Bacteriophages for the Targeted Control of Foodborne Pathogens
Abstract
:1. Introduction
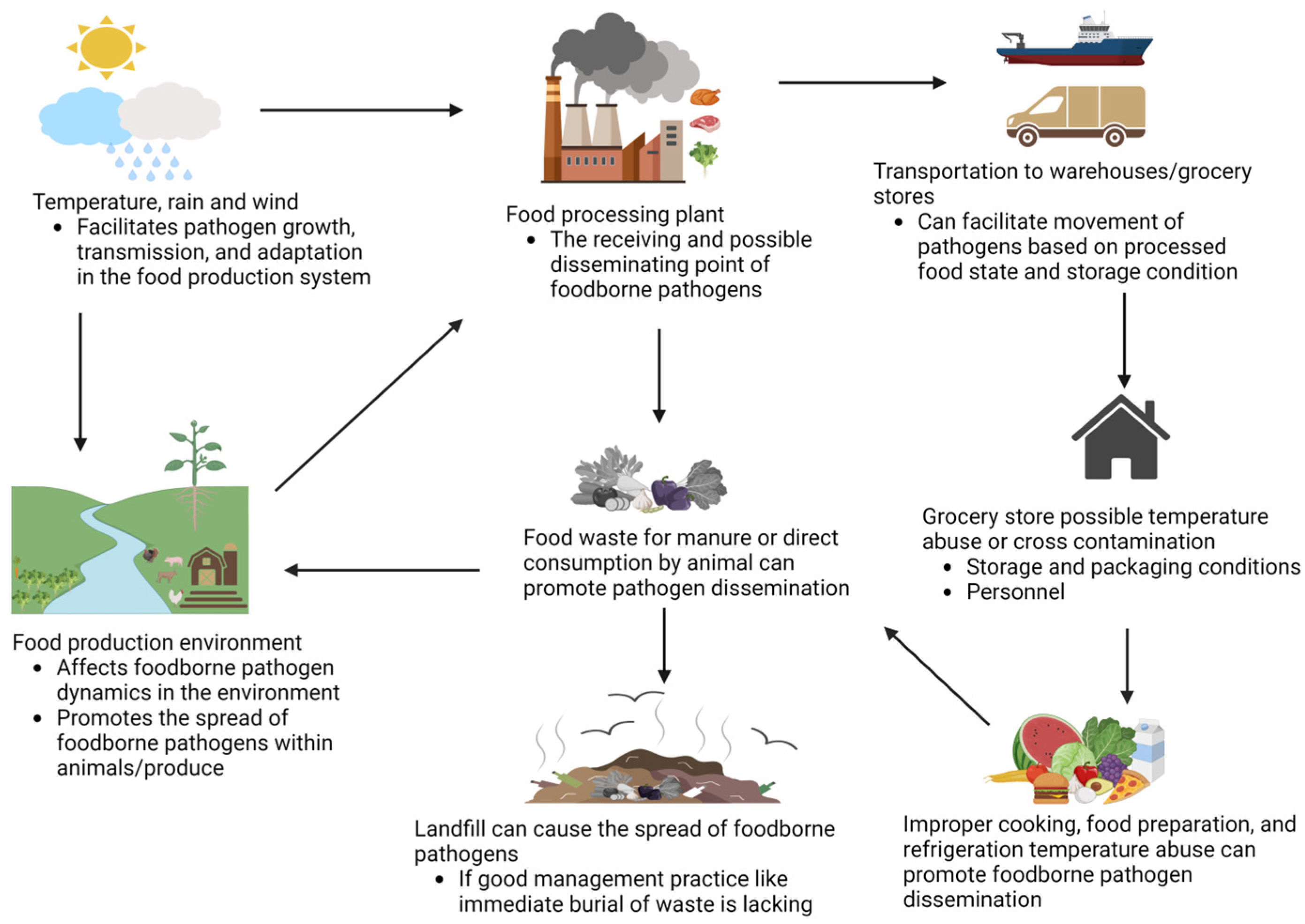
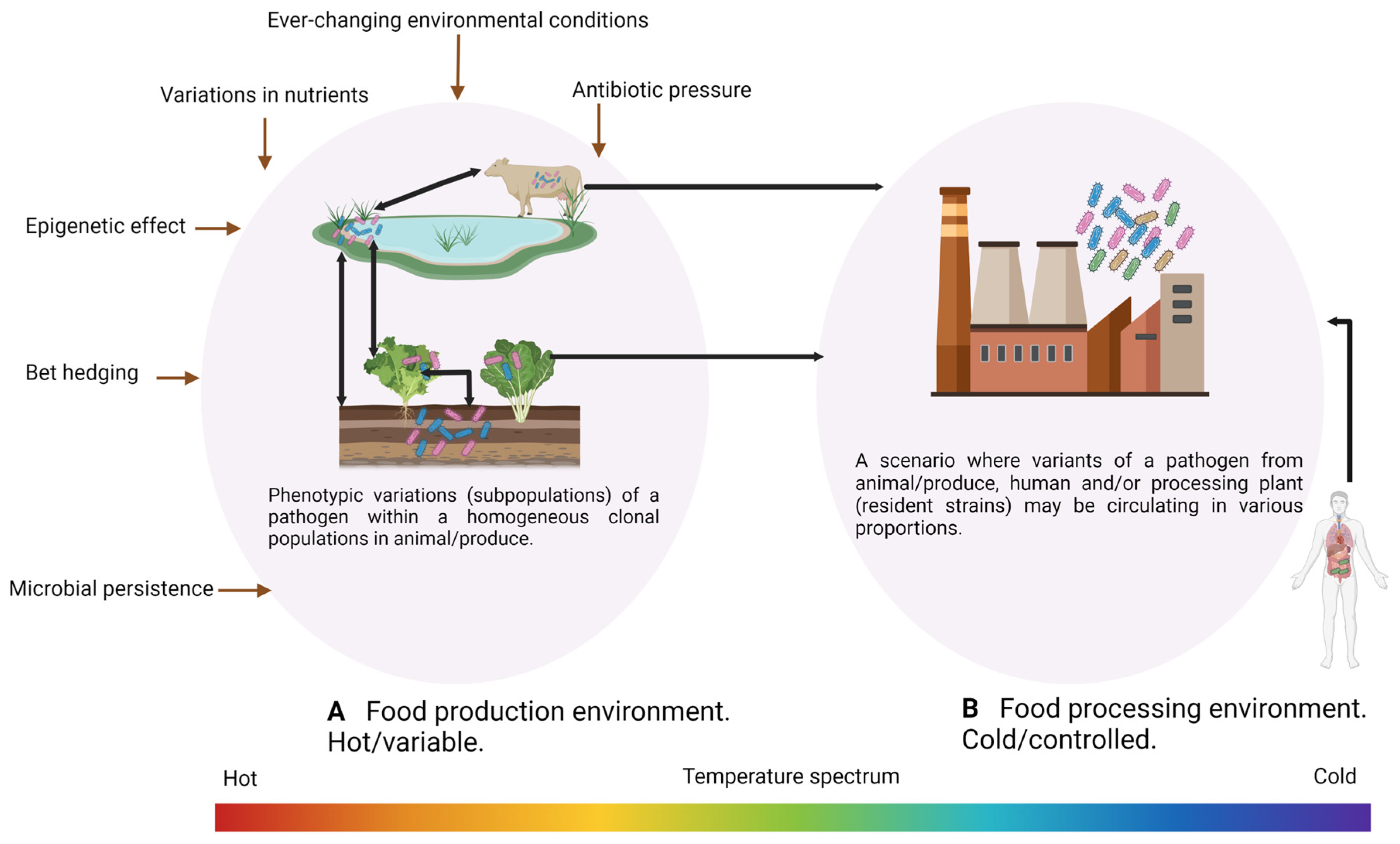
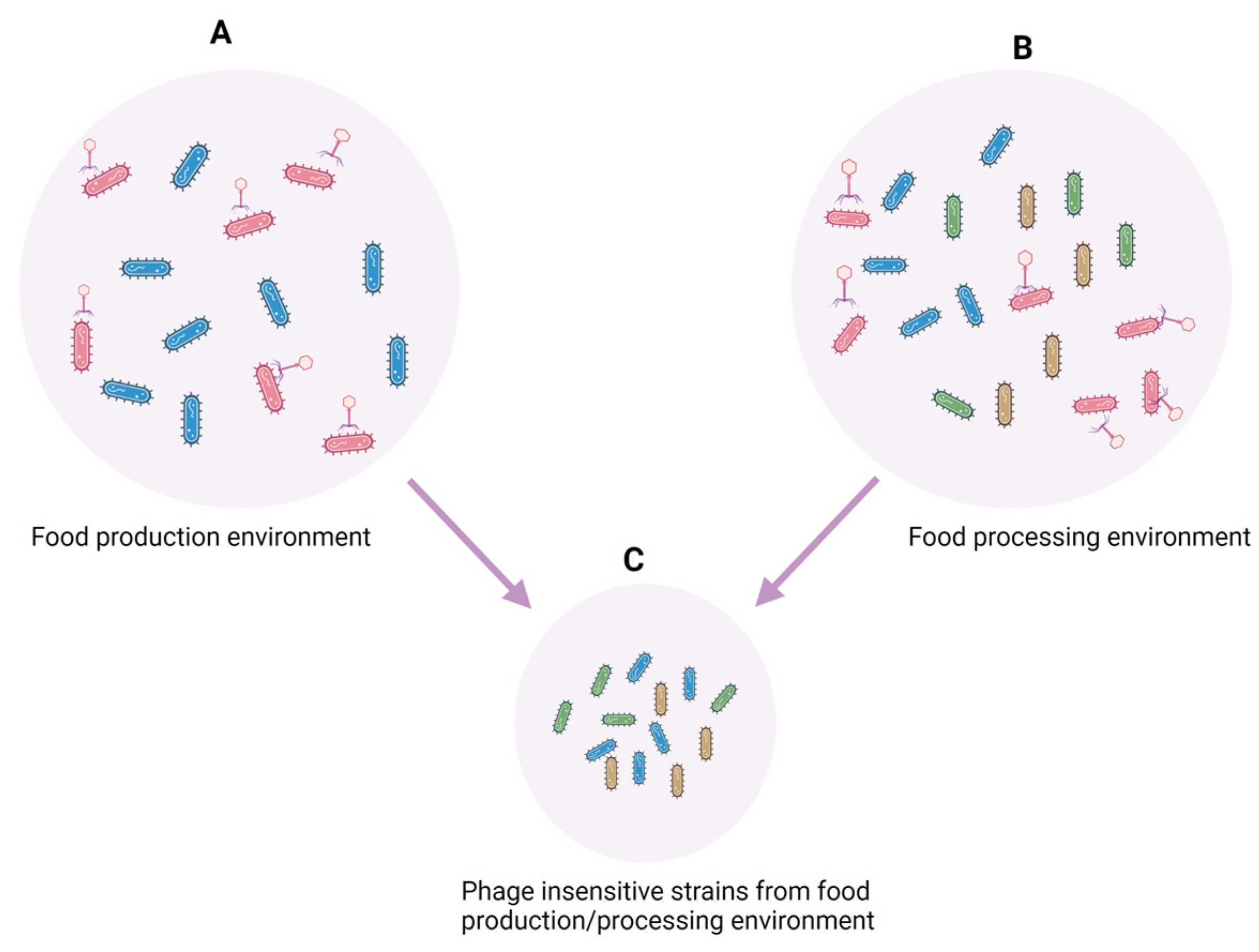
2. Factors Impacting Cross-Contamination of Pathogens Occurrence, Virulence, and Pathogenicity
3. Bacteriophages
4. Food-Production Animals, Leafy Green Produce, and Foodborne Pathogens
4.1. Application of Phages for Target Control of Foodborne Pathogens in Food-Production Animals
| Target Animal | Target Bacteria | Phage/Family | Phage/Mixture | Phage Dose | Phage Delivery Route | Settings | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|
| 24-day-old broiler chicken | C. jejuni | CP20 and CP30A/Myoviridae | Cocktail | 107 PFU/mL | Oral | In vivo controlled | A reduction of up to 2.4 log10 CFU/g 2 days post-treatment | [94] |
| 9-day-old broiler chicken | C. jejuni | NCTC12673, 12674, 12678, and 12672/Nd1 | Single and cocktail | 107 PFU/mL | Oral | In vivo controlled | A 2.8 log10 CFU/g reduction 21 days post-treatment | [109] |
| 36-day-old commercial broiler chicken | C. jejuni | NCTC12672, 12673, 12674, and 12678/Myoviridae | Cocktail | 7.2 and 7.9 PFU/mL | Oral | Commercial farm | A 3.2 log10 CFU/g reduction at slaughter | [101] |
| 25-day-old broiler chicken | C. jejuni | CP220/Myoviridae | Single | 107 and 109 PFU/mL | Oral | In vivo controlled | A 2.0 log10 CFU/g reduction 2 days post-treatment | [110] |
