Sea Bass Side Streams Extracts Obtained by Pulsed Electric Fields: Nutritional Characterization and Effect on SH-SY5Y Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Optimization Process
2.4. Pulsed Electric Fields (PEF) Treatment
2.5. Supplementary Aqueous Extraction
2.6. Minerals and Heavy Metals Determination and Quantification
2.7. Proteins
2.7.1. Total Protein Content
2.7.2. Molecular Weight Distribution
2.7.3. Bioactive Peptides Identification
2.8. Total Antioxidant Capacity Determination
2.9. Cell Cultures and Assessment of Cell Viability
2.9.1. Cell Culture
2.9.2. Assessment of Cell Viability
2.10. Statistical Analysis
3. Results
3.1. Optimization Process and Comparison with Control Sample
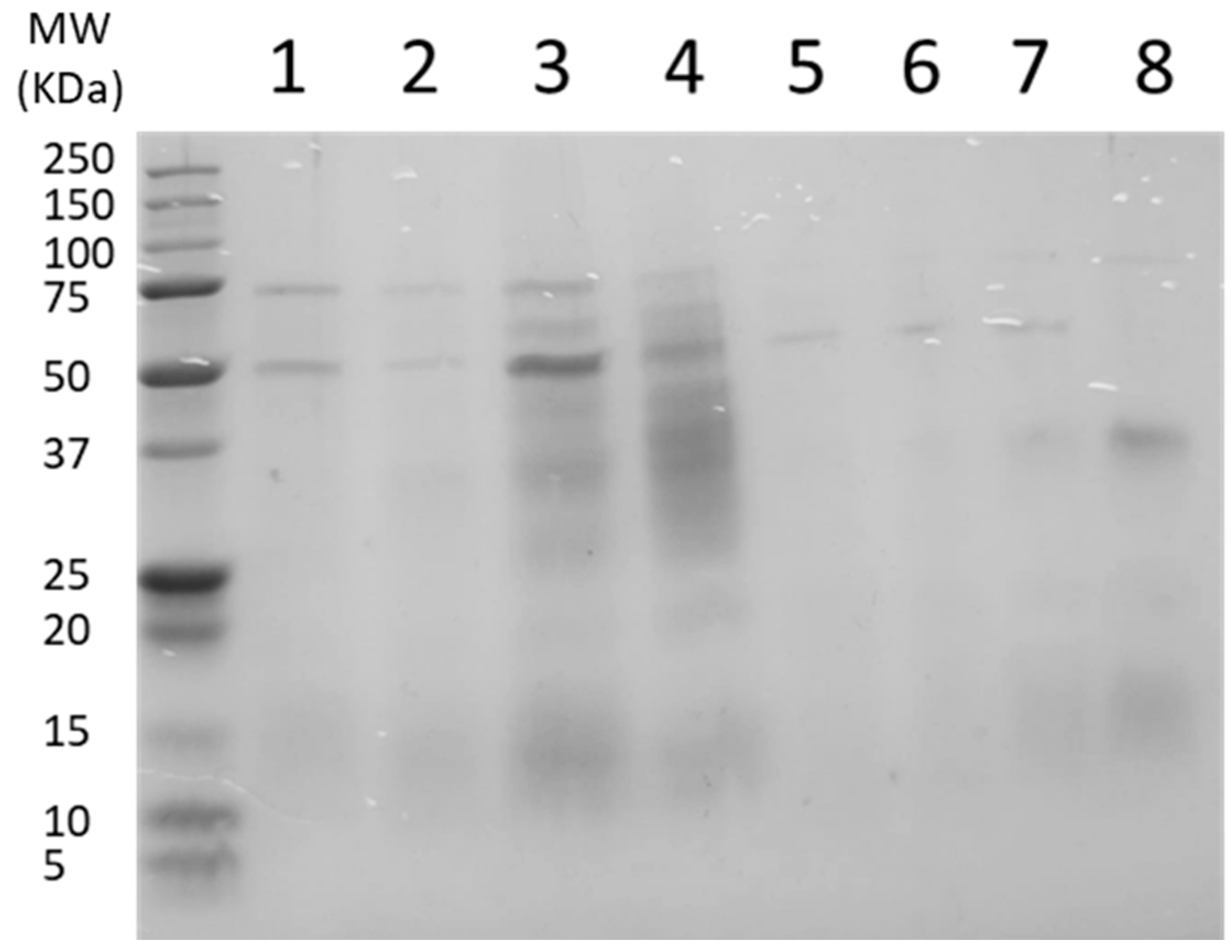
3.2. Distribution of Protein Molecular Weight
3.3. Bioactive Peptides Identification
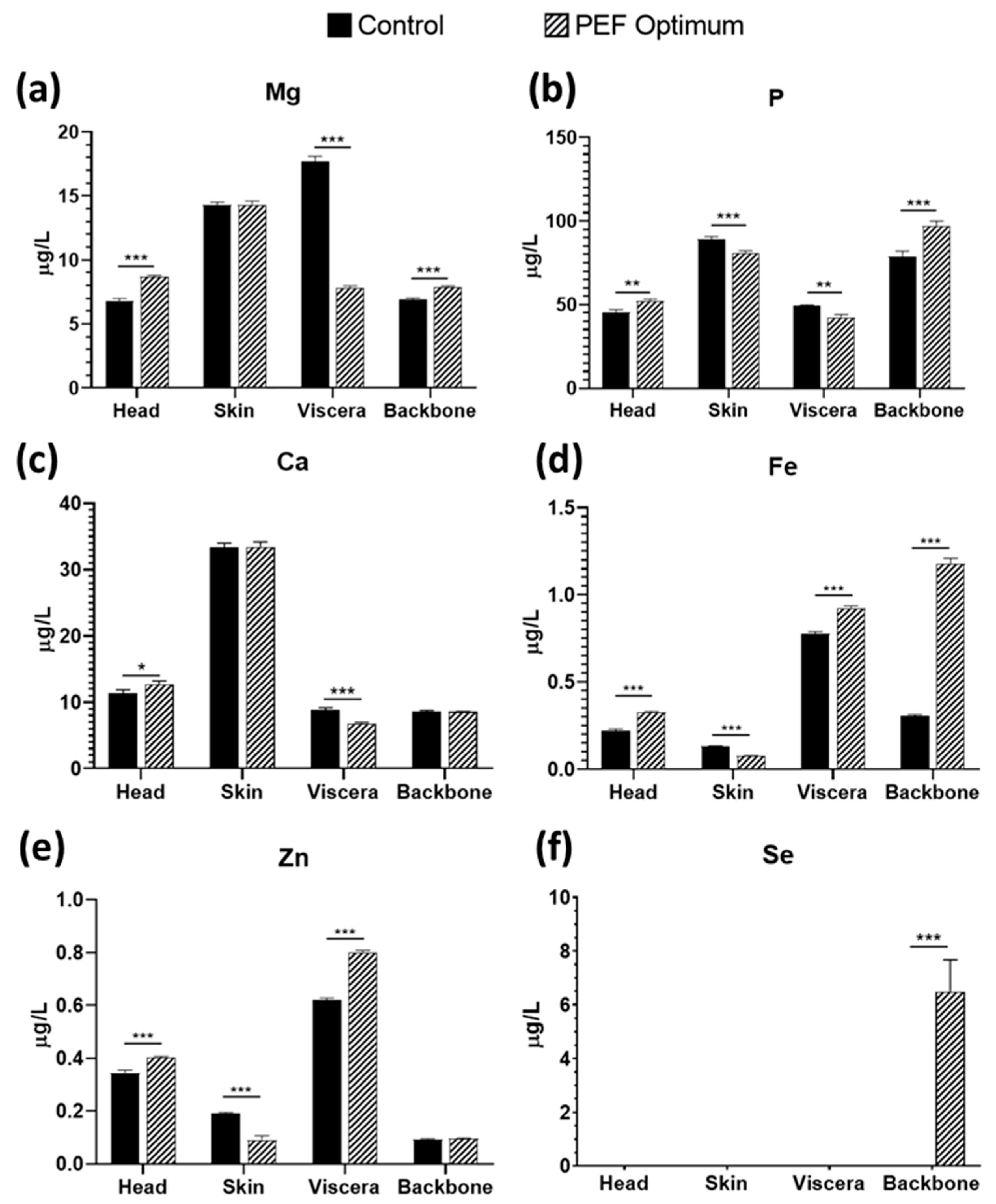
3.4. Mineral Content
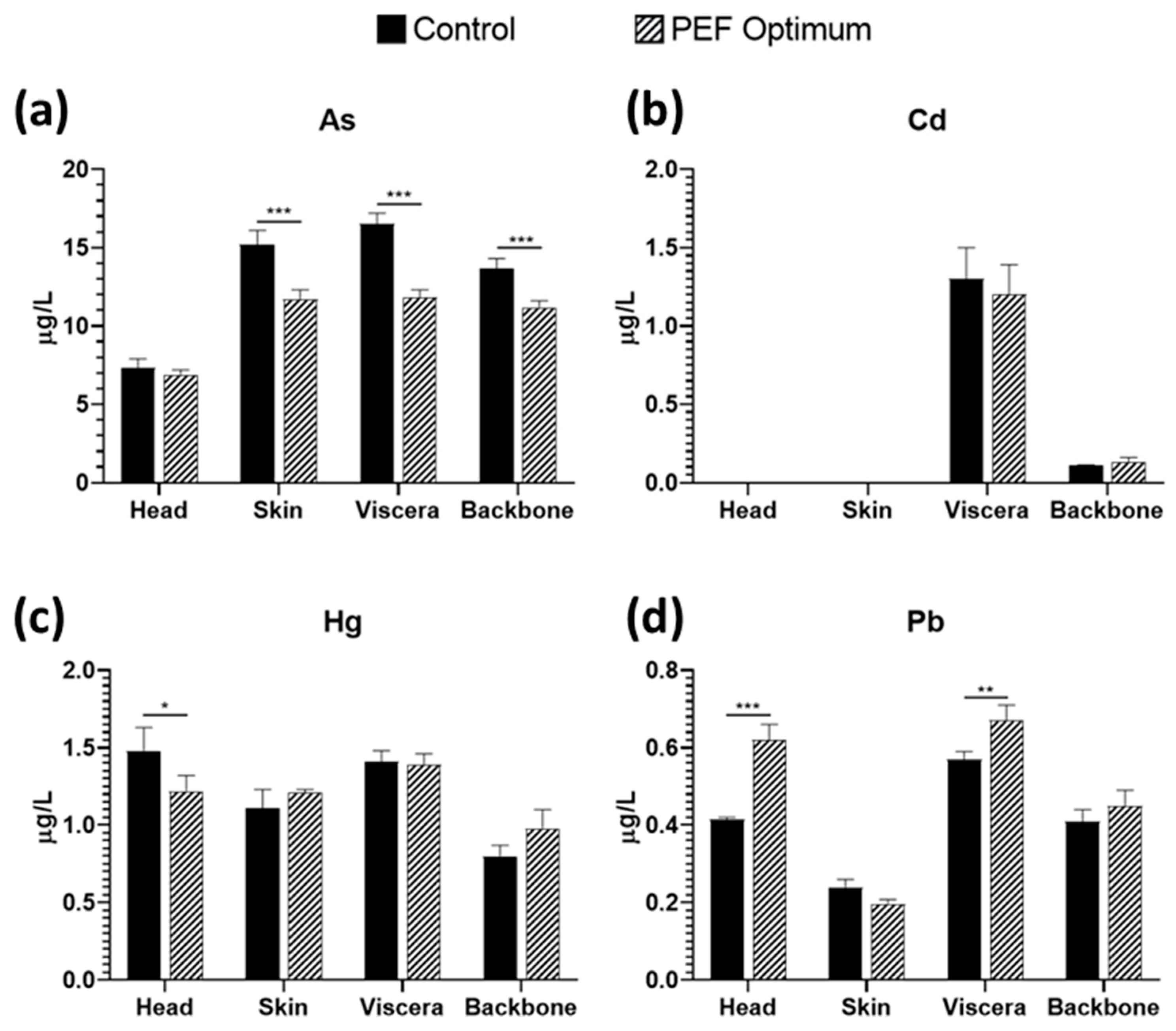
3.5. Heavy Metals Quantification
3.6. Effect of Fish Side Stream Extracts on Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Union. Circular Economy Action Plan. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 4 January 2023).
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization (FAO): Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Siddiqui, S.A.; Smaoui, S.; Ucak, İ.; Arshad, R.N.; Garcia-Oliveira, P.; Prieto, M.A.; Aït-Kaddour, A.; Perestrelo, R.; Câmara, J.S.; et al. Seafood processing, preservation, and analytical techniques in the Age of industry 4.0. Appl. Sci. 2022, 12, 1703. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [Green Version]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Collado, M.C.; Barba, F.J. Accelerated solvent extraction and pulsed electric fields for valorization of rainbow trout (Oncorhynchus mykiss) and sole (Dover sole) by-products: Protein content, molecular weight distribution and antioxidant potential of the extracts. Mar. Drugs 2021, 19, 207. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Pallarés, N.; Bäuerl, C.; Collado, M.C.; Dar, B.N.; Barba, F.J. Role of extracts obtained from rainbow trout and sole side streams by accelerated solvent extraction and pulsed electric fields on modulating bacterial and anti-inflammatory activities. Separations 2021, 8, 187. [Google Scholar] [CrossRef]
- Janssen, K.; Chavanne, H.; Berentsen, P.; Komen, H. Impact of selective breeding on European aquaculture. Aquaculture 2017, 472, 8–16. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Zhou, J.; Barba, F.J.; Lorenzo, J.M. Nutritional characterization of sea bass processing by-products. Biomolecules 2020, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Martí-Quijal, F.J.; Tornos, A.; Príncep, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.J.; Barba, F.J. Impact of fermentation on the recovery of antioxidant bioactive compounds from sea bass byproducts. Antioxidants 2020, 9, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Khawli, F.; Pallarés, N.; Martí-Quijal, F.J.; Ferrer, E.; Barba, F.J. Sea bass side streams valorization assisted by ultrasound. LC-MS/MS-IT determination of mycotoxins and evaluation of protein yield, molecular size distribution and antioxidant recovery. Appl. Sci. 2021, 11, 2160. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pallarés, N.; Barba, F.J.; Berrada, H. An integrated approach for the valorization of sea bass (Dicentrarchus labrax) side streams: Evaluation of contaminants and development of antioxidant protein extracts by pressurized liquid extraction. Foods 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.; Ahmmed, M.K.; Carne, A.; Tian, H.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Bekhit, A.E.D.A. Effect of pulsed electric fields on the lipidomic profile of lipid extracted from hoki fish male gonad. Foods 2022, 11, 610. [Google Scholar] [CrossRef]
- Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of pulsed electric fields for obtaining antioxidant extracts from fish residues. Antioxidants 2020, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Development of antioxidant protein extracts from gilthead sea bream (Sparus aurata) side streams assisted by pressurized liquid extraction (PLE). Mar. Drugs 2021, 19, 199. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Salmon (Salmo salar) side streams as a bioresource to obtain potential antioxidant peptides after applying pressurized liquid extraction (PLE). Mar. Drugs 2021, 19, 323. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Zingales, V.; Sirerol-Piquer, M.S.; Fernández-Franzón, M.; Ruiz, M.J. Role of quercetin on sterigmatocystin-induced oxidative stress-mediated toxicity. Food Chem. Toxicol. 2021, 156, 112498. [Google Scholar] [CrossRef]
- Li, M.; Lin, J.; Chen, J.; Fang, T. Pulsed electric field-assisted enzymatic extraction of protein from abalone (Haliotis discus hannai Ino) Viscera. J. Food Process. Eng. 2016, 39, 702–710. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Kim, S.K. Antioxidant peptide isolated from muscle protein of bullfrog, Rana catesbeiana shaw. J. Med. Food 2007, 10, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Sun, L.; Ju, H.; Bao, Z.; Zeng, X.A.; Lin, S. Research advances and application of pulsed electric field on proteins and peptides in food. Food Res. Int. 2021, 139, 109914. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Lima, K.O.; da Costa de Quadros, C.; da Rocha, M.; Jocelino Gomes de Lacerda, J.T.; Juliano, M.A.; Dias, M.; Mendes, M.A.; Prentice, C. Bioactivity and bioaccessibility of protein hydrolyzates from industrial byproducts of stripped weakfish (Cynoscion guatucupa). LWT 2019, 111, 408–413. [Google Scholar] [CrossRef]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘Geographical and geological influences on nutrition’ factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010, 69, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.K. Health benefits of seafood; Is it just the fatty acids? Food Chem. 2013, 140, 413–420. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The antioxidant properties of selenium and vitamin E; Their role in periparturient dairy cattle health regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef]
- Chan, J.M.; Darke, A.K.; Penney, K.L.; Tangen, C.M.; Goodman, P.J.; Lee, G.S.M.; Sun, T.; Peisch, S.; Tinianow, A.M.; Rae, J.M.; et al. Selenium- or Vitamin E-related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in SELECT. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Marreiro, D.d.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; Soares de Oliveira, A.R. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawarazuka, N. The contribution of fish intake, aquaculture, and small-scale fisheries to improving food and nutrition security: A literature review. WorldFish Cent. Work. Pap. 2010, 2106, 51. [Google Scholar]
- Ishikawa, M.; Kato, M.; Mihori, T.; Watanabe, H.; Sakai, Y. Effect of vapor pressure on the rate of softening of fish bone by super-heated steam cooking. Nippon Suisan Gakkaishi 1990, 56, 1687–1691. [Google Scholar] [CrossRef] [Green Version]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Regulation, C. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006. Available online: https://eur-lex.europa.eu/lexuriserv/lexuriserv.do?uri=oj:l:2006:364:0005:0024:en:pdf (accessed on 20 March 2023).
