Preparation and Characterization of Novel Chitosan Coatings to Reduce Changes in Quality Attributes and Physiochemical and Water Characteristics of Mongolian Cheese during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Coating Treatment
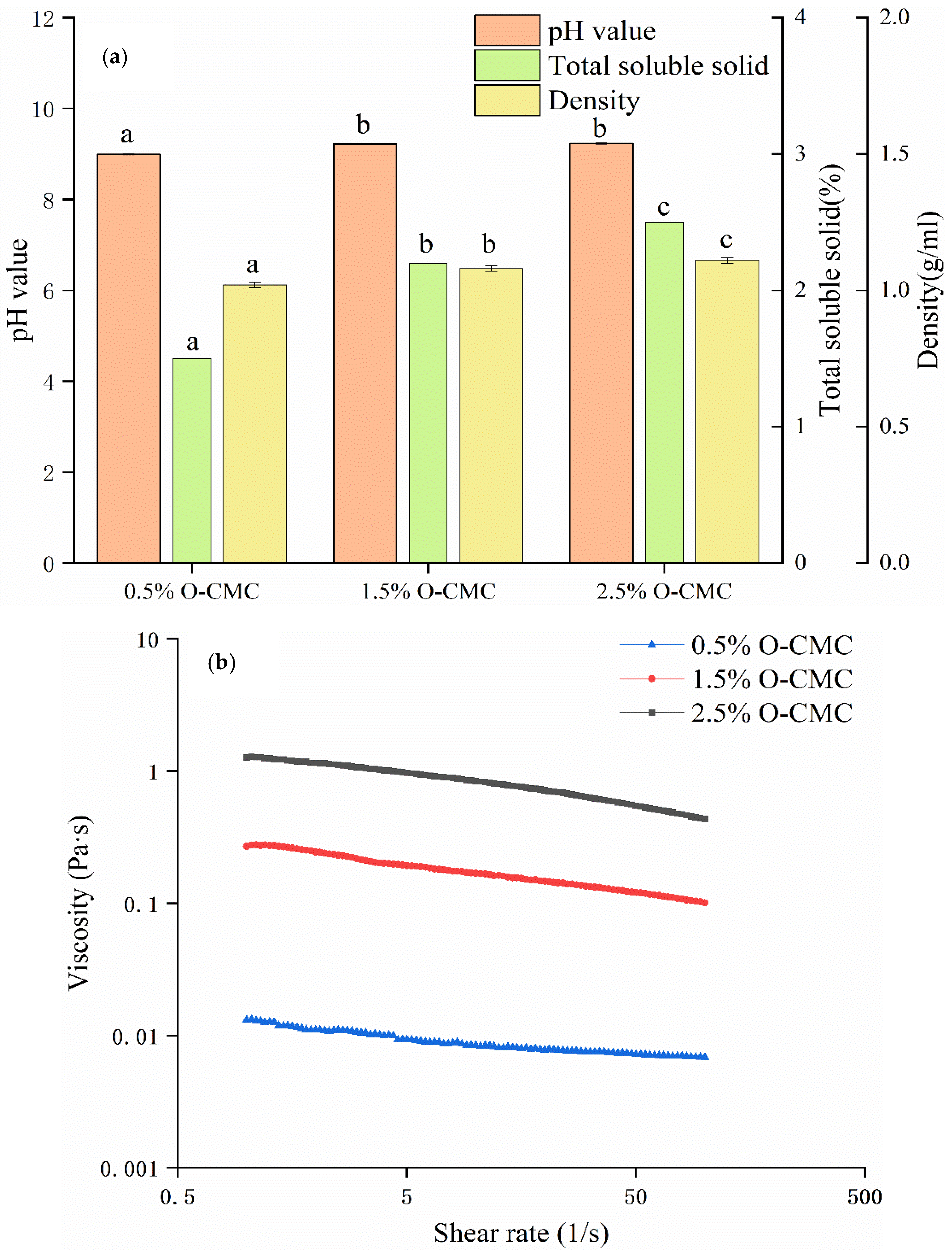
2.4. pH, Total Soluble Solid, Density, and Viscosity Properties of the Coating Solutions
2.5. Microbiological Analysis
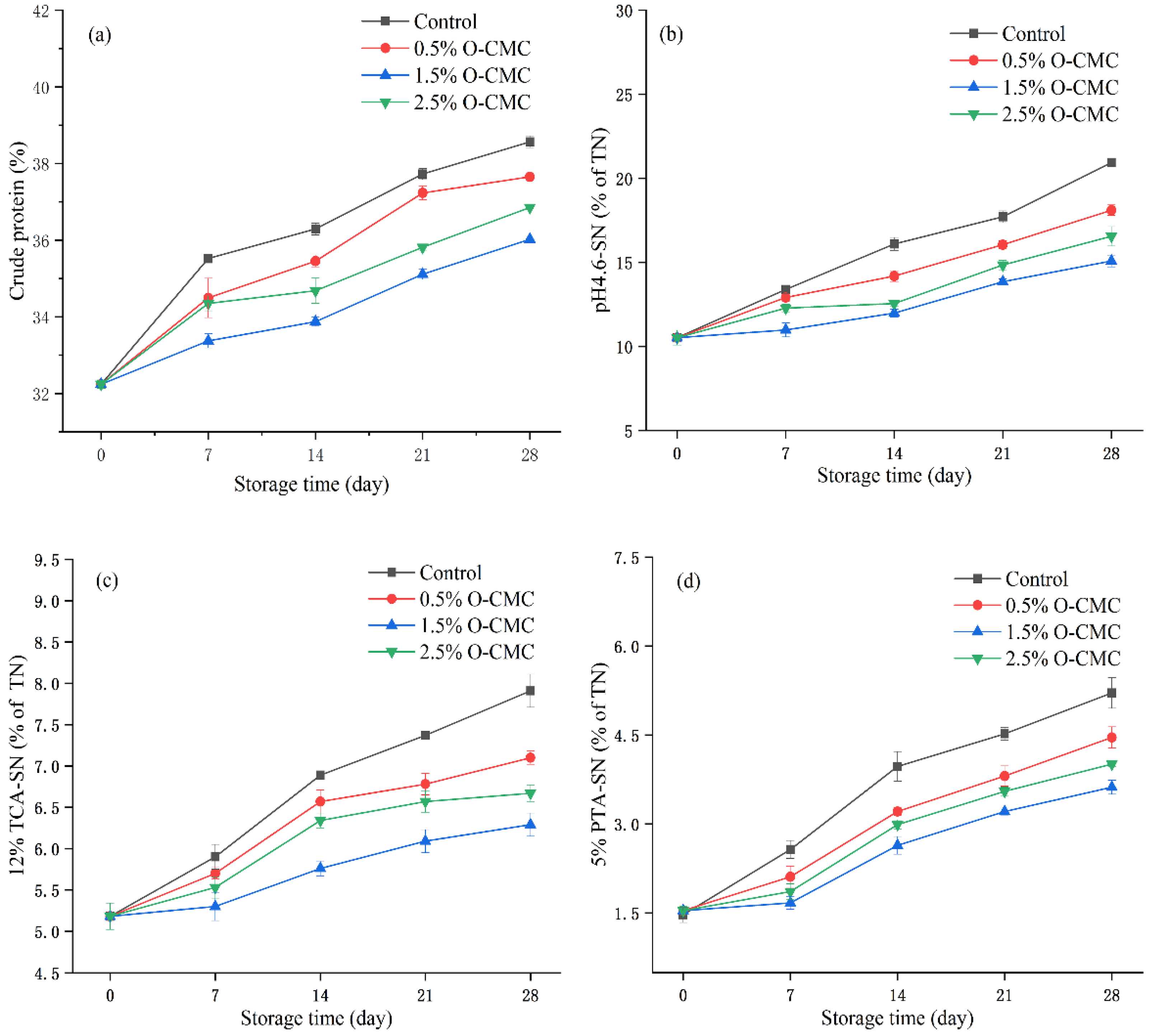
2.6. Protein and Non-Protein Nitrogen
2.7. Physicochemical Analysis
2.8. Color
2.9. Texture Profile Analysis (TPA)
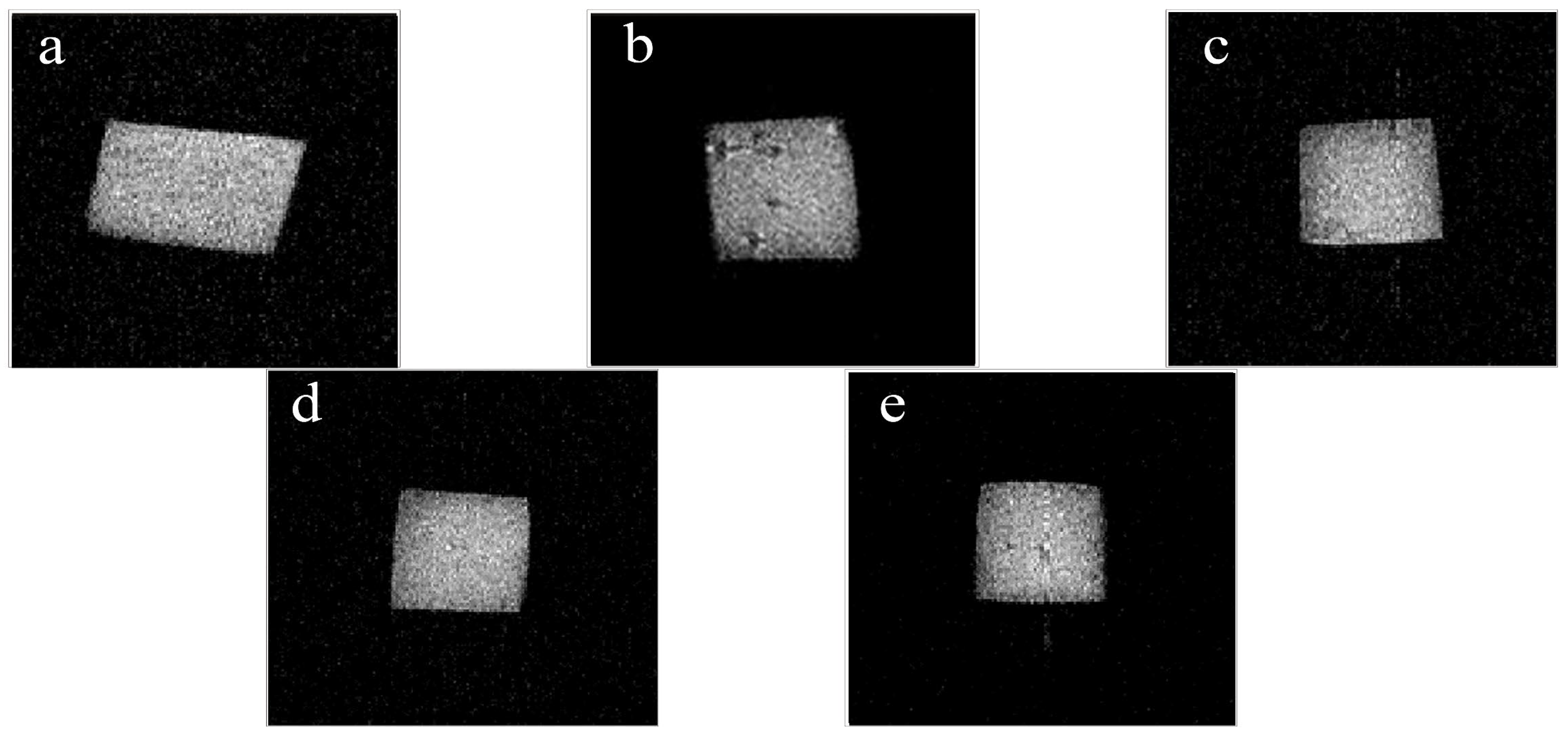
2.10. Nuclear Magnetic Resonance (NMR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. pH Value, Total Soluble Solid, Density, and Viscosity Properties of Coating Solutions
3.2. Microbiological Analysis
3.3. Protein and Non-Protein Nitrogen Analysis
3.4. Physicochemical Analyses
3.5. Color Analysis
3.6. TPA
3.7. NMR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zheng, Y.; Zhou, R.; Ma, M. Comprehensive Identification of Molecular Profiles Related to Sensory and Nutritional Changes in Mongolian Cheese during Storage by Untargeted Metabolomics Coupled with Quantification of Free Amino Acids. Food Chem. 2022, 386, 132740. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zheng, Y.; Ma, R.; Wan, J.; Zhou, R.; Ma, M. Kinetics of Proteolysis in Stored Mongolian Cheese at Ice-Temperatures and Split-Split-Plot Analysis of Storage Factors Affecting Cheese Quality. Food Res. Int. 2021, 140, 109850. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Lacroix, C.; Meile, L.; Jans, C. Enterococci and Pseudomonads as Quality Indicators in Industrial Production and Storage of Mozzarella Cheese from Raw Cow Milk. Int. Dairy J. 2018, 82, 28–34. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Bourbon, A.I.; Costa, M.J.; Muñoz-Tebar, N.; Carmona, M.; Molina, A.; Sánchez-Vioque, R.; Berruga, M.I.; Vicente, A.A. Optimization of a Chitosan Solution as Potential Carrier for the Incorporation of Santolina Chamaecyparissus L. Solid By-Product in an Edible Vegetal Coating on ‘Manchego’ Cheese. Food Hydrocoll. 2019, 89, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zheng, Y.; Zhang, X.; Zhou, R.; Ma, M. Effect of Supercritical Carbon Dioxide on Bacterial Community, Volatile Profiles and Quality Changes during Storage of Mongolian Cheese. Food Control 2023, 143, 109225. [Google Scholar] [CrossRef]
