Insights into the Flavor Differentiation between Two Wild Edible Boletus Species through Metabolomic and Transcriptomic Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom Materials
2.2. Metabolites Extraction
2.3. UHPLC-MS Analysis
2.4. RNA Sequencing and De Novo Transcriptomic Analysis
2.5. Correlation Analysis of Transcriptomic and Metabolomic Data
3. Results
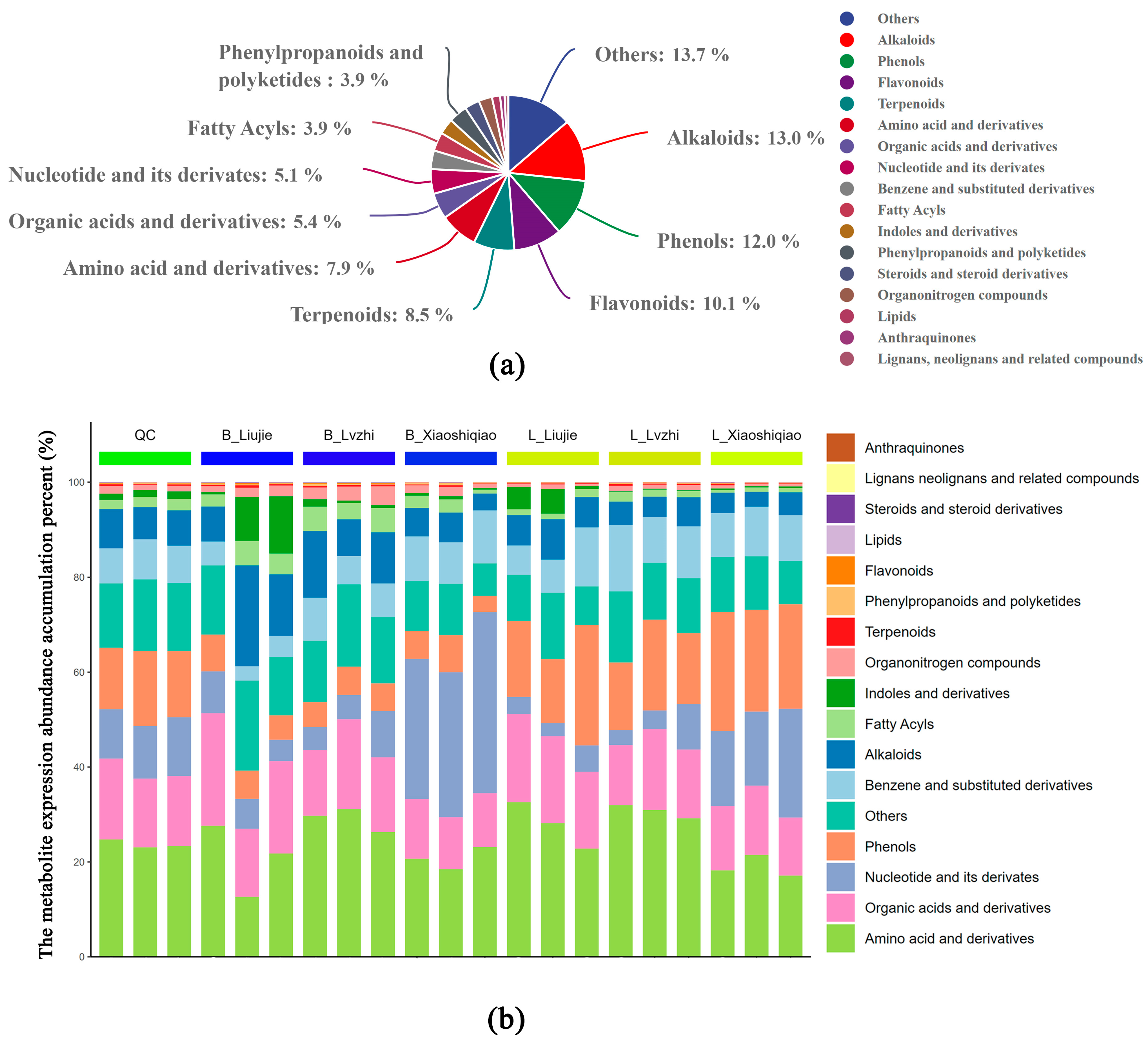
3.1. Metabolic Profiling
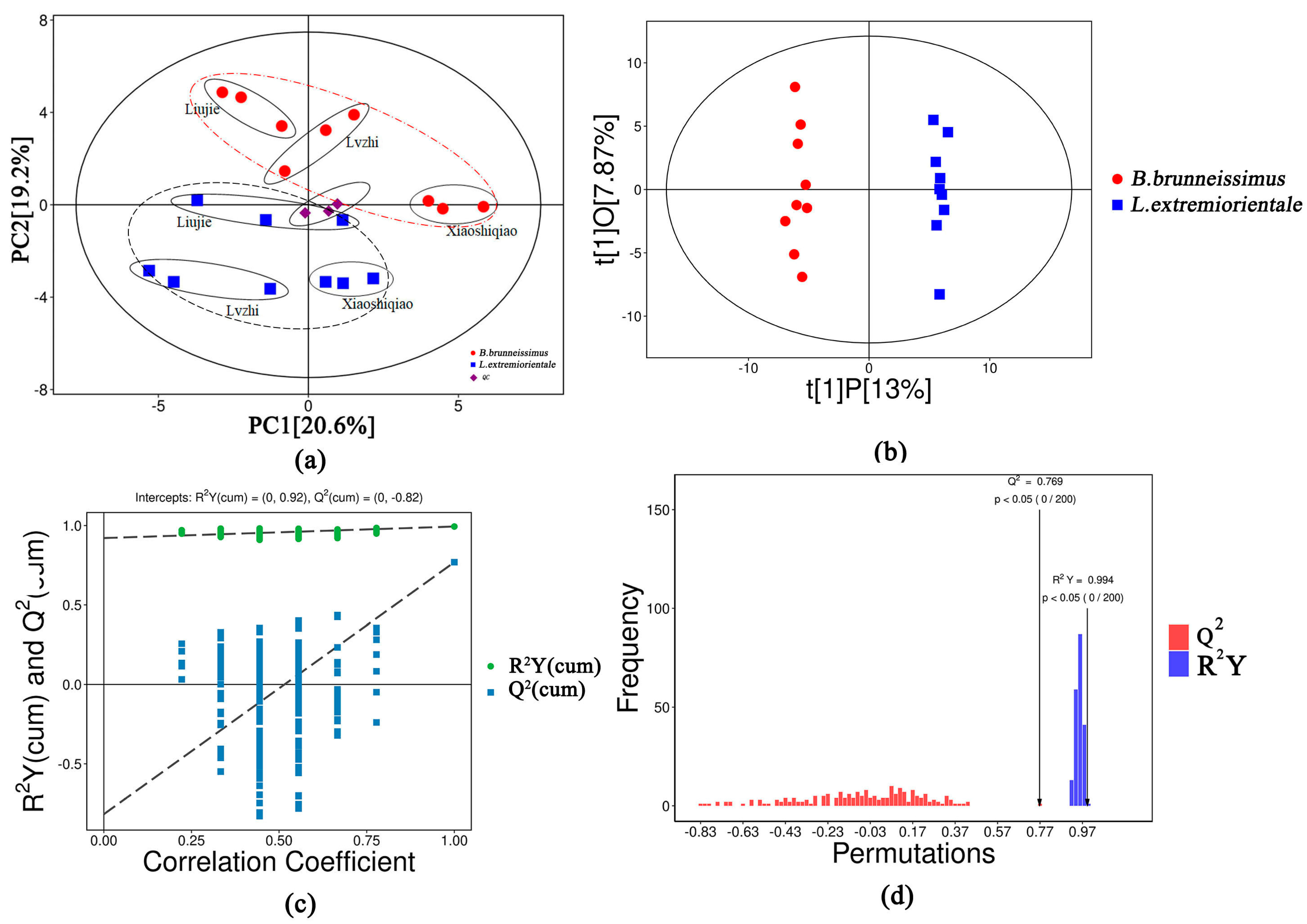
3.2. Analysis of the Applicability of the OPLS-DA Model to This Study
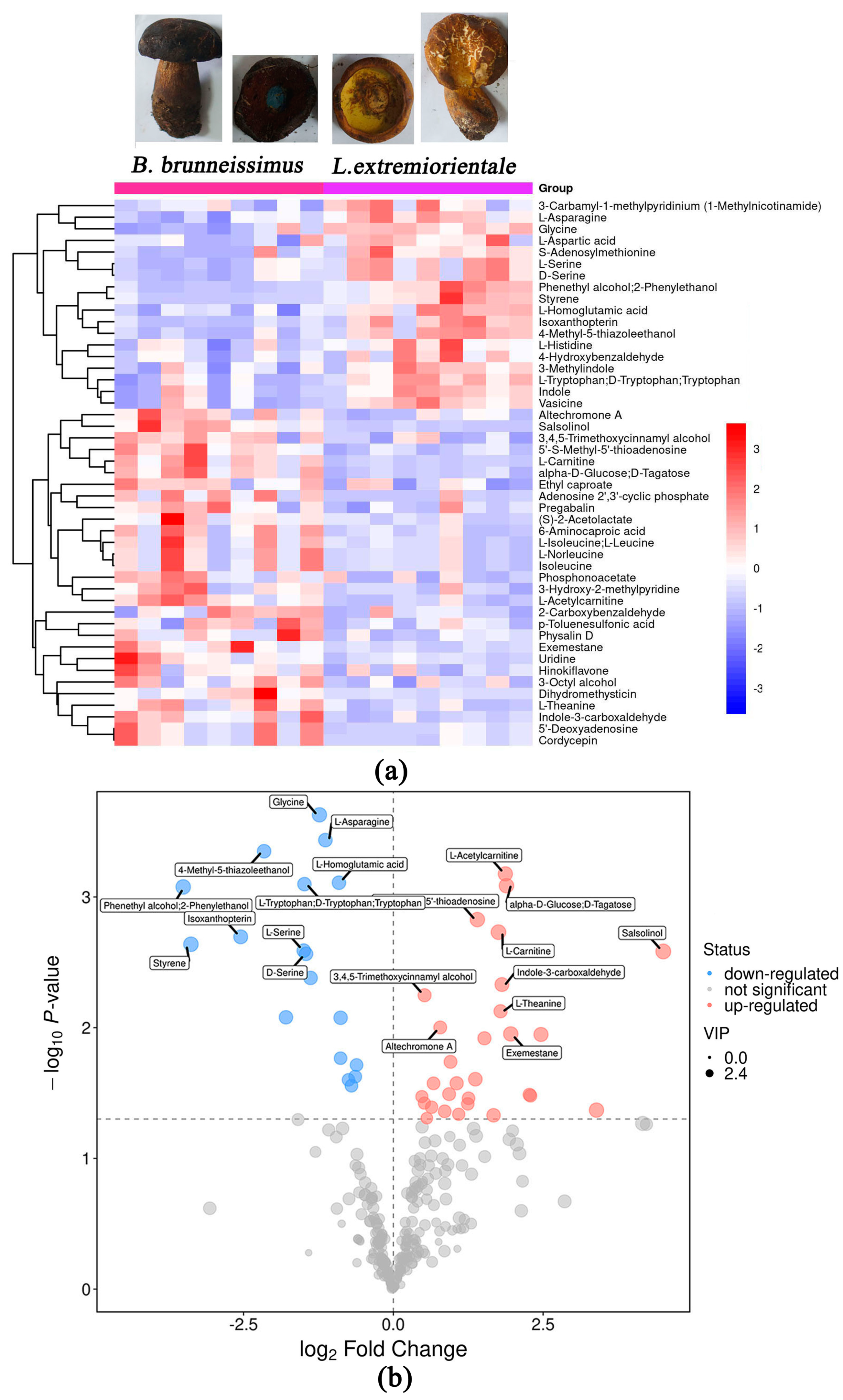
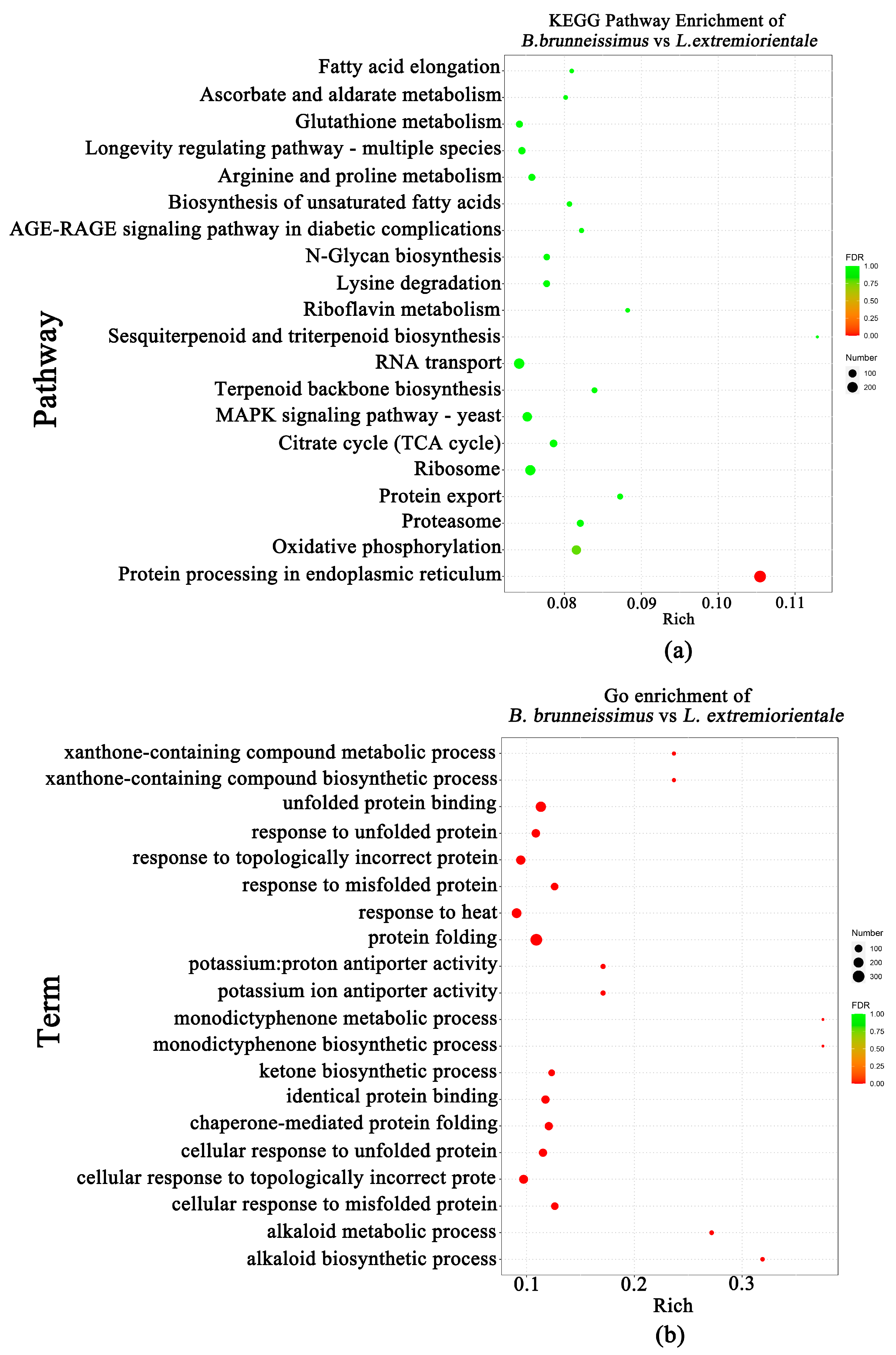
3.3. Difference of Flavor Metabolite between B. brunneissimus and L. extremiorientale
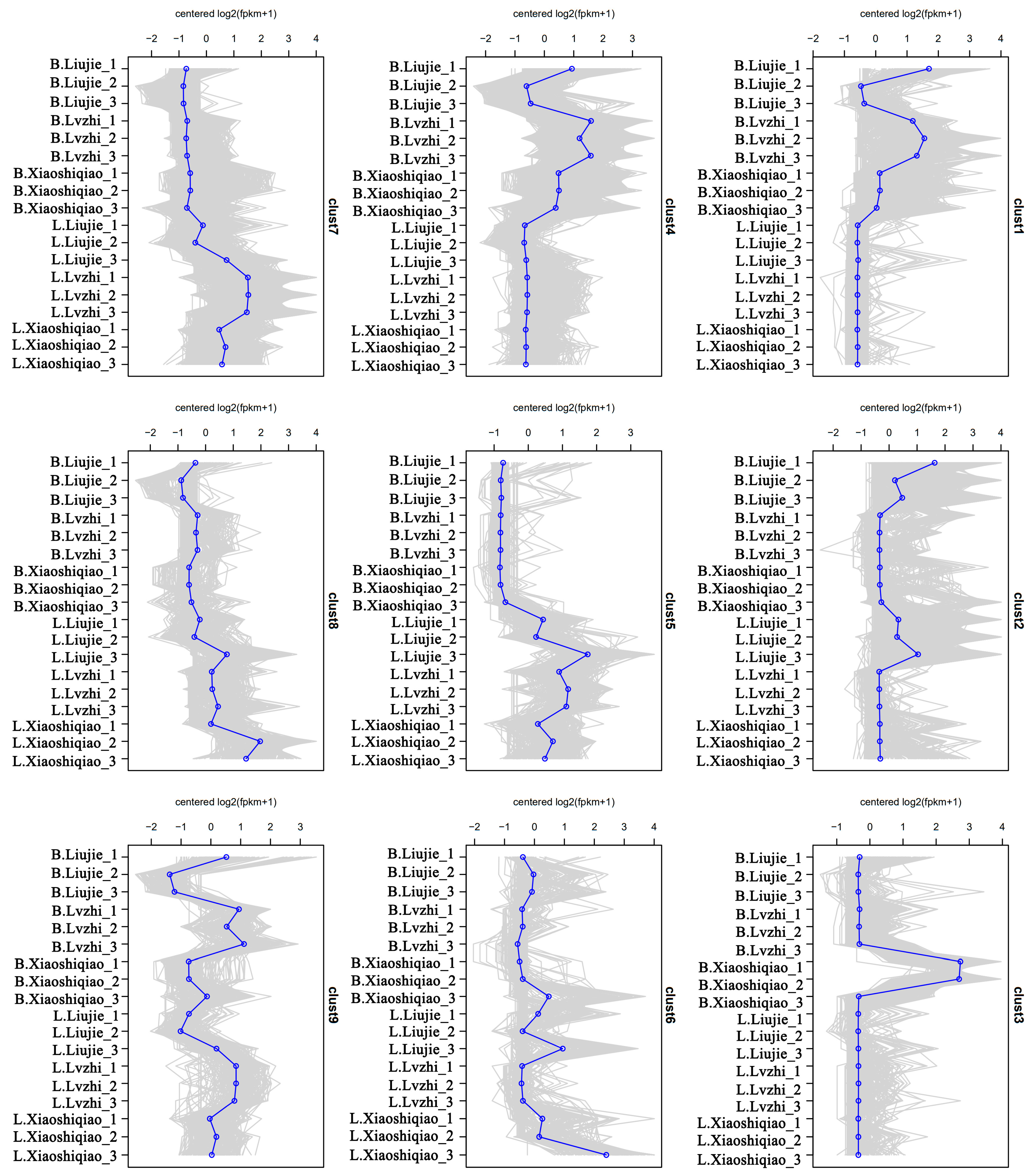
3.4. Transcriptomic Difference of the Fruiting Bodies of Two Wild Edible Boletus
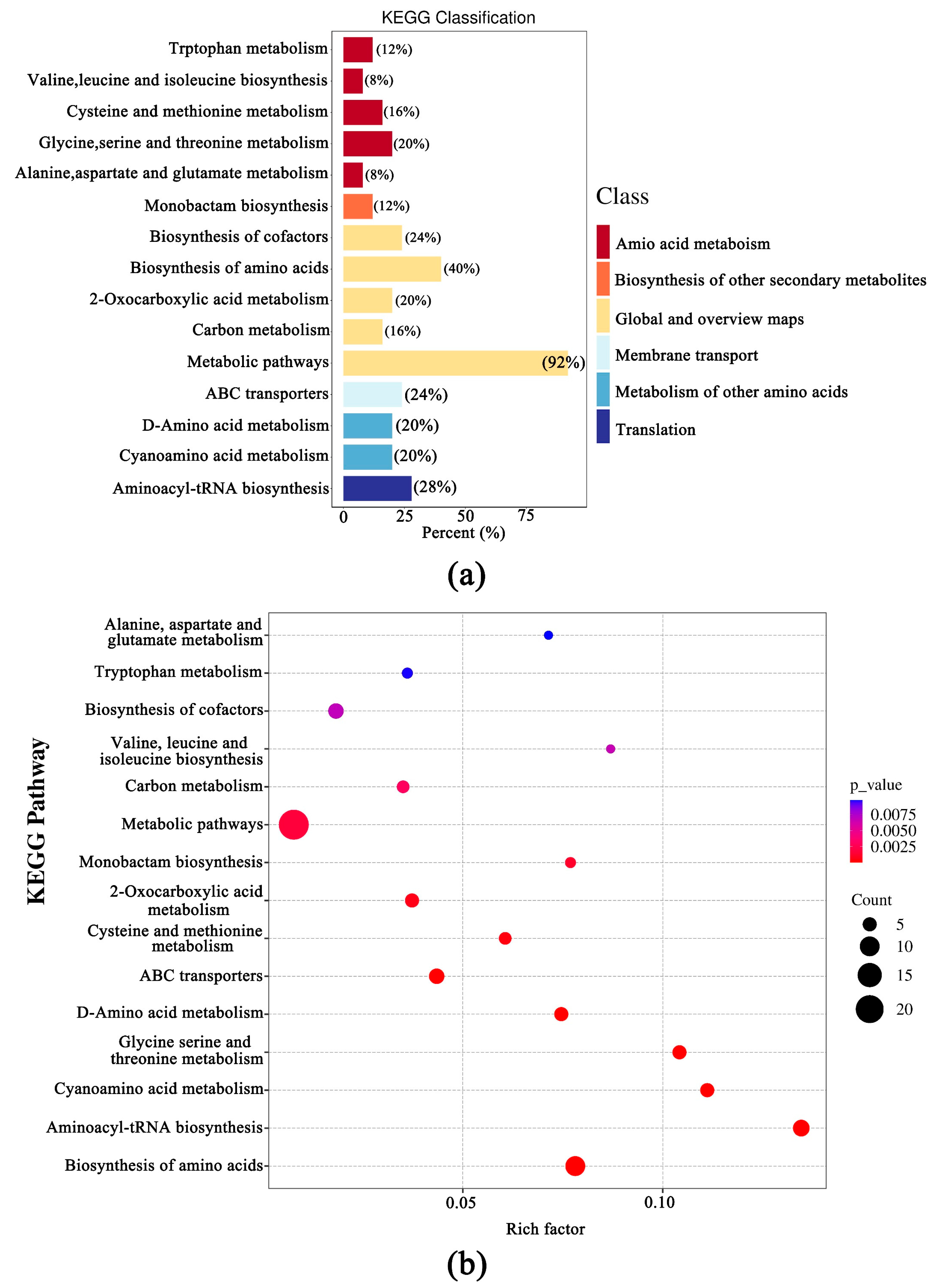
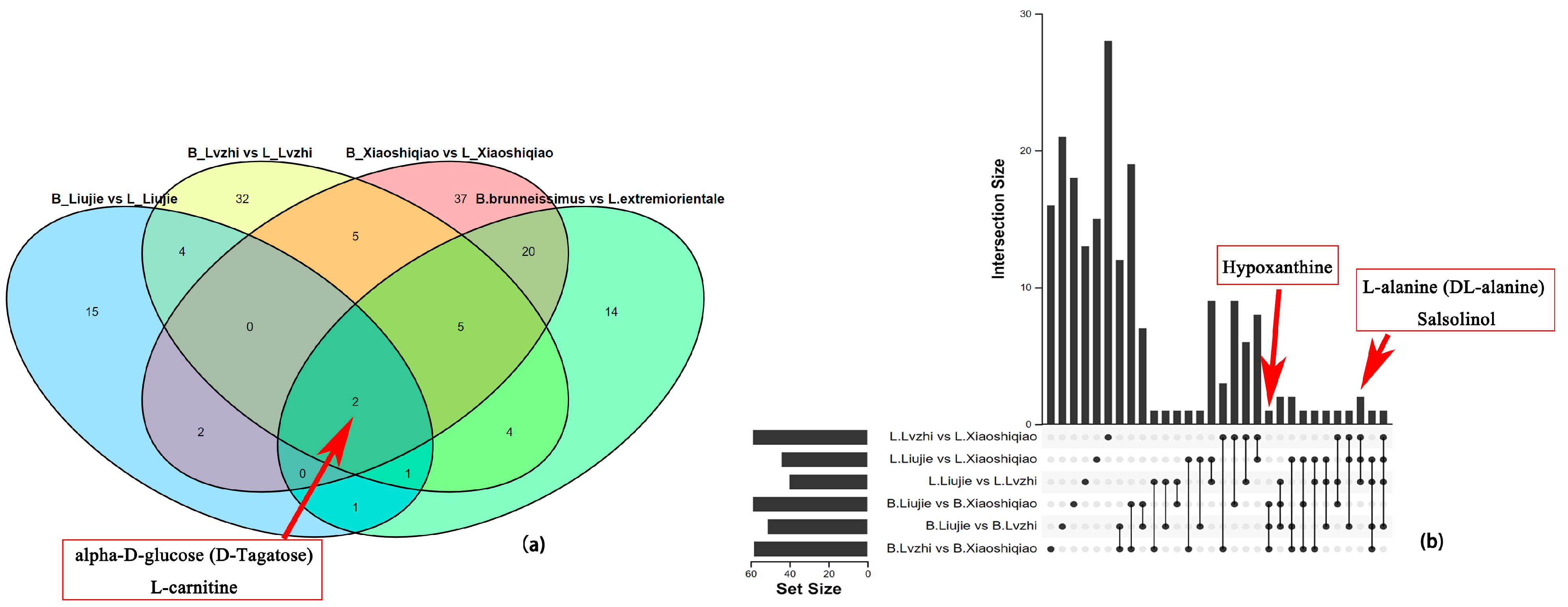
3.5. Analysis of DEMs and DEGs in the Two Boletus Fruiting Bodies in Different Locations
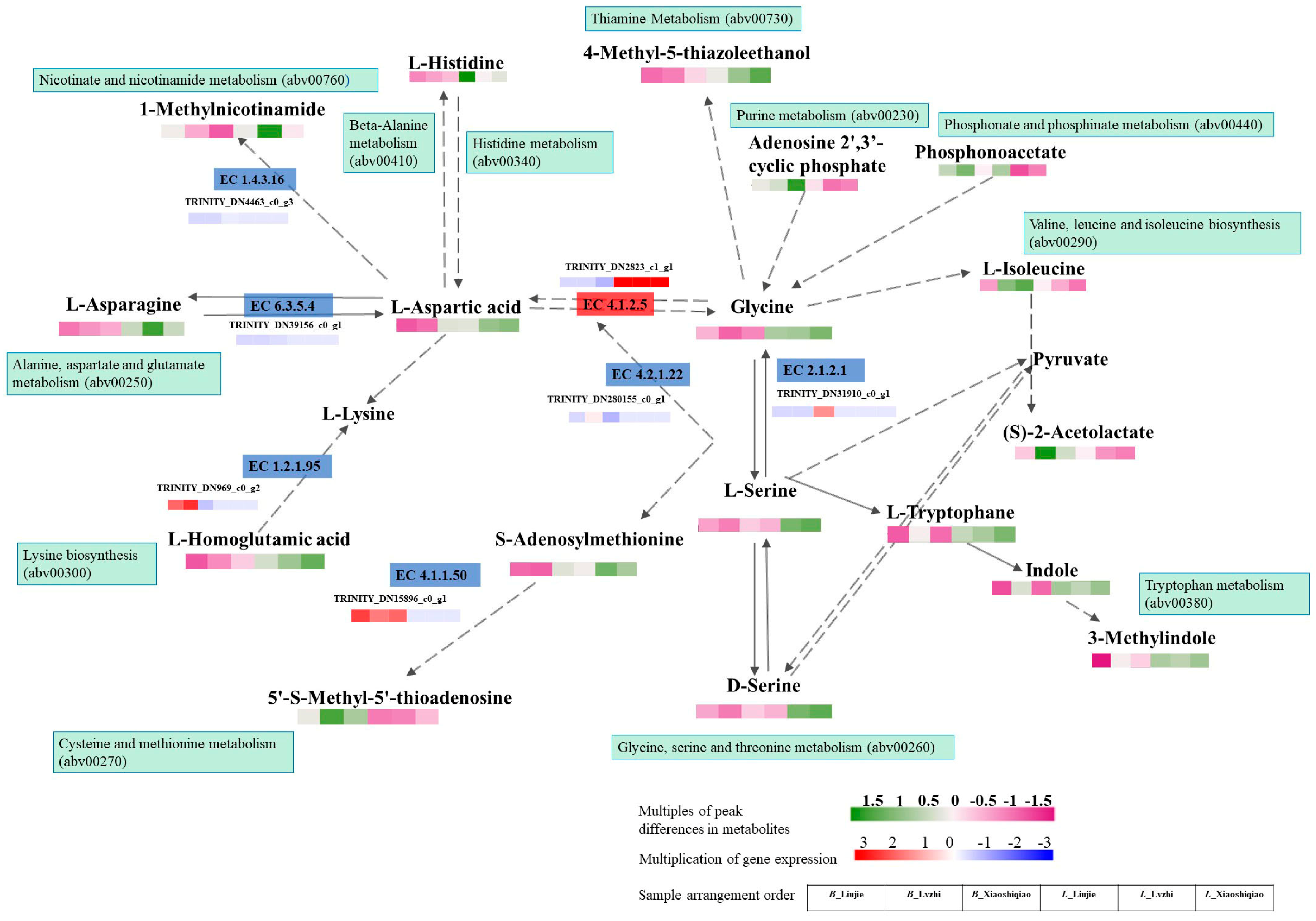
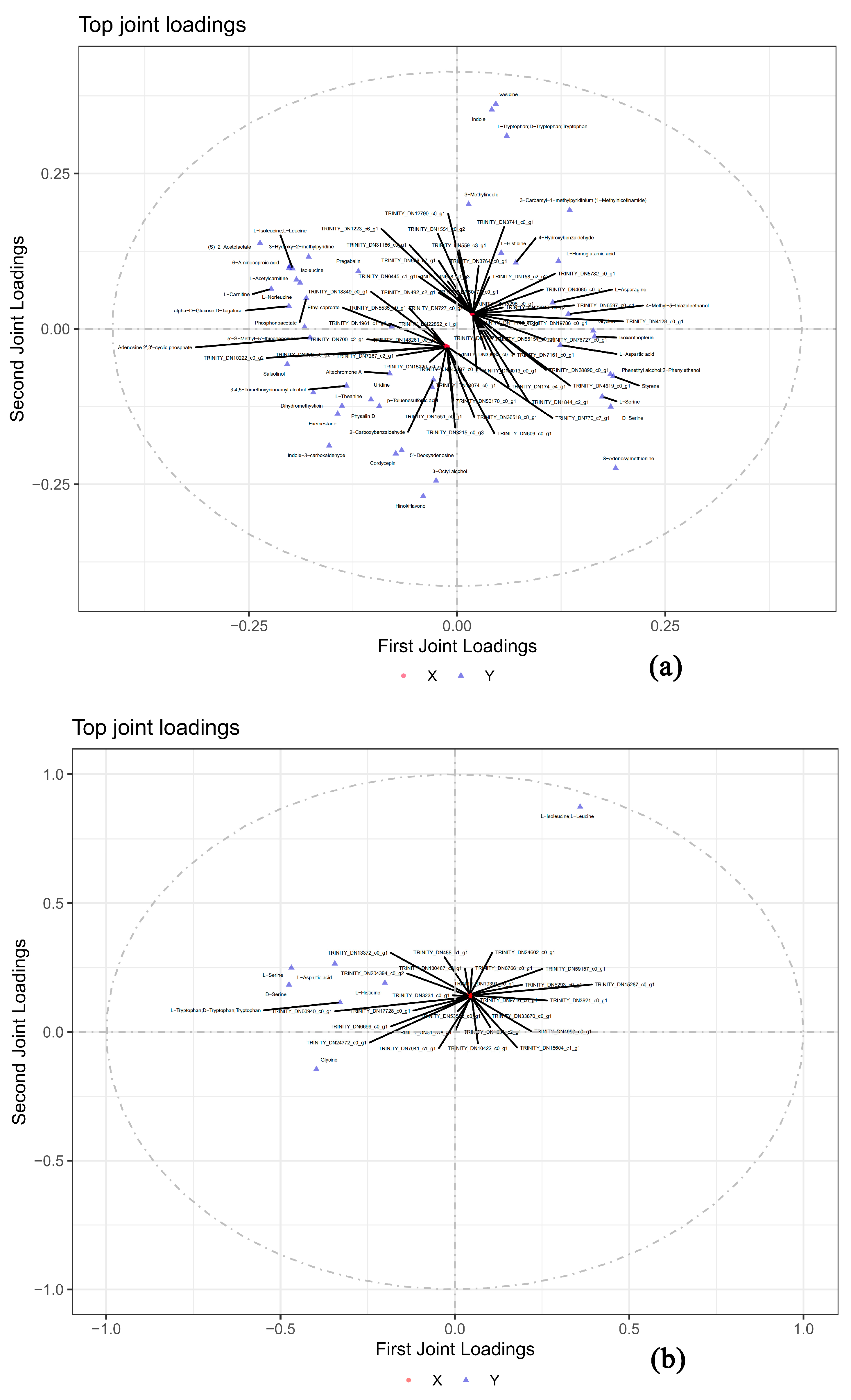
3.6. Correlation Analysis of Metabolomic and Transcriptomic Data
| Index | Compound name | Class | Taste | Peak Area | VIP | p-Value | B. brunneissimus vs L. extremiorientale | |
|---|---|---|---|---|---|---|---|---|
| B. brunneissimus | L. extremiorientale | |||||||
| 1 | L-Aspartic acid | Free amino acids | Umami taste (MSG-like) | 394,035.09 ± 192,939.3 | 618,031.63 ± 180,516.1 | 1.51 | 0.02 | UP |
| 2 | D-Aspartic acid | Free amino acids | Umami taste (MSG-like) | 433,700.93 ± 232,571.85 | 632,223.46 ± 316,790.67 | 1.03 | 0.13 | - |
| 3 | L-Glutamic acid | Free amino acids | Umami taste (MSG-like) | 6,201,893.95 ± 3,509,410.93 | 6,921,258.43 ± 1,860,205.86 | 0.62 | 0.76 | - |
| 4 | L-Alanine; DL-Alanine | Free amino acids | Sweet taste | 658,758.14 ± 413,661.32 | 1,382,503.87 ± 922,614.21 | 0.06 | 0.22 | - |
