Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Chemicals
2.2. Honey Sample Collection
2.3. Sample Preparation
2.4. HS-SPME-GC-MS Analysis
2.5. Data Analysis
3. Results and Discussion
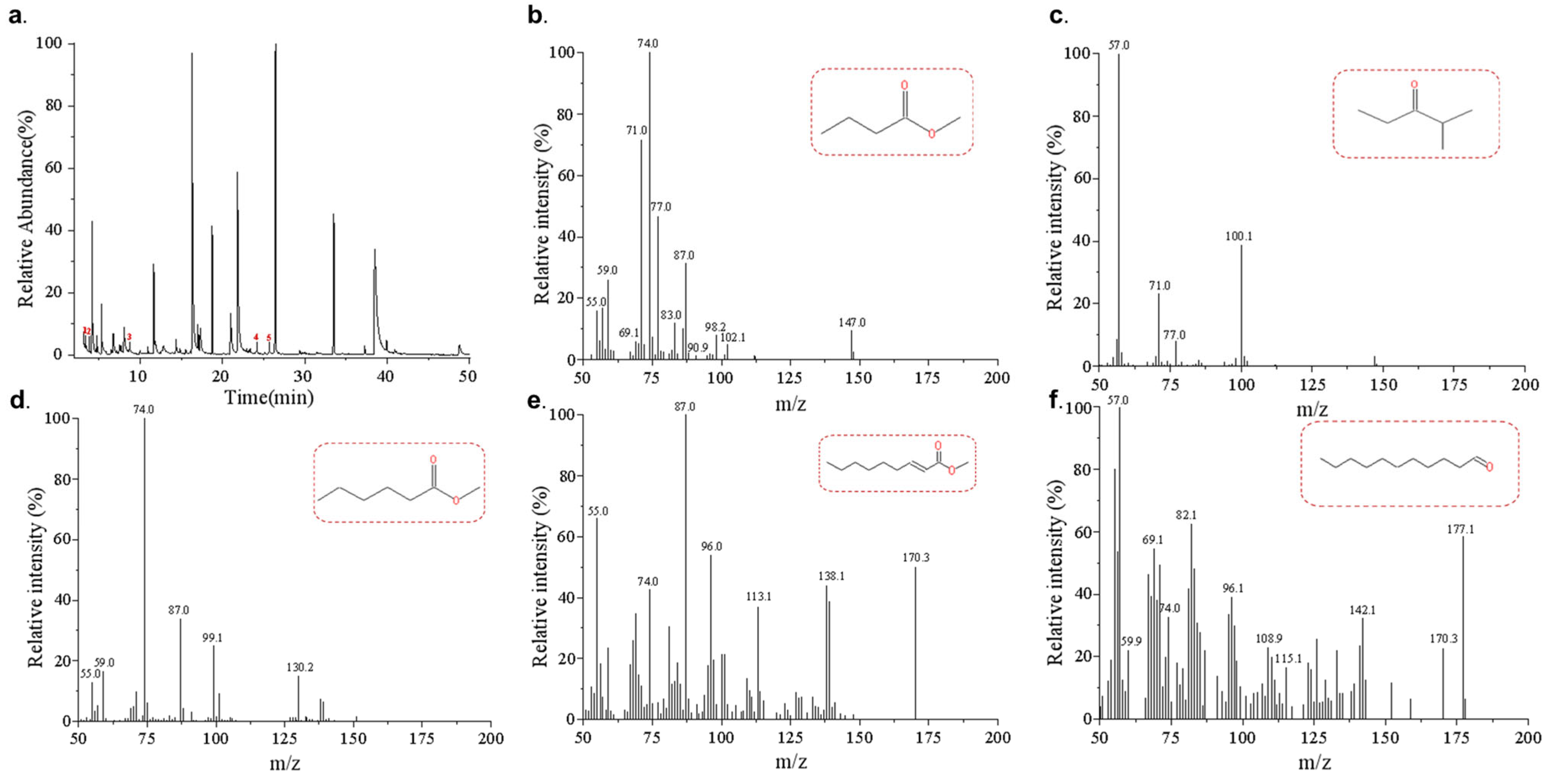
3.1. Volatiles in Zygosaccharomyces rouxii-Contaminated Jujube Honey Identified via HS-SPME-GC–MS
3.2. Identification of Characteristic VOCs by HS-GC-IMS
3.2.1. Alcohols
3.2.2. Esters
3.2.3. Aldehydes and Ketones
3.2.4. Hydrocarbons and Furan
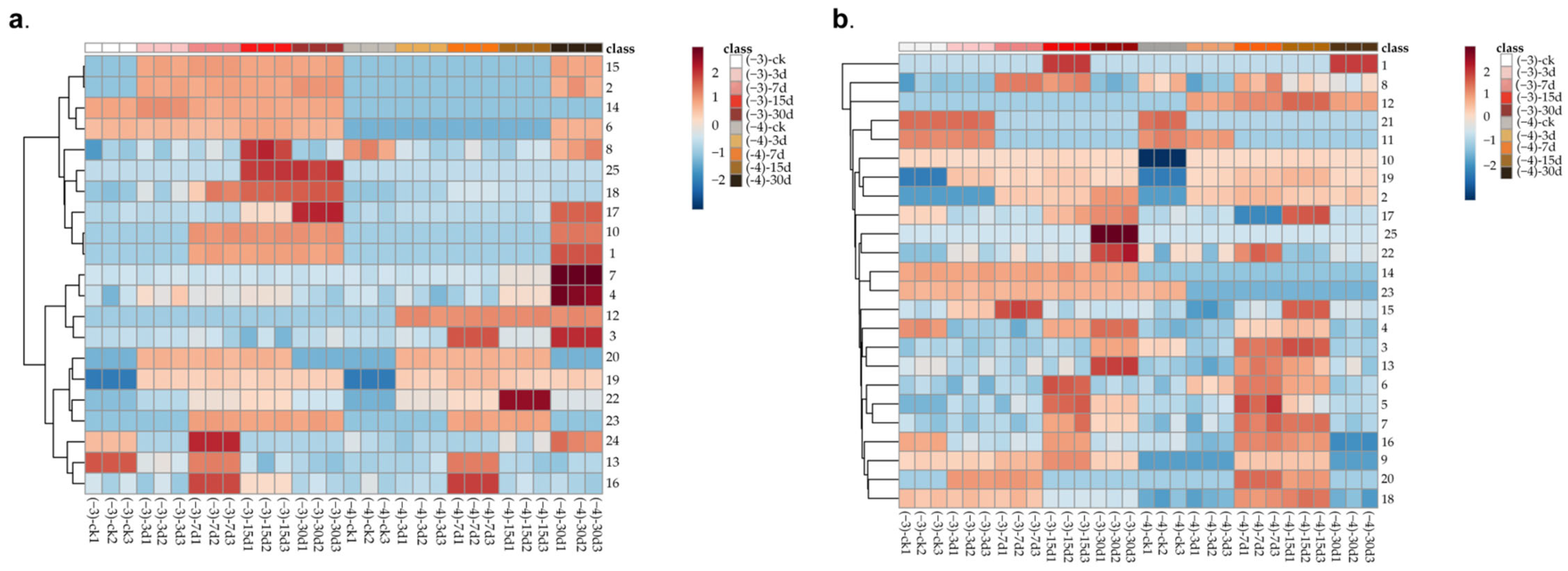
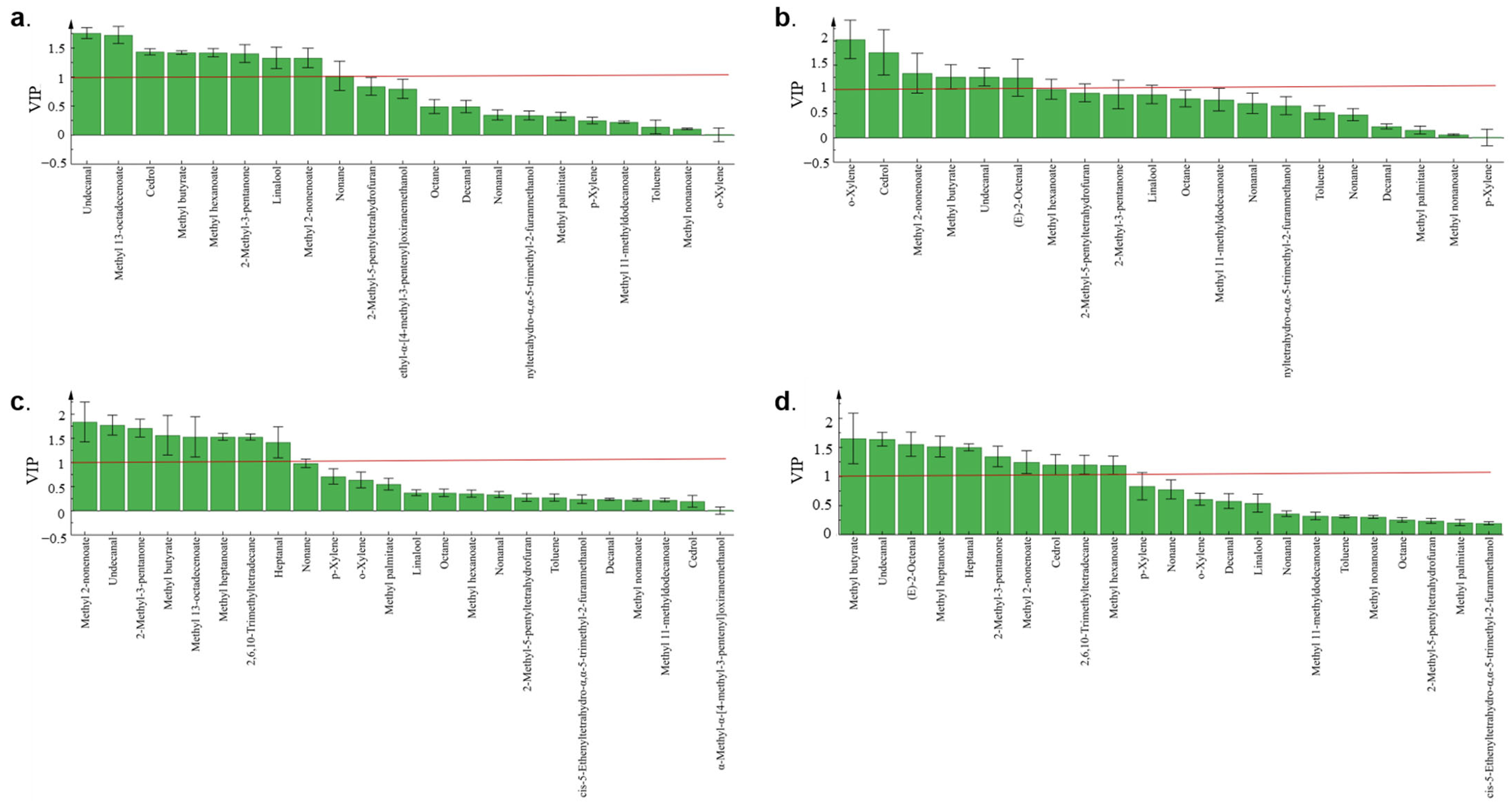
3.3. Determination of Violate Markers in Z. rouxii-Contaminated Jujube Honey
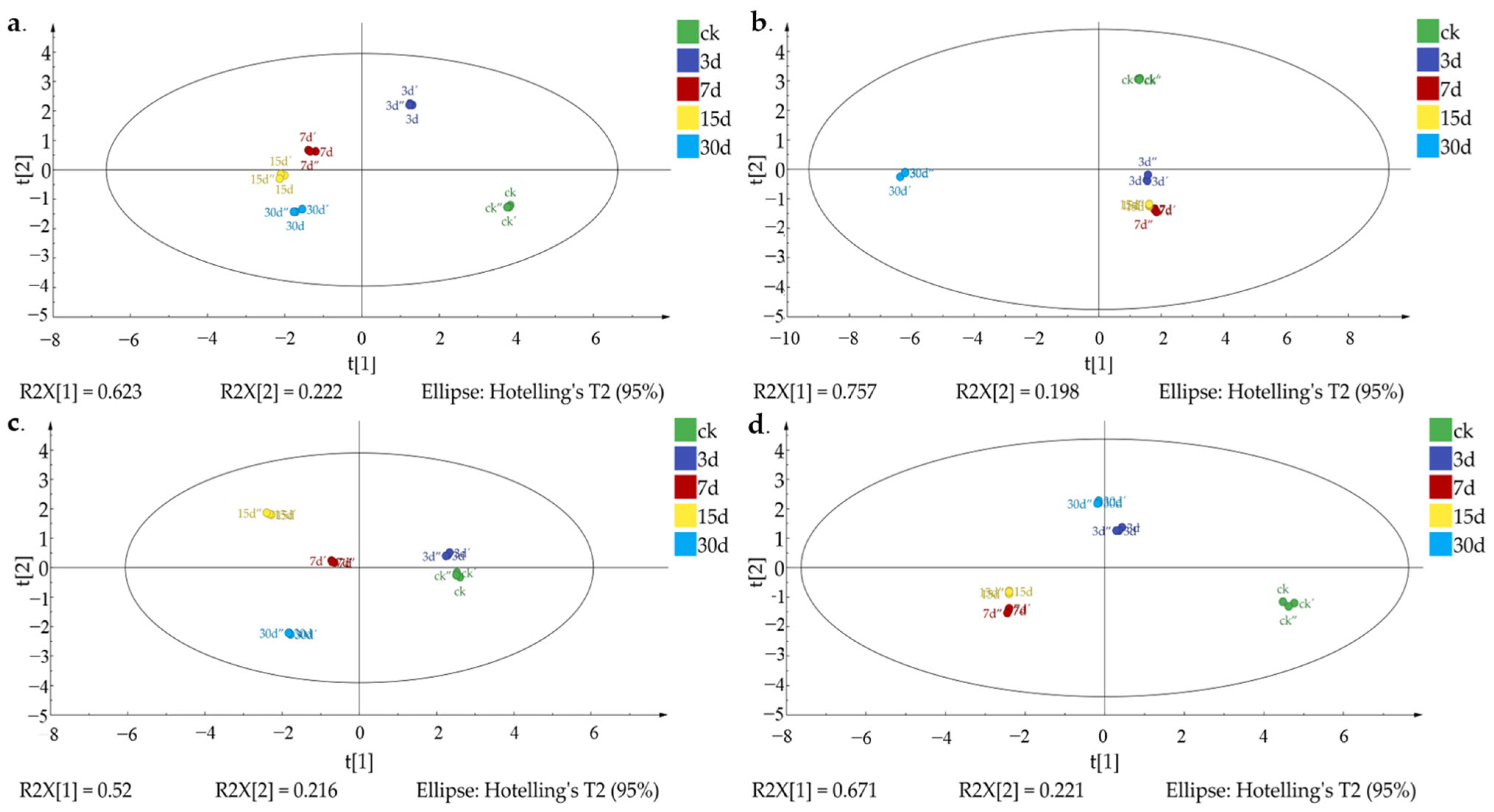
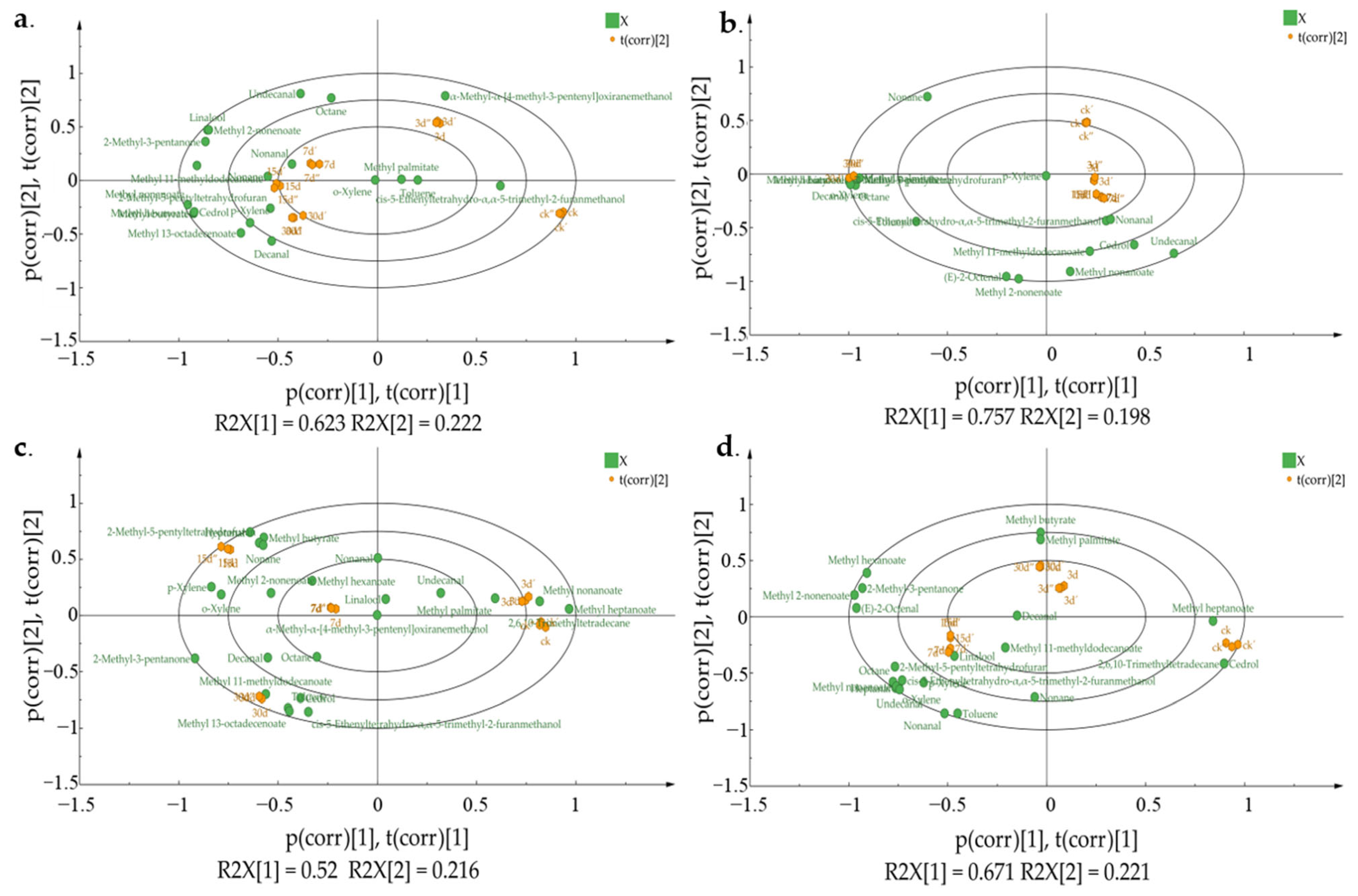
3.4. VOC Variation Analysis among Different Z. rouxii Concentration Contamination in MH and IH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Q.; Huang, X.Z.; Ji, X.X.; Jia, G.Q.; Sang, Y.X.; Cui, Z.Y.; Zhang, J.J. Comprehensive investigation of psicose in Chinese honeys and the assessment of its potential as a new marker for honey adulteration detection. J. Food Compos. Anal. 2022, 108, 104444. [Google Scholar] [CrossRef]
- Chen, L.; Wei, X.; Liu, G.L.; Hu, Z.; Chi, Z.M.; Chi, Z. Glycerol, trehalose and vacuoles had relations to pullulan synthesis and osmotic tolerance by the whole genome duplicated strain Aureobasidium melanogenum TN3-1 isolated from natural honey. Int. J. Biol. Macromol. 2020, 165, 131–140. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Scariot, F.J.; Foresti, L.; Schwarz, L.V.; Rocha, R.K.M.; da Silva, G.P.; Moreira, J.P.; Delamare, A.P.L. Yeast biodiversity in honey produced by stingless bees raised in the highlands of southern Brazil. Int. J. Food Microbiol. 2021, 347, 109200. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Andrade, E.; Stchigel, A.M.; Terrab, A.; Guarro, J.; Cano-Lira, J.F. Diversity of xerotolerant and xerophilic fungi in honey. IMA Fungus 2019, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Z.; Tian, J.; Zhang, Y.Z.; Li, S.S.; Zheng, H.Q.; Hu, F.L. Investigation of the Maturity Evaluation Indicator of Honey in Natural Ripening Process: The Case of Rape Honey. Foods 2021, 10, 2882. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.A.; Wu, F.H.; Zhu, M.; Zhang, Y.; Cheng, N.; Xue, X.F.; Wu, L.M.; Cao, W. Molecular Mechanism of Mature Honey Formation by GC-MS- and LC-MS-Based Metabolomics. J. Agric. Food Chem. 2021, 69, 3362–3370. [Google Scholar] [CrossRef]