- Ozturk, M.; Govindasamy-Lucey, S.; Jaeggi, J.J.; Johnson, M.E.; Lucey, J.A. Investigating the Properties of High-Pressure-Treated, Reduced-Sodium, Low-Moisture, Part-Skim Mozzarella Cheese during Refrigerated Storage. J. Dairy Sci. 2018, 101, 6853–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Khamrai, M.; Samanta, S.; Kumari, K.; Kundu, P.P. Microbial, Physicochemical, and Sensory Analyses-Based Shelf Life Appraisal of White Fresh Cheese Packaged into PET Waste-Based Active Packaging Film. J. Packag. Technol. Res. 2018, 2, 125–147. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Dell’Olmo, E.; Arciello, A.; Porta, R. Improved Shelf-Life of Nabulsi Cheese Wrapped with Hydrocolloid Films. Food Hydrocoll. 2019, 96, 29–35. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Sedaghat, N. Effect of Active Edible Coating and Temperature on Quality Properties of Roasted Pistachio Nuts during Storage. J. Food Process. Preserv. 2019, 43, e14121. [Google Scholar] [CrossRef]
- Mendes, C.G.; Martins, J.T.; Ludtke, F.L.; Geraldo, A.; Pereira, A.; Vicente, A.A.; Vieira, J.M. Chitosan Coating Functionalized with Flaxseed Oil and Green Tea Extract as a Bio-Based Solution for Beef Preservation. Foods 2023, 12, 1447. [Google Scholar] [CrossRef]
- O’Connor, L.; Favreau-Farhadi, N.; Barrett, A. Use of Edible Barriers in Intermediate Moisture Food Systems to Inhibit Moisture Migration. J. Food Process. Preserv. 2017, 42, e13512. [Google Scholar] [CrossRef]
- Anitha, A.; Sreeranganathan, M.; Narayanan, D.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Efficient Water Soluble O-Carboxymethyl Chitosan Nanocarrier for the Delivery of Curcumin to Cancer Cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Chen, X.; Park, H. Chemical Characteristics of O-Carboxymethyl Chitosans Related to the Preparation Conditions. Carbohyd. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Tang, X.; Wu, J.; Yang, J.; Lin, B. Preparation of Nano-Silver-Carboxymethyl Chitosan Composite and Its Effect on Fruit Preservation. J. Guangxi Univ. 2023, 48, 425–432. [Google Scholar]
- Liu, X.; Guan, Y.; Yang, D.; Li, Z.; Yao, K. Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohyd. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Cai, M.; Duan, Y.; Shi, T.; Su, J.; Chen, K.; Ma, D.; Wang, F.; Qin, J.; Wei, S.; Gao, Z. Multiple Effects Achieved with a Single Agent of O-Carboxymethyl Chitosan Exhibiting Cross-Linking and Antibacterial Properties. Prog. Org. Coat. 2023, 175, 107345. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Zhou, X.; Hu, Y.; Ma, M. Influence of Hot Water Treatment and O-Carboxymethyl Chitosan Coating on Postharvest Quality and Ripening in Hami Melons (Cucumis melo var. saccharinus) under Long-Term Simulated Transport Vibration. J. Food Biochem. 2020, 44, e13328. [Google Scholar]