| 5 | Glycine | Free amino acids | Sweet taste | 48,034.62 ± 15,782.87 | 114,075.09 ± 16,451.79 | 2.19 | 0 | UP |
| 6 | D-Proline | Free amino acids | Sweet taste | 8,407,880.54 ± 583,9726 | 11,620,670.17 ± 4,494,533.82 | 0.99 | 0.3 | - |
| 7 | D-Serine | Free amino acids | Sweet taste | 114,362.85 ± 68,449.37 | 316,275.73 ± 147,831.59 | 1.82 | 0 | UP |
| 8 | L-Serine | Free amino acids | Sweet taste | 111,179.47 ± 70,914.14 | 316,275.73 ± 147,831.59 | 1.81 | 0 | UP |
| 9 | L-Threonine | Free amino acids | Sweet taste | 576,328.69 ± 392,016.92 | 692,317.82 ± 153,705.11 | 0.88 | 0.44 | - |
| 10 | L-Histidine | Free amino acids | Bitter taste | 5,495,343.35 ± 2,751,086.31 | 9,208,274.72 ± 3,538,910.94 | 1.47 | 0.03 | UP |
| 11 | L-Isoleucine; L-Leucine | Free amino acids | Bitter taste | 378,672.76 ± 218,557.65 | 188,871.1 ± 108,314.07 | 1.3 | 0.46 | DOWN |
| 12 | Isoleucine | Free amino acids | Bitter taste | 400,448.58 ± 258,714.71 | 176,708.49 ± 108,579.05 | 1.43 | 0.03 | DOWN |
| 13 | L-Methionine | Free amino acids | Bitter taste | 106,123.63 ± 97,300.41 | 47,486.83 ± 42,604.14 | 1.28 | 0.13 | - |
| 14 | L-Phenylalanine;D-(+)-Phenylalanine; DL-Phenylalanine | Free amino acids | Bitter taste | 21,820,440.32 ± 12,846,566.66 | 19,022,942.97 ± 13,038,839.53 | 0.47 | 0.61 | - |
| 15 | Tryptophan(L-Tryptophan; D-Tryptophan) | Free amino acids | Bitter taste | 17,996.51 ± 16,094.24 | 53,685.79 ± 18,460.91 | 1.79 | 0 | UP |
| 16 | L-Valine | Free amino acids | Bitter taste | 409,058.87 ± 139,076.01 | 401,702.04 ± 71,429.27 | 0.05 | 0.69 | - |
| 17 | L-Arginine | Free amino acids | Bitter taste | 12,571,655.39 ± 1,273,7601.53 | 21,443,582.43 ± 13,314,464.99 | 1.2 | 0.2 | - |
| 18 | L-Lysine;L-Glutamine | Free amino acids | Tasteless | 18,074,414.78 ± 7,399,759.51 | 23,351,362.08 ± 8,585,564.65 | 0.9 | 0.2 | - |
| 19 | L-Tyrosine | Free amino acids | Tasteless | 1,958,434.87 ± 1,135,802.84 | 2,938,080.2 ± 1,187,109.94 | 1.16 | 0.19 | - |