- Xu, X.L.; Zhu, Y.X.; Li, Y.J.; Yang, W.C.; Zhou, H.; Chen, X.C. Proteomics Analysis of Zygosaccharomyces mellis in Response to Sugar Stress. Processes 2022, 10, 1193. [Google Scholar] [CrossRef]
- Matraxia, M.; Alfonzo, A.; Prestianni, R.; Francesca, N.; Gaglio, R.; Todaro, A.; Alfeo, V.; Perretti, G.; Columba, P.; Settanni, L.; et al. Non-conventional yeasts from fermented honey by-products: Focus on Hanseniaspora uvarum strains for craft beer production. Food Microbiol. 2021, 99, 103806. [Google Scholar] [CrossRef]
- Cai, R.; Hu, M.M.; Zhang, Y.J.; Niu, C.; Yue, T.L.; Yuan, Y.H.; Wang, Z.L. Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT-Food Sci. Technol. 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Wang, H.X.; Sun, H.M. Potential use of electronic tongue coupled with chemometrics analysis for early detection of the spoilage of Zygosaccharomyces rouxii in apple juice. Food Chem. 2019, 290, 152–158. [Google Scholar] [CrossRef]
- Chen, S.Q.; Tang, Q.Y.; Geng, J.Q.; Liu, Y.Q.; Jiang, J.; Cai, X.F.; Cao, H.; Wu, Y.T.; Ren, Y.; Liu, K.; et al. Detection of Viable Zygosaccharomyces rouxii in Honey and Honey Products via PMAXX-qPCR. Food Qual. 2022, 2022, 8670182. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Epping, R.; Koch, M. On-Site Detection of Volatile Organic Compounds (VOCs). Molecules 2023, 28, 1598. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, J.; Zhao, H.A.; Wu, F.H.; Xue, X.F.; Wu, L.M.; Cao, W. Volatile compounds of five types of unifloral honey in Northwest China: Correlation with aroma and floral origin based on HS-SPME/GC-MS combined with chemometrics. Food Chem. 2022, 384, 132461. [Google Scholar] [CrossRef] [PubMed]