- Zhang, X.; Zheng, Y.; Feng, J.; Zhou, R.; Ma, M. Integrated Metabolomics and High-Throughput Sequencing to Explore the Dynamic Correlations between Flavor Related Metabolites and Bacterial Succession in the Process of Mongolian Cheese Production. Food Res. Int. 2022, 160, 111672. [Google Scholar] [CrossRef]
- Yang, C.; Xu, C. Brief Introduction of On-Line Liquid Density Measurement Technology. Auto. Technol. Its Appl. 2022, 41, 1–4. [Google Scholar]
- Shebis, Y.; Fallik, E.; Rodov, V.; Sagiri, S.S.; Poverenov, E. Oligomers of Carboxymethyl Cellulose for Postharvest Treatment of Fresh Produce: The Effect on Fresh-Cut Strawberry in Combination with Natural Active Agents. Foods 2022, 11, 1117. [Google Scholar] [CrossRef] [PubMed]
- Verruck, S.; Prudêncio, E.S.; Müller, C.M.O.; Fritzen-Freire, C.B.; Amboni, R.D.d.M.C. Influence of Bifidobacterium Bb-12 on the Physicochemical and Rheological Properties of Buffalo Minas Frescal Cheese during Cold Storage. J. Food Eng. 2015, 151, 34–42. [Google Scholar] [CrossRef]
- Diezhandino, I.; Fernández, D.; González, L.; McSweeney, P.L.; Fresno, J.M. Microbiological, Physico-Chemical and Proteolytic Changes in a Spanish Blue Cheese during Ripening (Valdeón Cheese). Food Chem. 2015, 168, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Inácio, R.S.; Monteiro, M.J.P.; Lopes-Da-Silva, J.A.; Gomes, A.M.P.; Saraiva, J.A. Comparing Different Packaging Conditions on Quality Stability of High-Pressure Treated Serra da Estrela Cheeses during Cold Storage. Foods 2023, 12, 1935. [Google Scholar] [CrossRef]
- Kumar, S.; Kanawjia, S.; Kumar, S.; Khatkar, S. Comparative Study of Buffalo and Cow Milk Feta-Type Cheese with Respect to Sensory and Biochemical Characteristics during Ripening: Comparison of Buffalo and Cow Milk Feta Cheese. J. Food Process. Preserv. 2014, 38, 823–829. [Google Scholar] [CrossRef]
- King, R.L. Oxidation of Milk Fat Globule Membrane Material. I. Thiobarbituric Acid Reaction as a Measure of Oxidized Flavor in Milk and Model Systems. J. Dairy Sci. 1962, 45, 1165–1171. [Google Scholar] [CrossRef]
- Makhal, S.; Kanawjia, S.K.; Giri, A. Effect of MicroGARD on Keeping Quality of Direct Acidified Cottage Cheese. J. Food Sci. Technol. Mys. 2015, 52, 936–943. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.D.S.O.d.; Dias, C.O.; Pinto, S.S.; Pereira, L.C.; Verruck, S.; Fritzen-Freire, C.B.; Amante, E.R.; Prudêncio, E.S.; Amboni, R.D.M.C. Probiotic Mascarpone-Type Cheese: Characterisation and Cell Viability During Storage and Simulated Gastrointestinal Conditions. Int. J. Dairy Technol. 2018, 71, 195–203. [Google Scholar] [CrossRef]