| 20 | Inositol | Soluble sugars; Polyols | Sweet taste | 3100.23 ± 1151.09 | 2397.99 ± 1140.6 | 1 | 0.16 | - |
| 21 | D-Xylulose | Soluble sugars; Reducing sugars | Sweet taste | 73,430.52 ± 62,704.61 | 27,330.13 ± 25,050.39 | - | - | - |
| 22 | alpha-D-Glucose; D-Tagatose | Soluble sugars; Reducing sugars | Sweet taste | 301,634.92 ± 128,026.33 | 83,124.42 ± 34,406.66 | 2.25 | 0 | UP |
| 23 | Beta-D-Glucose; alpha-D-Glucose | Soluble sugars; Reducing sugars | Sweet taste | 3648.68 ± 1313.4 | 5118.91 ± 2292.21 | - | - | - |
| 24 | Sucrose | Soluble sugars; Non-reducing sugars | Sweet taste | 92,833.83 ± 62,857.55 | 349,897.24 ± 510,820.3 | - | - | - |
| 25 | L-Malic acid | Organic acids | sour taste | 2013.11 ± 1300.98 | 1580.5 ± 1538.06 | - | - | - |
| 26 | Ascorbic acid | Organic acids | sour taste | 7771.71 ± 2522.87 | 6542.41 ± 2207.86 | 0.83 | 0.2 | - |
| 27 | Adenosine 5′-monophosphate | 5′-nucleotides | - | 1,703,525.03 ± 710,544.37 | 2,947,050.82 ± 3,418,622.63 | 0.12 | 0.32 | - |
| 28 | Ethyl caproate | Miscellaneous | - | 64,844.26 ± 21,913.66 | 41,651.35 ± 22,101.1 | 1.48 | 0.03 | DOWN |
| The top 10 DEGs Associated with All DEMs by O2PLS Analysis | ||||
|---|---|---|---|---|
| DEGs | Loading_1 | Loading_2 | Loading2 1 | NR Annotation of DEGs |
| TRINITY_DN343297_c0_g1 | −0.011662098 | −0.031064851 | 0.00110103 | gi|1000864728|gb|KXN90516.1| Vesicle transport protein SFT2B [Leucoagaricus sp. SymC.cos] |
| TRINITY_DN15220_c0_g1 | −0.013141608 | −0.030371976 | 0.001095159 | gi|751055722|gb|KIK90884.1| hypothetical protein PAXRUDRAFT_831297 [Paxillus rubicundulus Ve08.2h10] |
| TRINITY_DN7287_c2_g1 | −0.013351728 | −0.030237734 | 0.001092589 | gi|914258129|gb|KNZ73472.1| WD repeat-containing protein 5 [Termitomyces sp. J132] |
| TRINITY_DN50170_c0_g1 | −0.011257006 | −0.030734214 | 0.001071312 | gi|750997013|gb|KIK39305.1| hypothetical protein CY34DRAFT_89493 [Suillus luteus UH-Slu-Lm8-n1] |