- Madas, N.M.; Marghitas, L.A.; Dezmirean, D.S.; Bonta, V.; Bobis, O.; Fauconnier, M.L.; Francis, F.; Haubruge, E.; Nguyen, K.B. Volatile Profile and Physico-Chemical Analysis of Acacia Honey for Geographical Origin and Nutritional Value Determination. Foods 2019, 8, 445. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Maslowiecka, J.; Pellizzer, N.; Miranda, D.; Bakier, S. Chemical composition of volatile components in the honey of some species of stingless bees. Food Control 2023, 146, 109545. [Google Scholar] [CrossRef]
- Chitarrini, G.; Debiasi, L.; Stuffer, M.; Ueberegger, E.; Zehetner, E.; Jaeger, H.; Robatscher, P.; Conterno, L. Volatile Profile of Mead Fermenting Blossom Honey and Honeydew Honey with or without Ribes nigrum. Molecules 2022, 25, 1818. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.Z.; Dan, M.L.; Zhao, G.H.; Wang, D.M. Recent advances in chromatography-mass spectrometry and electronic nose technology in food flavor analysis and detection. Food Chem. 2023, 405, 134814. [Google Scholar] [CrossRef]
- Verma, D.K.; Al-Sahlany, S.T.G.; Niamah, A.K.; Thakur, M.; Shah, N.; Singh, S.; Baranwal, D.; Patel, A.R.; Utama, G.L.; Aguilar, C.N. Recent trends in microbial flavour Compounds: A review on Chemistry, synthesis mechanism and their application in food. Saudi J. Biol. Sci. 2022, 29, 1565–1576. [Google Scholar] [CrossRef]
- Carraturo, F.; Libralato, G.; Esposito, R.; Galdiero, E.; Aliberti, F.; Amoresano, A.; Fontanarosa, C.; Trifuoggi, M.; Guida, M. Metabolomic profiling of food matrices: Preliminary identification of potential markers of microbial contamination. J. Food Sci. 2020, 85, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. of volatile organic compounds from four Plant Growth-Promoting Rhizobacteria by SPME–GC–MS: A metabolomics study. Metabolites 2022, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Shi, F.F.; He, X.J.; Cai, Y.J.; Yu, Y.L.; Yao, D.; Zhou, J.H.; Wei, X.P. Establishment and application of quantitative method for 22 organic acids in honey based on SPE-GC-MS. Eur. Food Res. Technol. 2022, 249, 473–484. [Google Scholar] [CrossRef]
- Wang, X.R.; Rogers, K.M.; Li, Y.; Yang, S.P.; Chen, L.Z.; Zhou, J.H. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC-MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef]