- Tas, O.; Ertugrul, U.; Grunin, L.; Oztop, M.H. Investigation of the Hydration Behavior of Different Sugars by Time Domain-NMR. Foods 2022, 11, 1148. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, X.; Hu, Y.; Zhang, G.; Yang, P.; Huang, B. Reduction in Hami Melon (Cucumis melo var. saccharinus) Softening Caused by Transport Vibration by Using Hot Water and Shellac Coating. Postharvest Biol. Technol. 2015, 110, 214–223. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of Different Coating Application Methods on the Performance of Edible Coatings on Mozzarella Cheese. LWT 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and Interactions in Chitosan Hydrogels Formed by Complexation or Aggregation for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Thomas, R.L.; Lee, C.; Park, H.J. Antimicrobial Activity of Native Chitosan, Degraded Chitosan, and O-Carboxymethylated Chitosan. J. Food Prot. 2003, 66, 1495–1498. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of Edible Starch–Chitosan Film and Its Application in the Storage of Mongolian Cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Chernova, V.V.; Valiev, D.R.; Bazunova, M.V.; Kulish, E.I. Features of the Rheological Behavior of Polymer-Colloidal Dispersions Based on a Sodium Salt of Carboxymethyl Cellulose and Silver Iodide Sols. Russ. J. Phys. Chem. B 2018, 12, 701–708. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, S.; Rhim, J.W.; Alias, A.K.; Ariffin, F.; Mahmud, S. Application of Antimicrobial Active Packaging Film Made of Semolina Flour, Nano Zinc Oxide and Nano-Kaolin to Maintain the Quality of Low-Moisture Mozzarella Cheese During Low-Temperature Storage. J. Sci. Food Agric. 2019, 99, 2716–2725. [Google Scholar] [CrossRef]
- Gumiero, M.; Peressini, D.; Pizzariello, A.; Sensidoni, A.; Iacumin, L.; Comi, G.; Toniolo, R. Effect of TiO2 Photocatalytic Activity in a HDPE-Based Food Packaging on the Structural and Microbiological Stability of a Short-Ripened Cheese. Food Chem. 2013, 138, 1633–1640. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Le Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Gobbetti, M.; Burzigotti, R.; Smacchi, E.; Corsetti, A.; De Angelis, M. Microbiology and Biochemistry of Gorgonzola Cheese during Ripening. Int. Dairy J. 1997, 7, 519–529. [Google Scholar] [CrossRef]