| TRINITY_DN18074_c0_g1 | −0.011755046 | −0.030544198 | 0.001071129 | gi|751064906|gb|KIK99469.1| hypothetical protein PAXRUDRAFT_131997 [Paxillus rubicundulus Ve08.2h10] |
| TRINITY_DN3215_c0_g3 | −0.012219336 | −0.03025251 | 0.001064527 | gi|749895491|gb|KIJ63208.1| hypothetical protein HYDPIDRAFT_29895 [Hydnomerulius pinastri MD-312] |
| TRINITY_DN262_c0_g2 | −0.014473261 | −0.029082473 | 0.001055266 | - 2 |
| TRINITY_DN700_c2_g1 | −0.014895131 | −0.028608579 | 0.001040316 | gi|749834671|gb|KIJ12323.1| hypothetical protein PAXINDRAFT_156963 [Paxillus involutus ATCC 200175] |
| TRINITY_DN10222_c0_g2 | −0.013377283 | −0.028754748 | 0.001005787 | gi|751062861|gb|KIK97499.1| hypothetical protein PAXRUDRAFT_136196 [Paxillus rubicundulus Ve08.2h10] |
| TRINITY_DN5782_c0_g1 | 0.019328067 | 0.024823556 | 0.000989783 | gi|749846545|gb|KIJ21256.1| hypothetical protein PAXINDRAFT_64023 [Paxillus involutus ATCC 200175] |
| The top 10 DEGs associated with flavor related DEMs by O2PLS analysis | ||||
| DEGs | Loading_1 | Loading_2 | Loading2 1 | NR annotation of DEGs |
| TRINITY_DN455_c1_g1 | 0.040883277 | 0.149953165 | 0.024157394 | gi|1035381610|gb|OAX38220.1| hypothetical protein K503DRAFT_850105 [Rhizopogon vinicolor AM-OR11-026] |
| TRINITY_DN24602_c0_g1 | 0.044125314 | 0.147860791 | 0.023809857 | gi|749890950|gb|KIJ58942.1| hypothetical protein HYDPIDRAFT_162871 [Hydnomerulius pinastri MD-312] |
| TRINITY_DN6766_c0_g1 | 0.043559178 | 0.147316192 | 0.023599462 | gi|628854451|ref|XP_007774347.1| hypothetical protein CONPUDRAFT_112209 [Coniophora puteana RWD-64-598 SS2] |
| TRINITY_DN19391_c0_g1 | 0.043673434 | 0.146674332 | 0.023420729 | gi|749903138|gb|KIJ70603.1| hypothetical protein HYDPIDRAFT_23695 [Hydnomerulius pinastri MD-312] |