| Sheep | E. coli O157:H7 | CEV1 and CEV2/Siphoviridae | Single and cocktail | 1011 PFU/mL | Oral | In vivo controlled | Cocktail had 99.9% reduction compared to 99% in single 2 days post inoculation | [111] |
| One-day-old Ross broiler chicks | C. coli and C. jejuni | phiCcoIBB35, phiCcoIBB37 and phiCcoIBB12/Myoviridae | Cocktail | 106 or 107 PFU/mL | Oral | Commercial farm | A 2.0 log10 CFU/g reduction 2 days post-treatment | [112] |
| 16-month- and 8–9-year-old cattle | E. coli O157:H7 | e11/2 and e4/1c/Myoviridae | Cocktail | 1011 PFU/mL | Oral | In vivo controlled | No significant difference compared to control 2 days post inoculation | [113] |
| ≥1-year-old cattle (steer) | E. coli O157:H7 | rV5, wV7, wV8, and wV11/Nd1 | Cocktail | 1010 PFU/bolus and 1011 PFU/feed | Oral bolus or phage mixed in cattle feed | In vivo controlled | The duration of shedding was reduced by 14 days in bolus-fed steers as compared with control steers, but phage did not reduce E. coli O157:H7 shedding overall | [99] |
| Cattle before passing through the lairage | E. coli O157:H7 | Finalyse®/Nd1 | Cocktail | 1010 PFU/gallon of water | Sprayed on hide | Commercial farm | No significant reduction after 3 days of application compared to control | [103] |
| Cattle | E. coli O157:H7 | rV5, wV7, wV8, and wV11/Myoviridae | Cocktail | 1011 PFU/mL | Oral and rectal | In vivo controlled | No significant difference compared to control over 83 days post inoculation | [114] |
| Six-month-old Holstein steers | E. coli O157:H7 | KH1 and SH1/Nd1 | Cocktail | 1011 PFU/mL | Recto-anal junction | In vivo controlled | Reduction in the average number of E. coli O157:H7 among phage-treated steers compared to control steers | [115] |