- Nega, A.; Moatsou, G. Proteolysis and Related Enzymatic Activities in Ten Greek Cheese Varieties. Dairy Sci. Technol. 2012, 92, 57–73. [Google Scholar] [CrossRef] [Green Version]
- Unalan, I.U.; Arcan, I.s.; Korel, F.; Yemenicioğlu, A. Application of Active Zein-Based Films with Controlled Release Properties to Control Listeria Monocytogenes Growth and Lipid Oxidation in Fresh Kashar Cheese. Innov. Food Sci. Emerg. Technol. 2013, 20, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/Whey Protein Film as Active Coating to Extend Ricotta Cheese Shelf-Life. LWT 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft Cheese Supplemented with Black Cumin Oil: Impact on Food Borne Pathogens and Quality during Storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zheng, Y.; Zhou, R.; Ma, M. Characterization of Chitosan Films Incorporated with Different Substances of Konjac Glucomannan, Cassava Starch, Maltodextrin and Gelatin, and Application in Mongolian Cheese Packaging. Coatings 2021, 11, 84. [Google Scholar] [CrossRef]
- Junior, J.; Schllemer, M.; Weber, C.; Tonial, I.; Senter, L.; Prado, N.; Pedrão, M.; Machado, A. Microbiological Quality and Physicochemical Characteristics of Head Cheeses Produced by Different Manufacturers. J. Food Process. Preserv. 2020, 44, e14416. [Google Scholar]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Novel Intelligent Detection of Safer Water Activity by LF-NMR Spectra for Selected Fruits and Vegetables during Drying. Food Bioprocess Technol. 2019, 12, 1093–1101. [Google Scholar] [CrossRef]
- Gianferri, R.; D’Aiuto, V.; Curini, R.; Delfini, M.; Brosio, E. Proton NMR Transverse Relaxation Measurements to Study Water Dynamic States and Age-Related Changes in Mozzarella di Bufala Campana Cheese. Food Chem. 2007, 105, 720–726. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Cardarelli, H.R. Composition, Microstructure and Chemical Interactions during the Production Stages of Mozzarella Cheese. Int. Dairy J. 2019, 88, 34–41. [Google Scholar] [CrossRef]
- Silva, H.L.A.; Balthazar, C.F.; Esmerino, E.A.; Neto, R.P.C.; Rocha, R.d.S.; Moraes, J.; Cavalcanti, R.N.; Franco, R.M.; Tavares, M.I.B.; Santos, J.S.; et al. Partial Substitution of NaCl by KCl and Addition of Flavor Enhancers on Probiotic Prato Cheese: A Study Covering Manufacturing, Ripening and Storage Time. Food Chem. 2018, 248, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T. Potential Uses of LF-NMR and MRI in the Study of Water Dynamics and Quality Measurement of Fruits and Vegetables. J. Food Process. Preserv. 2019, 43, e14202. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Cardarelli, H.R. Changes in Water Mobility and Protein Stabilization of Mozzarella Cheese Made under Different Stretching Temperatures. LWT 2019, 104, 16–23. [Google Scholar] [CrossRef]