| TRINITY_DN59157_c0_g1 | 0.04454385 | 0.146046036 | 0.023313599 | gi|763724697|gb|KJA22132.1| hypothetical protein HYPSUDRAFT_41267 [Hypholoma sublateritium FD-334 SS-4] |
| TRINITY_DN5293_c0_g1 | 0.043968374 | 0.145400401 | 0.023074494 | gi|749841699|gb|KIJ16704.1| acetolactate synthase [Paxillus involutus ATCC 200175] |
| TRINITY_DN15287_c0_g1 | 0.047024185 | 0.142239221 | 0.02244327 | gi|749892543|gb|KIJ60404.1| hypothetical protein HYDPIDRAFT_177471 [Hydnomerulius pinastri MD-312] |
| TRINITY_DN3921_c0_g1 | 0.046666069 | 0.141663398 | 0.02224624 | gi|749830483|gb|KIJ08773.1| glycoside hydrolase family 89 protein [Paxillus involutus ATCC 200175] |
| TRINITY_DN130487_c0_g1 | 0.04132318 | 0.142957832 | 0.022144547 | gi|749845521|gb|KIJ20338.1| hypothetical protein PAXINDRAFT_108316 [Paxillus involutus ATCC 200175] |
| TRINITY_DN9716_c0_g1 | 0.046452929 | 0.141217718 | 0.022100318 | gi|749901809|gb|KIJ69300.1| hypothetical protein HYDPIDRAFT_145192 [Hydnomerulius pinastri MD-312] |
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, G.; Goggi, S.; Giampieri, F.; Baroni, L. A review of mushrooms in human nutrition and health. Trends Food Sci. Technol. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Mau, J.L. The Umami taste of edible and medicinal mushrooms. Int. J. Med. Mushrooms 2005, 7, 119–126. [Google Scholar] [CrossRef]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef]
- Komata. The taste and constituents of foods. Nippon. Sho-Kuhin Kogyo Gakkaishi 1969, 3, 26. [Google Scholar]
- Poojary, M.M.; Orlien, V.; Passamonti, P.; Olsen, K. Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food Chem. 2017, 234, 236–244. [Google Scholar] [CrossRef]
- Samarasiri, M.; Chen, W.N. Variations of nonvolatile taste components of mushrooms with different operating conditions and parameters from farm to fork. Crit. Rev. Food Sci. 2022, 1–20. [Google Scholar] [CrossRef]