- Mania, M.; Rebeniak, M.; Szynal, T.; Wojciechowska-Mazurek, M.; Starska, K.; Ledzion, E.; Postupolski, J. Total and inorganic arsenic in fish, seafood and seaweeds--Exposure assessment. Rocz. Panstw. Zakl. Hig. 2015, 66, 203–210. [Google Scholar] [PubMed]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Aspevik, T.; Kousoulaki, K.; Barba, F.J.; Ruiz, M.-J.; Rodríguez-Carrasco, M.; Aspevik, Y.; Kousoulaki, T.; Barba, K.; et al. Cytoprotective effects of fish protein hydrolysates against H2O2-induced oxidative stress and mycotoxins in Caco-2/TC7 cells. Antioxidants 2021, 10, 975. [Google Scholar] [CrossRef]
- Zhong, S.; Ma, C.; Lin, Y.C.; Luo, Y. Antioxidant properties of peptide fractions from silver carp (Hypophthalmichthys molitrix) processing by-product protein hydrolysates evaluated by electron spin resonance spectrometry. Food Chem. 2011, 126, 1636–1642. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfish (Lophius litulon) muscle: Purification, identification, and cytoprotective function on HepG2 cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [Green Version]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, X.; Xu, B.; Yuan, F.; Gong, J.; Yang, Z. Collagen peptides from swim bladders of giant croaker (Nibea japonica) and their protective effects against H2O2-induced oxidative damage toward human umbilical vein endothelial cells. Mar. Drugs 2020, 18, 430. [Google Scholar] [CrossRef] [PubMed]
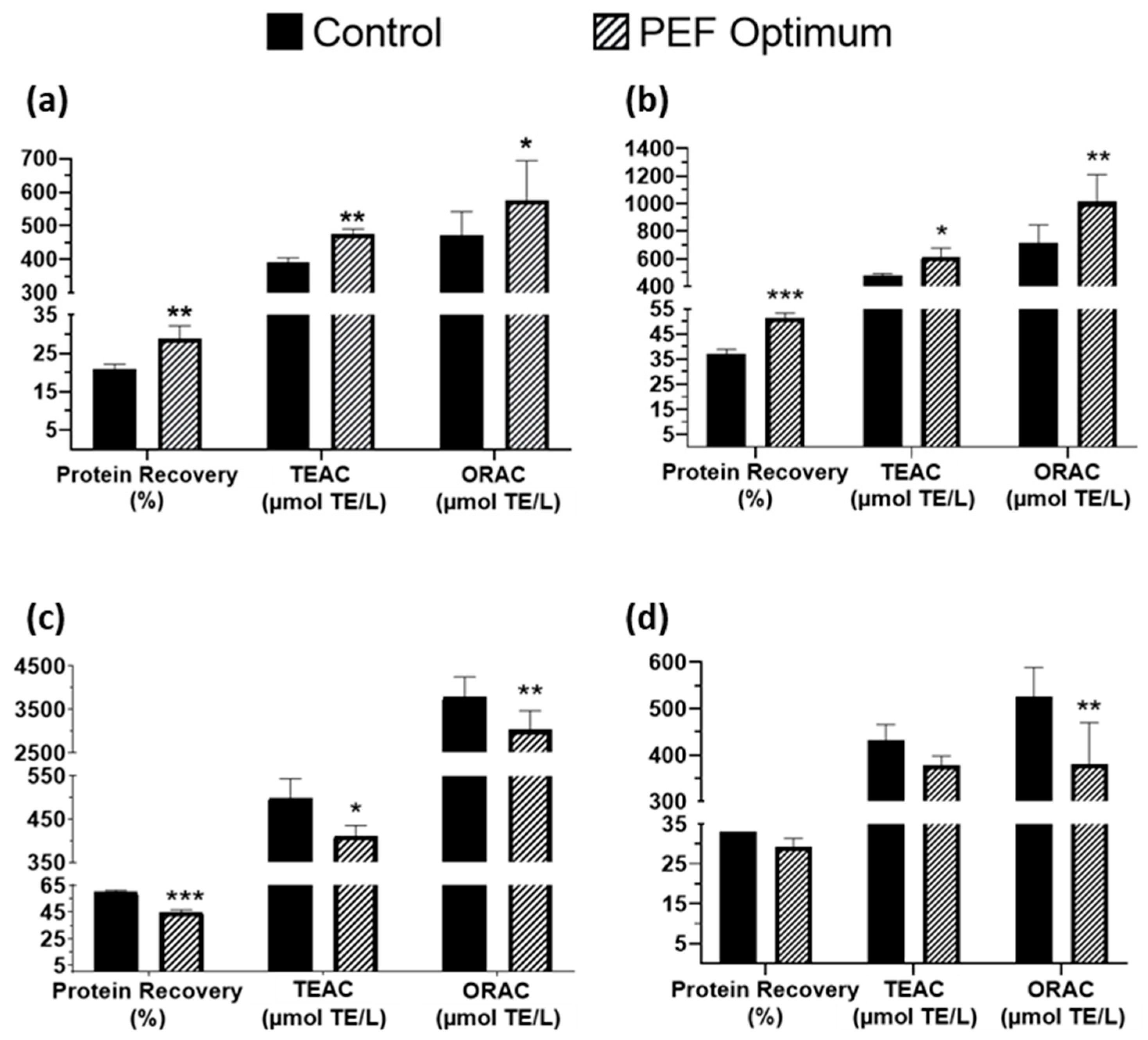

| Side Stream | Specific Energy (kJ/kg) | Field Strength (kV/cm) | Time of Extraction (h) |
|---|---|---|---|
| Head | 220 | 1 | 21.35 |
| Skin | 300 | 3 | 24 |
| Viscera | 123.72 | 3 | 15.17 |
| Backbone | 300 | 1 | 21 |
| Sequence | Involved Amino Acids | |
|---|---|---|
| HEAD CONTROL | ATDGGAHGVINVSVSEAAIEASTR | AH; GAH |
| IIDQDKSGFIEEDELKL | EL; LK | |
| IIDQDKSGFIEEDELKLFLQN | EL; LK | |
| MEHDPQARDRKAQEL | EL | |
| SANLMAGHWVAISGAAGGLGSLAVQYAK | GAA | |
| HEAD PEF OPT | AISEELDHALNDMTSI | EL |
| AVRNDEELNKLLGGVTI | EL | |
| AVRNDEELNKLLGGVTIAQGGVLPNIQA | EL | |
| AVRNDEELNKLLGGVTIAQGGVLPNIQAVLLPK | EL | |
| ENKNLQQEISDLTEQLGETGKSIHELEK | EL | |
| GTEDELDKYSEALKDAQEKLELAEKKATDAEGD-VAS | DAQEKLE; EL; KD; LK | |
| GTEDELDKYSEALKDAQEKLELAEKKATDAEGD-VASLNR | DAQEKLE; EL; KD; LK | |
| ISEELDHALNDMTSI | EL | |
| LLIVYPWTQR | PWT; PW; VY; YPW; YPWT | |
| MSADAMLKALLGSK | LK | |
| NDEELNKLLGGVT | EL | |