| Parameters | Time (day) | Control | 0.50% O-CMC | 1.50% O-CMC | 2.50% O-CMC |
|---|---|---|---|---|---|
| Total viable count (log CFU/g) | 0 | 5.20 ± 0.14a | 5.20 ± 0.14a | 5.20 ± 0.14a | 5.20 ± 0.14a |
| 7 | 5.45 ± 0.13a | 5.39 ± 0.12a | 5.27 ± 0.18a | 5.28 ± 0.03a | |
| 14 | 6.50 ± 0.00a | 5.91 ± 0.02b | 5.49 ± 0.01c | 5.54 ± 0.00d | |
| 21 | 6.98 ± 0.00a | 6.31 ± 0.01b | 5.92 ± 0.09c | 6.07 ± 0.10d | |
| 28 | 7.08 ± 0.01a | 6.68 ± 0.07b | 6.39 ± 0.01c | 6.49 ± 0.01d | |
| Molds & yeasts (log CFU/g) | 0 | 5.80 ± 0.12a | 5.80 ± 0.12a | 5.80 ± 0.12a | 5.80 ± 0.12a |
| 7 | 6.22 ± 0.12a | 6.12 ± 0.08a | 6.00 ± 0.00a | 6.10 ± 0.11a | |
| 14 | 6.87 ± 0.00a | 6.40 ± 0.01b | 6.06 ± 0.09c | 6.25 ± 0.08d | |
| 21 | 7.31 ± 0.00a | 6.57 ± 0.05b | 6.22 ± 0.06c | 6.37 ± 0.09d | |
| 28 | 7.35 ± 0.03a | 6.84 ± 0.05b | 6.43 ± 0.05c | 6.59 ± 0.02d | |
| Moisture (%) | 0 | 51.90 ± 0.23a | 51.90 ± 0.23a | 51.90 ± 0.23a | 51.90 ± 0.23a |
| 7 | 45.06 ± 0.18d | 45.74 ± 0.13c | 48.00 ± 0.21a | 47.10 ± 0.17b | |
| 14 | 42.69 ± 0.20d | 43.60 ± 0.04c | 45.83 ± 0.10a | 44.65 ± 0.28b | |
| 21 | 39.67 ± 0.21d | 40.40 ± 0.29c | 43.17 ± 0.05a | 42.68 ± 0.10b | |
| 28 | 36.46 ± 0.15d | 37.52 ± 0.06c | 40.81 ± 0.15a | 39.58 ± 0.28b | |
| TBA (mg/kg) | 0 | 0.27 ± 0.00a | 0.27 ± 0.00a | 0.27 ± 0.00a | 0.27 ± 0.00a |
| 7 | 0.37 ± 0.01a | 0.36 ± 0.01a | 0.34 ± 0.01a | 0.35 ± 0.00a | |
| 14 | 0.43 ± 0.01b | 0.39 ± 0.00a | 0.38 ± 0.01a | 0.39 ± 0.01a | |
| 21 | 0.46 ± 0.00c | 0.43 ± 0.01b | 0.38 ± 0.01a | 0.42 ± 0.01b | |
| 28 | 0.47 ± 0.00b | 0.43 ± 0.01a | 0.40 ± 0.01a | 0.42 ± 0.01a | |
| pH | 0 | 5.47 ± 0.00a | 5.47 ± 0.00a | 5.47 ± 0.00a | 5.47 ± 0.00a |
| 7 | 5.30 ± 0.02c | 5.31 ± 0.01c | 5.40 ± 0.02a | 5.34 ± 0.00b | |
| 14 | 5.26 ± 0.02b | 5.29 ± 0.01b | 5.38 ± 0.01a | 5.33 ± 0.02b | |
| 21 | 5.22 ± 0.01a | 5.25 ± 0.01a | 5.28 ± 0.02a | 5.25 ± 0.01a | |
| 28 | 5.15 ± 0.00b | 5.18 ± 0.01b | 5.22 ± 0.01a | 5.20 ± 0.01a | |
| Titratable acidity (%) | 0 | 0.50 ± 0.06a | 0.50 ± 0.06a | 0.50 ± 0.06a | 0.50 ± 0.06a |
| 7 | 0.88 ± 0.01a | 0.80 ± 0.03a | 0.77 ± 0.02b | 0.77 ± 0.02b | |
| 14 | 0.99 ± 0.04a | 0.88 ± 0.02b | 0.78 ± 0.05c | 0.86 ± 0.00b | |
| 21 | 1.08 ± 0.03a | 0.91 ± 0.01b | 0.86 ± 0.07b | 0.90 ± 0.02b | |