- Sun, L.-B.; Zhang, Z.-Y.; Xin, G.; Sun, B.-X.; Bao, X.-J.; Wei, Y.-Y.; Zhao, X.-M.; Xu, H.-R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Wen, X.; Geng, F.; Xu, Y.; Li, X.; Liu, D.; Liu, Z.; Luo, Z.; Wang, J. Quantitative transcriptomic and metabolomic analyses reveal the changes in Tricholoma matsutake fruiting bodies during cold storage. Food Chem. 2022, 381, 132292. [Google Scholar] [CrossRef]
- Dong, J.E.; Zuo, Z.T.; Zhang, J.; Wang, Y.Z. Geographical discrimination of Boletus Edulis using two dimensional correlation spectral or integrative two dimensional correlation spectral image with ResNet. Food Control 2021, 129, 108132. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Wang, L.; Huang, Y.; Wang, L. Key taste components in two wild edible Boletus mushrooms using widely targeted metabolomics. Biochem. Syst. Ecol. 2021, 96, 104268. [Google Scholar] [CrossRef]
- Feng, B.; Yang, Z. Studies on diversity of higher fungi in Yunnan, southwestern China: A review. Plant Divers. 2018, 40, 165–171. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Yu, F. Color Atlas of Wild Commercial Mushrooms in Yunnan; Yunnan Science and Technology Press: Kunming, China, 2004. (In Chinese) [Google Scholar]
- Liang, J.; Chen, L.; Li, Y.; Hu, X. Isolation and identification of umami-flavored peptides from Leccinum extremiorientale and their taste characteristic. J. Food Process. Preserv. 2021, 45, e15255. [Google Scholar] [CrossRef]
- Wang, X. Study of Taste Active Components of Five Wild Edible Mushrooms in Yunnan. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2015. (In Chinese). [Google Scholar]
- Falandysz, J.; Saba, M.; Strumińska-Parulska, D. 137Caesium, 40K and total K in Boletus edulis at different maturity stages: Effect of braising and estimated radiation dose intake. Chemosphere 2021, 268, 129336. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent developments on umami ingredients of edible mushrooms—A review. Trends Food Sci. Technol. 2013, 33, 78–92. [Google Scholar] [CrossRef]