- Liu, X.T.; Sun, J.; Ji, P.R.; Yang, C.C.; Wu, F.H.; Cheng, N.; El-Seedi, H.R.; Zhao, H.A.; Cao, W. Hydroxy Fatty Acids as Novel Markers for Authenticity Identification of the Honey Entomological Origin Based on the GC-MS Method. J. Agric. Food Chem. 2023, 71, 7163–7173. [Google Scholar] [CrossRef]
- Andrea, B. Springer Handbook of Odor, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 154–196. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, X.Y.; Fu, Y.; Zhang, Q.; Wang, X.H.; Cui, M.Y.; Ma, Y.Y.; Gao, X.L. Effects of Torulaspora delbrueckii co-fermented with Saccharomyces cerevisiae on physicochemical and aromatic profiles of blueberry fermented beverage. Food Chem. 2023, 409, 135284. [Google Scholar] [CrossRef]
- Han, Y.Y.; Song, Z.L.; Du, J.H. Effects of the yeast endogenous β-glucosidase on hawthorn (Crataegus pinnatifida Bunge) wine ethyl carbamate and volatile compounds. J. Food Compos. Anal. 2021, 103, 104084. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Francesca, N.; Corona, O.; Di Gerlando, R.; Columba, P.; Moschetti, G. Production of the Sicilian distillate “Spiritu re fascitrari” from honey by-products: An interesting source of yeast diversity. Int. J. Food Microbiol. 2017, 261, 62–72. [Google Scholar] [CrossRef]
- Rodriguez-Flores, M.S.; Falcao, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Description of the volatile fraction of Erica honey from the northwest of the Iberian Peninsula. Food Chem. 2021, 336, 127758. [Google Scholar] [CrossRef]
- Guo, X.Y.; Ho, C.T.; Wan, X.C.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef]
- Mantzourani, C.; Kokotou, M.G. Targeted and Suspect Fatty Acid Profiling of Royal Jelly by Liquid Chromatography-High Resolution Mass Spectrometry. Biomolecules 2023, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Jimenez, S.M.; Angoa-Perez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Montanez- Soto, J.L.; Oregel-Zamudio, E. Identification of Organic Volatile Markers Associated with Aroma during Maturation of Strawberry Fruits. Molecules 2021, 26, 504. [Google Scholar] [CrossRef]