| 28 | 1.31 ± 0.08a | 1.09 ± 0.07b | 0.96 ± 0.04b | 1.00 ± 0.04b | |
| L* | 0 | 93.11 ± 0.10a | 93.11 ± 0.10a | 93.11 ± 0.10a | 93.11 ± 0.10a |
| 7 | 83.04 ± 0.16c | 80.87 ± 0.35b | 81.99 ± 1.01a | 83.37 ± 1.88a | |
| 14 | 82.82 ± 0.80c | 80.29 ± 1.25b | 84.26 ± 1.29a | 82.95 ± 1.67b | |
| 21 | 77.18 ± 4.09b | 77.65 ± 3.92b | 82.23 ± 1.12a | 81.23 ± 0.68a | |
| 28 | 81.01 ± 0.59b | 74.97 ± 0.45c | 84.31 ± 0.00a | 81.21 ± 0.07b | |
| a* | 0 | −3.71 ± 0.10a | −3.71 ± 0.10a | −3.71 ± 0.10a | −3.71 ± 0.10a |
| 7 | −4.74 ± 0.26b | −5.04 ± 0.28a | −5.23 ± 0.15a | −5.09 ± 0.14a | |
| 14 | −5.18 ± 0.24b | −5.47 ± 0.15a | −5.39 ± 0.17a | −5.30 ± 0.16a | |
| 21 | −5.77 ± 0.10a | −5.79 ± 0.54a | −5.40 ± 0.41a | −5.77 ± 0.16a | |
| 28 | −5.79 ± 0.02d | −5.59 ± 0.13c | −5.03 ± 0.18a | −5.28 ± 0.16b | |
| b* | 0 | 13.41 ± 0.16a | 13.41 ± 0.16a | 13.41 ± 0.16a | 13.41 ± 0.16a |
| 7 | 15.26 ± 0.20d | 14.60 ± 0.38c | 16.17 ± 0.10a | 16.33 ± 0.46b | |
| 14 | 15.94 ± 0.66b | 16.73 ± 0.32b | 17.74 ± 0.30a | 17.04 ± 0.24a | |
| 21 | 15.56 ± 0.93b | 17.63 ± 0.37a | 17.20 ± 0.67a | 17.92 ± 0.41a | |
| 28 | 15.73 ± 0.16d | 18.48 ± 0.36c | 18.52 ± 0.00a | 19.78 ± 0.00b | |
| ∆E* | 0 | 9.78 ± 0.14a | 9.78 ± 0.14a | 9.78 ± 0.14a | 9.78 ± 0.14a |
| 7 | 12.74 ± 0.14a | 15.4 ± 1.31b | 14.51 ± 4.47b | 13.5 ± 0.69ab | |
| 14 | 13.82 ± 0.19a | 15.78 ± 0.86b | 13.97 ± 0.19a | 14.29 ± 0.96ab | |
| 21 | 20.66 ± 4.61a | 19.8 ± 3.09a | 15.88 ± 1.63a | 12.45 ± 5.59a | |
| 28 | 16.79 ± 3.64a | 20.98 ± 0.43a | 18.01 ± 3.18a | 22.16 ± 3.87a |
| Parameters | Time (day) | Control | 0.50% O-CMC | 1.50% O-CMC | 2.50% O-CMC |
|---|---|---|---|---|---|
| Hardness (N) | 0 | 129.29 ± 2.11a | 129.29 ± 2.11a | 129.29 ± 2.11a | 129.29 ± 2.11a |
| 7 | 113.14 ± 4.81d | 119.04 ± 5.53c | 126.51 ± 0.71a | 123.32 ± 2.39b | |
| 14 | 97.78 ± 3.79d | 107.04 ± 1.63c | 118.67 ± 1.98a | 114.23 ± 4.42b | |
| 21 | 96.18 ± 1.76d | 106.37 ± 2.83c | 113.10 ± 6.10a | 83.90 ± 6.95b | |
| 28 | 89.74 ± 1.66d | 101.59 ± 0.86c | 109.13 ± 3.69a | 102.80 ± 2.43b | |
| Cohesiveness | 0 | 0.35 ± 0.02a | 0.35 ± 0.02a | 0.35 ± 0.02a | 0.35 ± 0.02a |
| 7 | 0.42 ± 0.03a | 0.41 ± 0.03a | 0.41 ± 0.05a | 0.44 ± 0.09a | |
| 14 | 0.41 ± 0.04a | 0.42 ± 0.02a | 0.41 ± 0.03a | 0.42 ± 0.04a | |
| 21 | 0.42 ± 0.05a | 0.37 ± 0.09a | 0.38 ± 0.09a | 0.41 ± 0.06a | |
| 28 | 0.42 ± 0.04a | 0.42 ± 0.04a | 0.39 ± 0.04a | 0.43 ± 0.05a | |
| Springiness | 0 | 0.74 ± 0.04a | 0.74 ± 0.04a | 0.74 ± 0.04a | 0.74 ± 0.04a |
| 7 | 0.76 ± 0.04a | 0.75 ± 0.03a | 0.74 ± 0.02a | 0.76 ± 0.03a | |
| 14 | 0.74 ± 0.04a | 0.72 ± 0.03a | 0.74 ± 0.03a | 0.75 ± 0.03a | |