| NDEELNKLLGGVTIAQGGVLPNIQAVLLPK | EL | |
| NLQQEISDLTEQLGETGKSIHELEK | EL | |
| VAKLEKTIDDLEDELYAQK | LY; EL | |
| VFLENVIRDAVTYT | IR; TY; VFL | |
| VFLENVIRDAVTYTEHAK | IR; TY; VFL | |
| VFLENVIRDAVTYTEHAKR | IR; TY; VFL | |
| VGAGAPVYLAAVLEYLTAEILELAGNAAR | EL; VY | |
| VLEYLTAEILELAGNAARDNKKT | EL | |
| YKAISEELDHALNDMTSI | EL; KAI | |
| SKIN CONTROL | AFGLFDRVGDNKVAYNQ | AY |
| AGLLTSRSPTGSLWVVTA | LWV; LW | |
| AVGKVIPELNGKITGMA | EL; KVI; PEL | |
| AVGKVIPELDGKLTGMA | EL; KVI; PEL | |
| AVGKVIPELNGKITG | EL; KVI; PEL | |
| AVGKVIPELNGKLTG | EL; KVI; PEL | |
| AVGKVIPELNGKLTGMA | EL; KVI; PEL | |
| FAGDDAPRAVFPS | AGDDAPR | |
| FGLFDRVGDNKVAY | AY | |
| IIPASTGAAKAVGKVIPELNGK | EL; KVI; PEL; GAA | |
| IIPASTGAAKAVGKVIPELNGKITGMA | EL; KVI; PEL; GAA | |
| IIPASTGAAKAVGKVIPELNGKLTGMA | EL; KVI; PEL; GAA | |
| LRVFDKEGNGTVMGAELR | EL | |
| SSSLEKSYELPDGQVITIGNER | EL | |
| SVAELGEQIDNLQR | EL | |
| SVAELGEQIDNLQRVKQKLEKEKSE | EL | |
| SYELPDGQVITIGNER | EL | |
| TQQLEDLKRQLEEEVKAKN | LK | |
| TQQLEDLKRQLEEEVKAKNALAH | AH; LK | |
| VAELGEQIDNLQRVKQKLEKEKSE | EL | |
| VGKVIPELNGKITGMA | EL; KVI; PEL | |
| VGKVIPELNGKLTGMA | EL; KVI; PEL | |
| VLSGGTTMYPGIADRM | MY | |
| VLSGGTTMYPGIADRMQKE | MY | |
| VLSGGTTMYPGIADRMQKEITA | MY | |
| SKIN PEF OPT | AFGLFDRVGDNKVAYNQIADIMR | AY |
| DDEETTALVCDNGSGLVKAGFAGDDAPRA | AGDDAPR | |
| GAAAGAGAGAAGAGAAAGAEGPAGGPTGGP | GAA | |
| GKKMFGKQAGEDESDDFAIGGSTPTNKLK | LK | |
| GPAGAGAGDEAVDGATLYVKNLSF | LY | |
| IITNWDDMEKIWH | WDDMEK | |
| IITNWDDMEKIWHHT | HH; WHH; WDDMEK | |
| PIEHGIITNWDDMEKIWHHT | HH; WHH; WDDMEK | |
| QAGAAGGQPGAKAGGDDDVVDA | GAA | |
| VLSGGTTMYPGIADRMQKEITAL | MY | |
| YPIEHGIITNWDDMEKIWHHT | HH; WHH; WDDMK | |
| VISCERA CONTROL | AIGLPDELIQK | EL |
| AWGPGLEGGVVGK | AW; WG | |
| EVHLGWAAKGLGRKIQAMM | HL; MM | |
| FGGEHIPNSPF | GGE | |
| NIPTSGAEIGGAFGGEK | GGE | |
| VISCERA PEF OPT | AIGLPDELIQK | EL |
| AIIDQDKSGFIEEDEL | EL | |
| AIIDQDKSGFIEEDELK | EL; LK | |
| AWGPGLEGGVVGK | AW; WG | |
| GIELPYQDPAIK | EL | |
| GKDLFVSDLK | KD; LK; LFV | |
| IIDQDKSGFIEEDELK | EL; LK | |
| ILDQDKSGFIEEDELQ | EL | |
| IVNGEEAVPHSWPW | PHS; PW | |
| KEADAMAVDGGQVY | VY | |
| KVMGFVGIQTGFR | GFVG | |
| BACKBONE CONTROL | AAVPSGASTGVHEALELR | EL |
| AAVPSGASTGVHEALELRDGDKSRY | EL; RY | |
| AAVPSGASTGVHEALELRDGDKSRYLG | EL; RY; RYL; RYLG; YLG | |
| AAVPSGASTGVHEALELRDGDKSRYLGKGT | EL; RY; RYL; RYLG; YLG | |
| AAVPSGASTGVHEALELRDNDKANY | EL | |