- Li, Y.D.; Wang, T.; Li, S.; Yin, P.P.; Sheng, H.Y.; Wang, T.B.; Zhang, Y.; Zhang, K.L.; Wang, Q.L.; Lu, S.L.; et al. Influence of GABA-producing yeasts on cheese quality, GABA content, and the volatilome. LWT-Food Sci. Technol. 2022, 154, 112766. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gonzaga, L.V.; de Azevedo, M.S.; Biluca, F.C.; Schulz, M.; Costa, A.C.O.; Fett, R. Stability of volatile compounds of honey during prolonged storage. J. Food Sci. Technol.-Mysore 2020, 57, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; Garcia-Martinez, T. Using an electronic nose and volatilome analysis to differentiate sparkling wines obtained under different conditions of temperature, ageing time and yeast formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ekeanyanwu, R.C.; Chile-Agada, A.B. Phytocomponents from Anacardium occidentale, Psidium guajava, and Terminalia catappa altered membrane osmotic stability of sickle erythrocytes. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Pei, R.Q.; Lv, G.B.; Guo, B.R.; Li, Y.; Ai, M.Q.; He, B.; Wan, R.L. Physiological and transcriptomic analyses revealed the change of main flavor substance of Zygosaccharomyces rouxii under salt treatment. Front. Nutr. 2022, 9, 990380. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.P.; Chen, L.; Sheng, L.; Tong, Q.Y. Volatile Compounds Analysis and Off-Flavors Removing of Porcupine Liver. Food Sci. Technol. Res. 2016, 22, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.R.; Tian, Y.Q.; Chen, L.; Jin, Z.Y. Impact of cooling rates on the flavor of cooked rice during storage. Food Biosci. 2020, 35, 100563. [Google Scholar] [CrossRef]
- Zhao, T.F.; Cao, Z.Q.; Yu, J.; Weng, X.D.; Benjakul, S.; Guidi, A.; Ying, X.G.; Ma, L.K.; Xiao, G.S.; Deng, S.G. Gas-phase ion migration spectrum analysis of the volatile flavors of large yellow croaker oil after different storage periods. Curr. Res. Food Sci. 2022, 5, 813–822. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.Q.; Song, S.Q.; Wang, H.T.; Yao, L.Y.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Zeng, L.; Fu, Y.Q.; Huang, J.S.; Wang, J.R.; Jin, S.; Yin, J.F.; Xu, Y.Q. Comparative Analysis of Volatile Compounds in Tieguanyin with Different Types Based on HS-SPME-GC-MS. Foods 2022, 11, 1530. [Google Scholar] [CrossRef] [PubMed]
