemical analysis of phage adsorption. J. Appl. Microbiol. 2004, 96, 340–351. [Google Scholar] [CrossRef]
- Tomat, D.; Quiberoni, A.; Casabonne, C.; Balagué, C. Phage adsorption on Enteropathogenic and Shiga Toxin-Producing Escherichia coli strains: Influence of physicochemical and physiological factors. Food Res. Int. 2014, 66, 23–28. [Google Scholar] [CrossRef]
- Vörös, Z.; Csík, G.; Herényi, L.; Kellermayer, M. Temperature-Dependent Nanomechanics and Topography of Bacteriophage T7. J. Virol. 2018, 92, e01236-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sae-Ueng, U.; Bhunchoth, A.; Phironrit, N.; Treetong, A.; Sapcharoenkun, C.; Chatchawankanphanich, O.; Leartsakulpanich, U. Thermoresponsive C22 phage stiffness modulates the phage infectivity. Sci. Rep. 2022, 12, 13001. [Google Scholar] [CrossRef]
- Trucco, V.; Reinheimer, J.; Quiberoni, A.; Suárez, V.B. Adsorption of temperate phages of Lactobacillus delbrueckii strains and phage resistance linked to their cell diversity. J. Appl. Microbiol. 2011, 104, 371–379. [Google Scholar]
- Chen, X.; Xi, Y.; Zhang, H.; Wang, Z.; Fan, M.; Liu, Y.; Wu, W. Characterization and adsorption of Lactobacillus virulent phage P1. J. Dairy Sci. 2016, 99, 6995–7001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lan, Y.; Jiao, W.; Li, Y.; Tang, L.; Jiang, Y.; Cui, W. Isolation and characterization of a novel virulent phage of Lactobacillus casei ATCC 393. Food Environ. Virol. 2015, 7, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Cvirkaite-Krupovic, V.; Krupovic, M.; Daugelavicius, R.; Bamford, D.H. Calcium ion-dependent entry of the membrane-containing bacteriophage PM2 into its Pseudoalteromonas host. Virology 2010, 405, 120–128. [Google Scholar] [CrossRef]
- Sharma, S.; Datta, S.; Chatterjee, S.; Dutta, M.; Samanta, J.; Vairale, M.G.; Gupta, R.; Veer, V.; Dwivedi, S.K. Isolation and characterization of a lytic bacteriophage against Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 19393. [Google Scholar] [CrossRef]
- Geller, B.; Ngo, H.; Mooney, D.; Su, P.; Dunn, N. Lactococcal 936-Species Phage Attachment to Surface of Lactococcus lactis. J. Dairy Sci. 2005, 88, 900–907. [Google Scholar] [CrossRef] [Green Version]
- Veesler, D.; Spinelli, S.; Mahony, J.; Lichière, J.; Blangy, S.; Bricogne, G.; Legrand, P.; Ortiz-Lombardia, M.; Campanacci, V.; van Sinderen, D.; et al. Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 8954–8958. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.; Tremblay, D.M.; Labrie, S.J.; Moineau, S.; van Sinderen, D. Investigating the requirement for calcium during lactococcal phage infection. Int. J. Food Microbiol. 2015, 201, 47–51. [Google Scholar] [CrossRef]
- Kala, S.; Cumby, N.; Sadowski, P.D.; Hyder, B.Z.; Kanelis, V.; Davidson, A.R.; Maxwell, K.L. HNH proteins are a widespread component of phage DNA packaging machines. Proc. Natl. Acad. Sci. USA 2014, 111, 6022–6027. [Google Scholar] [CrossRef] [PubMed]
- Jarocki, P.; Komoń-Janczara, E.; Podleśny, M.; Kholiavskyi, O.; Pytka, M.; Kordowska-Wiater, M. Genomic and Proteomic Characterization of Bacteriophage BH1 Spontaneously Released from Probiotic Lactobacillus rhamnosus Pen. Viruses 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, D.; Huang, Y.; Zhu, X.; Rui, M.; Wan, T.; Zheng, X.; Shen, Y.; Chen, X.; Ma, K.; et al. Structural and functional characterization of deep-sea thermophilic bacteriophage GVE2 HNH endonuclease. Sci. Rep. 2017, 7, srep42542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokareddy, R.K.; Sankhala, R.S.; Roy, A.; Afonine, P.V.; Motwani, T.; Teschke, C.M.; Parent, K.N. Portal protein functions akin to a DNA-sensor that couples genome-packaging to icosahedral capsid maturation. Nat. Commun. 2017, 8, 14310. [Google Scholar] [CrossRef] [Green Version]
- Suhanovsky, M.M.; Teschke, C.M. Nature׳s favorite building block: Deciphering folding and capsid assembly of proteins with the HK97-fold. Virology 2015, 479–480, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, R.W. Bacteriophage HK97: Assembly of the Capsid and Evolutionary Connections. Adv. Virus Res. 2005, 64, 1–14. [Google Scholar] [CrossRef]