| AGTNGETTTQGLDGLYER | LY | |
| AHQQTLDDLQAEEDKVNT | AH | |
| AKAVGKVIPELNGKLTGMA | EL; KVI; PEL | |
| AKYGKDATNVGDEGGF | KD | |
| AVGKVIPELNGKLT | EL; KVI; PEL | |
| AVGKVIPELNGKLTG | EL; KVI; PEL | |
| AVGKVIPELNGKLTGMA | EL; KVI; PEL | |
| AVPSGASTGVHEALELRDGDKSRY | EL; RY | |
| AVPSGASTGVHEALELRDGDKSRYLGKGT | EL; RY; RYL; RYLG; YLG | |
| AVRNDEELNKLLGGVTIAQGGVLPN | EL | |
| EEALDHLETLKRENKNLQQEISDLTEQLGETGKSI-HELEKA | HL; EL; LK | |
| EHGIVNNWDDMEKIWHHT | HH; WHH; WDDMEK | |
| EKTIDDLEDELYAQK | LY; EL | |
| ELPDGQVITIGNER | EL | |
| FDMFDTDGGGDISTKELGT | EL | |
| FMLELDGTENKSK | EL | |
| GIITNWDDMEK | WDDMEK | |
| GPMKGILGYTEHQ | LGY | |
| HIADLAGHKDVILP | KD | |
| IADLAGNEDVILPVPAFNVINGGSHAGNK | GSH | |
| IITNWDDMEKIWH | WDDMEK | |
| IITNWDDMEKIWHHT | HH; WHH; WDDMEK | |
| ISDLTEQLGETGKSIHELE | EL | |
| ISDLTEQLGETGKSIHELEK | EL | |
| ISDLTEQLGETGKSIHELEKA | EL | |
| ITATQKTVDGPSGKLWR | LWR; LW | |
| IVNNWDDMEKIWH | WDDMEK | |
| KLEKTIDDLEDELY | LY; EL | |
| KYGKDATNVGDEGGF | KD | |
| LAGTNGETTTQGLDGLYER | LY | |
| LAVRNDEELNKLLGGVTIAQGGVLPN | EL | |
| LEKTIDDLEDELY | LY; EL | |
| LPDGKVITIGNER | KVI | |
| LQLAVRNDEELNKLLGGVTIAQGGVLPN | EL; LQL | |
| LTEQLGETGKSIHELEK | EL | |
| LTGCTDIQIVGDDLTVTNPKR | TGC | |
| LTKLEEAEKAADESERGMKVIENR | KVI | |
| MLELDGTENKSKFGANA | EL | |
| MLELDGTENKSKFGANAILGVS | EL | |
| QGGGGWGGGPGGGQQGGGAP | WG | |
| SEALKDAQEKLELAE | DAQEKLE; EL; KD; LK | |
| SEALKDAQEKLELAEK | DAQEKLE; EL; KD; LK | |
| SEALKDAQEKLELAEKKATDAEGDVASLNR | DAQEKLE; EL; KD; LK | |
| SEALKDAQEKLELAEKKATDAEGDVASLNRR | DAQEKLE; EL; KD; LK | |
| SELKKDIDDLELTL | EL; KD; LK | |
| SELKKDIDDLELTLAK | EL; KD; LK | |
| SELKKDIDDLELTLAKVEKE | EL; KD; LK | |
| SELKKDIDDLELTLAKVEKEKHATEN | EL; KD; LK | |
| SELKKDIDDLELTLAKVEKEKHATENK | EL; KD; LK | |
| SKQLEDDLVALQKKLKGTEDELDKYSE | EL; LK | |
| SVAELGEQIDNLQR | EL | |
| SYELPDGQVITIGNER | EL | |
| TEQLGETGKSIHELEKA | EL | |
| VAKLEKTIDDLEDELY | LY; EL | |
| VEEELDRAQERLATALTKLEEAEKAADESERG | EL | |
| VEEELDRAQERLATALTKLEEAEKAADESERGMK | EL | |
| VEEELDRAQERLATALTKLEEAEKAADESERGMKVIENR | EL; KVI | |
| VGKVIPELNGKLTGMA | EL; KVI; PEL | |
| VINGGSHAGNKLAMQEFM | GSH | |
| VLSGGTTMYPGIADR | MY | |
| VVESTGVFTTIEKASAH | AH | |
| VVESTGVFTTIEKASAHIKGGAKR | AH | |
| YELPDGQVITIGNER | EL | |
| BACKBONE PEF OPT | AALTGGAAAGVAGAAAAGPAGDIA | GAA |
| AHQQTLDDLQAEEDKVNT | AH | |
| AISEELDHALNDMTS | EL | |