- Mamat, A.; Tusong, K.; Xu, J. Identification of metabolic pathways related to rough-skinned fruit formation in Korla pear. Sci. Hortic-Amst. 2021, 288, 110414. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Tong, X.; Peng, Y.; Ma, P.; Zhang, M.-J.; Lu, H.-M.; Chen, X.-Q.; Liang, Y.-Z. Multiscale peak detection in wavelet space. Analyst 2015, 140, 7955–7964. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An Integrated Strategy for Compound Spectra Extraction and Annotation of Liquid Chromatography/Mass Spectrometry Data Sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lu, Q.; Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2022, 375, 131908. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal Component Analysis; Wiley Online Library: Hoboken, NJ, USA, 2002. [Google Scholar]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-Based Metabolomics Data for Identification of Biochemically Interesting Compounds Using OPLS Class Models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Powell, S.; Szklarczyk, D.; Trachana, K.; Roth, A.; Kuhn, M.; Muller, J.; Arnold, R.; Rattei, T.; Letunic, I.; Doerks, T.; et al. eggNOG v3.0: Orthologous groups covering 1133 organisms at 41 different taxonomic ranges. Nucleic. Acids Res. 2011, 40, D284–D289. [Google Scholar] [CrossRef] [Green Version]
- Bouhaddani, S.E.; Houwing-Duistermaat, J.; Salo, P.; Perola, M.; Jongbloed, G.; Uh, H.-W. Evaluation of O2PLS in Omics data integration. BMC Bioinform. 2016, 17, S11. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.H.; Kim, S.Y.; Choi, H.-K.; Kim, Y.-S. Characterization of Aroma-Active Compounds in Raw and Cooked Pine-Mushrooms (Tricholoma matsutake Sing.). J. Agr. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Dijkstra, F.Y. Studies on mushroom flavours 3. Some flavour compounds in fresh, canned and dried edible mushrooms. Eur. Food Res. Technol. 1976, 160, 401–405. [Google Scholar] [CrossRef]