| 38-day-old broiler chicken | S. enterica serotypes Enteritidis, Typhimurium, and Hadar | ϕ151/Myoviridae, ϕ10, and ϕ25/Siphoviridae | Single | 109 or 1011 PFU/mL | Oral | In vivo controlled | Phage ϕ151 had a 4.2 log10 CFU/g reduction 1 day post-treatment for both S. Enteritidis and Typhimurium. Phage ϕ10, a 2.19 log10 CFU/g reduction for S. Typhimurium. No reduction by ϕ25 on Hadar | [116] |
| 18-day-old commercial broiler chicken | Salmonella | SalmoFREE® (φ San15, φ San23, φ San24, and φ San25)/Myoviridae | Cocktail | 108 PFU/mL | Oral | In vivo controlled | 100% reduction on day 33 post-treatment compared to control | [102] |
| One-day-old broiler chicken | S. Typhimurium | Φst1/Siphoviridae | Single | 1010 or 1012 PFU/mL | Intracloacal | In vivo controlled | 100% reduction after 1 day post-treatment compared to control | [117] |
| 4-day-old broiler chicken | S. enterica serotype Enteritidis | CNPSA1, CNPSA3, and CNPSA4/Nd1 | Single | 1011 PFU/mL | Oral | In vivo controlled | A reduction of 3.5 orders of magnitude of CFU/g 5 days post treatment | [118] |
4.2. Application of Phages for Controlling Foodborne Pathogens in Leafy Green Vegetables at Pre-Harvest Level
5. Food-Processing Environment