- Raczyk, M.; Kmiecik, D.; Schieberle, P.; Przybylski, R.; Jelen, H.; Rudzinska, M. Model studies on the formation of volatile compounds generated by a thermal treatment of steryl esters with different fatty acid moieties. Food Res. Int. 2017, 97, 87–94. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Novel Insights into Dietary Phytosterol Utilization and Its Fate in Honey Bees (Apis mellifera L.). Molecules 2020, 25, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrignani, M.; Fagundez, G.A.; Tananaki, C.; Thrasyvoulou, A.; Lupano, C.E. Volatile compounds of Argentinean honeys: Correlation with floral and geographical origin. Food Chem. 2018, 246, 32–40. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | Formula | RT (min) | m/z | ck 1 | 3d 1 | 7d 1 | 15d 1 | 30d 1 | ck 2 | 3d 2 | 7d 2 | 15d 2 | 30d 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | cis-5-Ethenyltetrahydro-α,α-5-trimethyl-2-furanmethanol | C10H18O2 | 14.822 | 170.13 | + | + | + | + | + | + | − | + | + | − |
| α-Methyl-α-[4-methyl-3-pentenyl]oxiranemethanol | C10H18O2 | 15.522 | 170.13 | + | + | + | + | + | − | − | − | − | − | |
| Linalool | C10H18O | 16.143 | 154.14 | − | + | + | + | + | − | − | − | − | + | |
| Cedrol | C15H26O | 37.259 | 222.37 | − | − | + | + | + | − | − | + | + | − | |
| Aldehydes | (E)-2-Octenal | C8H14O | 14.316 | 126.20 | − | − | − | − | − | − | + | + | + | + |
| Nonanal | C9H18O | 16.377 | 142.24 | + | + | + | + | + | + | + | + | + | + | |
| Decanal | C10H20O | 21.024 | 156.27 | + | + | + | + | + | + | + | + | + | + | |
| Undecanal | C11H22O | 25.660 | 170.29 | − | + | + | + | − | − | + | + | + | − | |
| Ketones | 2-Methyl-3-pentanone | C6H12O | 3.834 | 316.04 | − | + | + | + | + | − | − | − | − | + |
| Benzophenone * | C13H10O | 38.511 | 182.22 | + | + | + | + | + | + | + | + | + | + | |
| Esters | Methyl butyrate | C5H10O2 | 3.396 | 102.13 | − | − | + | + | + | − | − | − | − | + |
| Methyl hexanoate | C7H14O2 | 8.675 | 130.19 | − | − | + | + | + | − | − | − | − | + | |
| Methyl nonanoate | C10H20O2 | 21.511 | 172.26 | + | + | + | + | + | + | + | + | + | + | |
| Methyl 2-nonenoate | C10H18O2 | 21.834 | 170.25 | − | + | + | + | + | − | + | + | + | + | |