- Aisala, H.; Manninen, H.; Laaksonen, T.; Linderborg, K.M.; Myoda, T.; Hopia, A.; Sandell, M. Linking volatile and non-volatile compounds to sensory profiles and consumer liking of wild edible Nordic mushrooms. Food Chem. 2020, 304, 125403. [Google Scholar] [CrossRef]
- Bozok, F.; Zarifikhosroshahi, M.; Kafkas, E.; Taşkin, H.; Buyukalaca, S. Comparison of Volatile Compounds of Fresh Boletus edulis and B. pinophilus in Marmara Region of Turkey. Not. Bot. Horti Agrobot. 2015, 43, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Balabanova, L.A.; Shkryl, Y.N.; Slepchenko, L.V.; Yugay, Y.A.; Gorpenchenko, T.Y.; Kirichuk, N.N.; Khudyakova, Y.V.; Bakunina, I.Y.; Podvolotskaya, A.B.; Bulgakov, V.P.; et al. Development of host strains and vector system for an efficient genetic transformation of filamentous fungi. Plasmid 2019, 101, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, K.; Qi, T.; Wan, Q.; Li, T. Insights into the Flavor Differentiation between Two Wild Edible Boletus Species through Metabolomic and Transcriptomic Analyses. Foods 2023, 12, 2728. https://doi.org/10.3390/foods12142728
Chao K, Qi T, Wan Q, Li T. Insights into the Flavor Differentiation between Two Wild Edible Boletus Species through Metabolomic and Transcriptomic Analyses. Foods. 2023; 12(14):2728. https://doi.org/10.3390/foods12142728
Chicago/Turabian StyleChao, Kaixiang, Tuo Qi, Qionglian Wan, and Tao Li. 2023. "Insights into the Flavor Differentiation between Two Wild Edible Boletus Species through Metabolomic and Transcriptomic Analyses" Foods 12, no. 14: 2728. https://doi.org/10.3390/foods12142728
APA StyleChao, K., Qi, T., Wan, Q., & Li, T. (2023). Insights into the Flavor Differentiation between Two Wild Edible Boletus Species through Metabolomic and Transcriptomic Analyses. Foods, 12(14), 2728. https://doi.org/10.3390/foods12142728