| 21 | 0.78 ± 0.06a | 0.74 ± 0.04a | 0.73 ± 0.05a | 0.75 ± 0.04a | |
| 28 | 0.77 ± 0.04a | 0.74 ± 0.05a | 0.73 ± 0.04a | 0.74 ± 0.05a | |
| Gumminess (N) | 0 | 45.00 ± 0.05a | 45.00 ± 0.05a | 45.00 ± 0.05a | 45.00 ± 0.05a |
| 7 | 47.28 ± 0.15d | 49.39 ± 0.16c | 51.92 ± 0.04b | 54.37 ± 0.22a | |
| 14 | 40.29 ± 0.16d | 44.72 ± 0.03c | 48.09 ± 0.06a | 47.56 ± 0.18b | |
| 21 | 40.12 ± 0.09b | 39.84 ± 0.27c | 42.74 ± 0.57a | 34.81 ± 0.41d | |
| 28 | 37.83 ± 0.06d | 42.62 ± 0.04c | 42.10 ± 0.16b | 43.69 ± 0.11a | |
| Chewiness (N) | 0 | 33.45 ± 0.00a | 33.45 ± 0.00a | 33.45 ± 0.00a | 33.45 ± 0.00a |
| 7 | 35.77 ± 0.01d | 36.91 ± 0.00c | 38.66 ± 0.00b | 41.07 ± 0.01a | |
| 14 | 29.92 ± 0.01d | 32.39 ± 0.00c | 35.34 ± 0.00b | 35.79 ± 0.00a | |
| 21 | 31.24 ± 0.01a | 29.50 ± 0.01b | 31.24 ± 0.03a | 26.24 ± 0.02c | |
| 28 | 29.15 ± 0.00d | 31.55 ± 0.00b | 30.67 ± 0.01c | 32.43 ± 0.01a |
| Fresh Cheese | Control | 0.5% O-CMC | 1.5% O-CMC | 2.5% O-CMC | |
|---|---|---|---|---|---|
| M21(%) | 11.54 ± 0.06a | 13.02 ± 0.69b | 13.29 ± 0.09b | 13.14 ± 0.18b | 13.45 ± 0.73b |
| M22(%) | 87.34 ± 0.85a | 75.36 ± 0.38d | 78.33 ± 1.56c | 80.93 ± 1.19b | 80.19 ± 1.42b |
| M23(%) | 0.73 ± 0.21d | 11.70 ± 0.41a | 8.38 ± 1.64b | 6.41 ± 0.46c | 6.90 ± 0.27c |
| T21(ms) | 0.66 ± 0.09a | 0.53 ± 0.08a | 0.53 ± 0.12a | 0.52 ± 0.04a | 0.58 ± 0.15a |
| T22(ms) | 18.74 ± 0.00a | 16.30 ± 0.00b | 17.41 ± 0.29c | 18.45 ± 0.24a | 18.15 ± 3.72a |
| T23(ms) | 267.34 ± 37.22a | 151.99 ± 0.00e | 172.69 ± 2.92c | 175.25 ± 9.96b | 163.48 ± 2.11d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zheng, Y.; Zhong, Y.; Zhou, R.; Li, B.; Ma, M. Preparation and Characterization of Novel Chitosan Coatings to Reduce Changes in Quality Attributes and Physiochemical and Water Characteristics of Mongolian Cheese during Cold Storage. Foods 2023, 12, 2731. https://doi.org/10.3390/foods12142731
Gao X, Zheng Y, Zhong Y, Zhou R, Li B, Ma M. Preparation and Characterization of Novel Chitosan Coatings to Reduce Changes in Quality Attributes and Physiochemical and Water Characteristics of Mongolian Cheese during Cold Storage. Foods. 2023; 12(14):2731. https://doi.org/10.3390/foods12142731
Chicago/Turabian StyleGao, Xin, Yuanrong Zheng, Yu Zhong, Ran Zhou, Bo Li, and Ming Ma. 2023. "Preparation and Characterization of Novel Chitosan Coatings to Reduce Changes in Quality Attributes and Physiochemical and Water Characteristics of Mongolian Cheese during Cold Storage" Foods 12, no. 14: 2731. https://doi.org/10.3390/foods12142731