| Methyl 11-methyldodecanoate | C13H26O2 | 34.535 | 214.34 | + | + | + | + | + | + | + | + | + | + | |
| Methyl palmitate | C17H34O2 | 48.825 | 270.45 | + | + | + | + | + | + | + | + | + | + | |
| Methyl 13-octadecenoate | C19H36O2 | 54.273 | 296.49 | − | − | − | + | + | − | − | − | − | − | |
| Aromatic | Toluene | C7H8 | 4.139 | 92.14 | + | + | + | + | + | + | + | + | + | + |
| p-Xylene | C8H10 | 6.722 | 106.17 | + | + | + | + | + | + | + | + | + | + | |
| o-Xylene | C8H10 | 7.465 | 106.17 | + | + | + | + | + | + | + | + | + | + | |
| Alkanes | Octane | C8H18 | 4.756 | 114.23 | + | + | + | + | + | + | + | + | + | + |
| Nonane | C9H20 | 7.677 | 128.26 | + | + | + | + | + | + | + | + | + | + | |
| Furan | 2-Methyl-5-pentyltetrahydrofuran | C10H20O | 7.367 | 156.26 | + | + | + | + | + | + | + | + | + | + |
| Metabolite | Formula | RT (min) | m/z | ck 1 | 3d 1 | 7d 1 | 15d 1 | 30d 1 | ck 2 | 3d 2 | 7d 2 | 15d 2 | 30d 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | cis-5-Ethenyltetrahydro-α,α-5-trimethyl-2-furanmethanol | C10H18O2 | 14.822 | 170.13 | + | + | + | + | + | + | + | + | + | + |
| α-Methyl-α-[4-methyl-3-pentenyl]oxiranemethanol | C10H18O2 | 15.522 | 170.13 | + | + | + | + | + | − | − | − | − | − | |
| Linalool | C10H18O | 16.143 | 154.14 | + | + | + | + | + | + | + | + | + | + | |
| Cedrol | C15H26O | 37.259 | 222.37 | + | + | + | + | + | + | − | − | − | − | |
| Aldehydes | Heptanal | C7H14O | 7.792 | 114.19 | + | + | + | + | + | − | − | + | + | − |
| (E)-2-Octenal | C8H14O | 14.316 | 126.20 | − | − | − | − | − | − | + | + | + | + | |
| Nonanal | C9H18O | 16.377 | 142.24 | + | + | + | + | + | + | + | + | + | + | |
| Decanal | C10H20O | 21.024 | 156.27 | + | + | + | + | + | + | + | + | + | + | |
| Undecanal | C11H22O | 25.660 | 170.29 | − | + | + | − | − | − | − | + | + | − | |
| Ketones | 2-Methyl-3-pentanone | C6H12O | 3.834 | 316.04 | − | − | + | + | + | − | + | + | + | + |
| Benzophenone * | C13H10O | 38.511 | 182.22 | + | + | + | + | + | + | + | + | + | + | |
| Esters | Methyl butyrate | C5H10O2 | 3.396 | 102.13 | − | − | − | + | + | − | − | − | − | + |