- Leprince, A.; Nuytten, M.; July, E.; Tesseur, C.; Mahillon, J. Getting outside the cell: Versatile holin strategies used by distinct phages toleave their Bacillus thuringiensis host. J. Virol. 2022, 96, e0069622. [Google Scholar] [CrossRef]
- Erill, I.; Campoy, S.; Barbé, J. Aeons of distress: An evolutionary perspective on the bacterial SOS response. FEMS Microbiol. Rev. 2007, 31, 637–656. [Google Scholar] [CrossRef] [Green Version]
- Fornelos, N.; Browning, D.F.; Pavlin, A.; Podlesek, Z.; Hodnik, V.; Salas, M.; Butala, M. Lytic gene expression in the temperate bacteriophage GIL01 is activated by a phage-encoded LexA homologue. Nucleic Acids Res. 2018, 46, 9432–9443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrin, S.; Akter, S.; Begum, S.; Hossain, M.N. The Prospects of Lactobacillus oris as a Potential Probiotic with Cholesterol-Reducing Property From Mother’s Milk. Front. Nutr. 2021, 8, 619506. [Google Scholar] [CrossRef] [PubMed]
- Botha, M.; Botes, M.; Loos, B.; Smith, C.; Dicks, L.M.T. Lactobacillus equigenerosi Strain Le1 Invades Equine Epithelial Cells. Appl. Environ. Microbiol. 2012, 78, 4248–4255. [Google Scholar] [CrossRef] [Green Version]
- Lysnyansky, I.; Calcutt, M.J.; Ben-Barak, I.; Ron, Y.; Levisohn, S.; Methé, B.A.; Yogev, D. Molecular characterization of newly identified IS3, IS4 and IS30 insertion sequence-like elements in Mycoplasma bovis and their possible roles in genome plasticity. FEMS Microbiol. Lett. 2009, 294, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Marcó, M.B.; Garneau, J.E.; Tremblay, D.; Quiberoni, A.; Moineau, S. Characterization of Two Virulent Phages of Lactobacillus plantarum. Appl. Environ. Microbiol. 2012, 78, 8719–8734. [Google Scholar] [CrossRef] [Green Version]
- Lima-Mendez, G.; Van Helden, J.; Toussaint, A.; Leplae, R. Reticulate representation of evolutionary and functional relationships between phage genomes. Mol. Biol. Evol. 2008, 25, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.H.; Chang, H.I. Complete genomic sequence of the Lactobacillus temperate phage LF1. Arch. Virol. 2011, 156, 1909–1912. [Google Scholar] [CrossRef]
- Wang, S.; Kong, J.; Zhang, X. Identification and characterization of the two-component cell lysis cassette encoded by temperate bacteriophage phiPYB5 of Lactobacillus fermentum. J. Appl. Microbiol. 2010, 105, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Mavrich, T.N.; Hatfull, G.F. Bacteriophage evolution differs by host, lifestyle and genome. Nat. Microbiol. 2017, 2, 17112. [Google Scholar] [CrossRef] [Green Version]
- Hatfull, G.F. Bacteriophage genomic. Curr. Opin. Microbiol. 2008, 11, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef] [PubMed]

| Temperature (°C) | 10 | 20 | 30 | 37 | 42 | 50 |
|---|---|---|---|---|---|---|
| Adsorption rate | 98.39% ± 1.30 b | 98.87% ± 1.70 a | 98.86% ± 2.00 a | 99.03% ± 0.50 a | 98.96% ± 1.30 a | 94.01% ± 1.12 c |
| pH | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|
| Adsorption rate | 97.00% ± 1.90 a | 97.10% ± 1.50 a | 98.60% ± 1.12 a | 99.30% ± 1.30 a | 99.00% ± 1.70 a | 98.40% ± 2.00 a | 95.90% ± 2.50 a | 95.10% ± 1.30 a |
| Group | Adsorption Rate | ||
|---|---|---|---|
| 0 min | 15 min | 30 min | |
| Control | 0% | 98.90% ± 0.12 a | 99.60% ± 0.03 a |
| Ca2+ | 0% | 97.90% ± 0.09 b | 99.00% ± 0.06 b |
| Mg2+ | 0% | 97.00% ± 0.32 c | 99.10% ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, R.; Gao, X.; Zhang, C.; Lian, W.; Quan, X.; Guo, S.; Chen, X. Characteristics and Whole-Genome Analysis of Limosilactobacillus fermentum Phage LFP02. Foods 2023, 12, 2716. https://doi.org/10.3390/foods12142716
Lv R, Gao X, Zhang C, Lian W, Quan X, Guo S, Chen X. Characteristics and Whole-Genome Analysis of Limosilactobacillus fermentum Phage LFP02. Foods. 2023; 12(14):2716. https://doi.org/10.3390/foods12142716
Chicago/Turabian StyleLv, Ruirui, Xin Gao, Can Zhang, Weiqi Lian, Xingyu Quan, She Guo, and Xia Chen. 2023. "Characteristics and Whole-Genome Analysis of Limosilactobacillus fermentum Phage LFP02" Foods 12, no. 14: 2716. https://doi.org/10.3390/foods12142716