| AVRNDEELNKLLGGVTIAQGGVLPN | EL | |
| DAQEKLELAEKKATDAEGDVAS | DAQEKLE; EL | |
| EKTIDDLEDELYAQK | LY; EL | |
| FMIELDGTENK | EL | |
| GIITNWDDMEK | WDDMEK | |
| GPAGAGAGDEAVDGATLYVKNLSF | LY | |
| GTEDELDKYSE | EL | |
| GTEDELDKYSEALKDAQEKLE | DAQEKLE; EL; KD; LK | |
| HLQLAVRNDEELNKLLGGVTIAQGGVLPN | HL; EL; LQL | |
| IIDQDKSGFIEEDELKL | EL; LK | |
| IITNWDDMEK | WDDMEK | |
| IITNWDDMEKIWHHT | HH; WHH; WDDMEK | |
| ILDQDKSGFIEEDELQLFLQN | EL; LQL | |
| ISDLTEQLGETGKSIHELEK | EL | |
| ISEELDHALNDMTS | EL | |
| ISEELDHALNDMTSI | EL | |
| KKQADSVAELGEQIDNLQR | EL | |
| KLKGTEDELDKYSEALKDAQEKLELAEKKATDAEGDVASLNR | DAQEKLE; EL; KD; LK | |
| LAVRNDEELNKLLGGVTIAQGGVLPN | EL | |
| LEKTIDDLEDELYAQK | LY; EL | |
| LTKLEEAEKAADESERGMKVIENR | KVI | |
| MSADAMLKALLGSK | LK | |
| NLQQEISDLTEQLGETGKSIHELEK | EL | |
| PGPNKGDSRGPPNHHMGP | HH; NHH; GPP | |
| PGSPAGAATSAPGAPAPG | GAA | |
| PIEHGIITNWDDMEK | WDDMEK | |
| RIQLVEEELDRAQERLATA | EL | |
| SADTLWGIQKDLKDL | LWG; KD; LK; LW; WG | |
| SEALKDAQEKLELAEKKATDAEGDVAS | DAQEKLE; EL; KD; LK | |
| SEALKDAQEKLELAEKKATDAEGDVASLNR | DAQEKLE; EL; KD; LK | |
| SEALKDAQEKLELAEKKATDAEGDVASLNRR | DAQEKLE; EL; KD; LK | |
| SKQLEDDLVALQKKLKGTEDELDKYSEALKDAQEKLELAEKKATDAEGDVASLNR | DAQEKLE; EL; KD; LK | |
| SQKEDKYEEEIKVLTDKLK | LK | |
| SQKEDKYEEEIKVLTDKLKEAETR | LK | |
| SQKEDKYEEEIKVLTDKLKEAETRAE | LK | |
| TIDDLEDELYAQK | LY; EL | |
| VAKLEKTIDDLEDELY | LY; EL | |
| VEEELDRAQERLATALTKLEEAEKAADESERGMK | EL | |
| VEEELDRAQERLATALTKLEEAEKAADESERG | EL | |
| VRNDEELNKLLGGVTIAQGGVLPN | EL | |
| VTIMPKDIQLAR | KD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martí-Quijal, F.J.; Castagnini, J.M.; Ruiz, M.-J.; Barba, F.J. Sea Bass Side Streams Extracts Obtained by Pulsed Electric Fields: Nutritional Characterization and Effect on SH-SY5Y Cells. Foods 2023, 12, 2717. https://doi.org/10.3390/foods12142717
Martí-Quijal FJ, Castagnini JM, Ruiz M-J, Barba FJ. Sea Bass Side Streams Extracts Obtained by Pulsed Electric Fields: Nutritional Characterization and Effect on SH-SY5Y Cells. Foods. 2023; 12(14):2717. https://doi.org/10.3390/foods12142717
Chicago/Turabian StyleMartí-Quijal, Francisco J., Juan Manuel Castagnini, María-José Ruiz, and Francisco J. Barba. 2023. "Sea Bass Side Streams Extracts Obtained by Pulsed Electric Fields: Nutritional Characterization and Effect on SH-SY5Y Cells" Foods 12, no. 14: 2717. https://doi.org/10.3390/foods12142717