| Methyl hexanoate | C7H14O2 | 8.675 | 130.19 | + | + | + | + | + | − | + | + | + | + | |
| Methyl heptanoate | C8H16O2 | 12.838 | 144.21 | + | + | − | − | − | + | + | − | − | − | |
| Methyl nonanoate | C10H20O2 | 21.511 | 172.26 | + | + | + | + | + | + | + | + | + | + | |
| Methyl 2-nonenoate | C10H18O2 | 21.834 | 170.25 | − | + | + | + | + | − | + | + | + | + | |
| Methyl 11-methyldodecanoate | C13H26O2 | 34.535 | 214.34 | + | + | + | + | + | + | + | + | + | + | |
| Methyl palmitate | C17H34O2 | 48.825 | 270.45 | + | + | + | + | + | + | + | + | + | + | |
| Methyl 13-octadecenoate | C19H36O2 | 54.273 | 296.49 | − | − | − | − | + | − | − | − | − | − | |
| Aromatic | Toluene | C7H8 | 4.139 | 92.14 | + | + | + | + | + | + | + | + | + | + |
| p-Xylene | C8H10 | 6.722 | 106.17 | + | + | + | + | + | + | + | + | + | + | |
| o-Xylene | C8H10 | 7.465 | 106.17 | + | + | + | + | + | + | + | + | + | + | |
| Alkanes | Octane | C8H18 | 4.756 | 114.23 | + | + | + | + | + | + | + | + | + | + |
| Nonane | C9H20 | 7.677 | 128.26 | + | + | + | + | + | + | + | + | + | + | |
| 2,6,10-Trimethyltetradecane | C17H36 | 31.890 | 240.47 | + | + | − | − | − | + | − | − | − | − | |
| Furan | 2-Methyl-5-pentyltetrahydrofuran | C10H20O | 7.367 | 156.26 | + | + | + | + | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, Y.; Cheng, N.; Zhao, H.; Zhang, Y.; Liu, C.; He, L.; Ma, T.; Li, Y.; Cao, W. Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey. Foods 2023, 12, 2730. https://doi.org/10.3390/foods12142730
Wang Y, Huang Y, Cheng N, Zhao H, Zhang Y, Liu C, He L, Ma T, Li Y, Cao W. Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey. Foods. 2023; 12(14):2730. https://doi.org/10.3390/foods12142730
Chicago/Turabian StyleWang, Yin, Yuanyuan Huang, Ni Cheng, Haoan Zhao, Ying Zhang, Cailing Liu, Liangliang He, Tianchen Ma, Yankang Li, and Wei Cao. 2023. "Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey" Foods 12, no. 14: 2730. https://doi.org/10.3390/foods12142730
APA StyleWang, Y., Huang, Y., Cheng, N., Zhao, H., Zhang, Y., Liu, C., He, L., Ma, T., Li, Y., & Cao, W. (2023). Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey. Foods, 12(14), 2730. https://doi.org/10.3390/foods12142730