| Environment or Surface Type | Target Bacteria | Phage/Family | Phage/Mixture | Phage Dose | Bacteria Dose | Mode of Application | Temperature Condition | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Ready-to-eat food manufacturing area (door frames/seals, floors/curbing, wheels/casters, walls/windows/curbing, drains, catch pan, water pipe, freezer doors/door seals, etc.) | L. monocytogenes | PhageGuard Listex™ | Cocktail | 107 and 108 PFU/mL | Not indicated | Spraying | 4 and 20 °C | Moderate application of 107 resulted in a 66% reduction in listeria prevalence at both 4 and 20 °C, whereas at concentration 108, a reduction of 43 and 32% was obtained at 4 and 20 °C, respectively. | [130] |
| Glass | C. jejuni (NCTC 11168 and PT14) | CP8 and CP30 | Single | 106 or 109 PFU/mL | 105 CFU/mL (initial cells for biofilm formation) | Spot inoculation | 37 °C | A 1 to 3 log10 CFU/cm2 reduction 24 h after phage treatment compared with control. | [142] |
| Stainless steel and polyurethane thermoplastic belting | Cocktail of L. monocytogenes and L. innocua | P100 | Cocktail | 107 and 108 PFU/cm2 | 104–105 CFU/cm2 | Spot inoculation | 4 and 20 °C | Overall, a reduction of 1.27–3.33 and 1.17–2.76 log10 CFU/cm2 on stainless steel and polyurethane thermoplastic belting, respectively, with a higher reduction at a high phage dilution of 108. | [143] |
| Spinach harvester blade | Cocktail of E. coli O157:H7 | Phages not specified | Cocktail | 108 PFU/mL | 105–106 CFU/mL | Spraying | 22 °C | Reduction in biofilm populations by 4.5 log10 CFU on blades after 2 h of phage treatment. | [144] |
| Stainless steel | L. monocytogenes (19CO9, 19DO3 and 19EO3) | LiMN4L, LiMN4p, and LiMN17 | Single or cocktail | 109 PFU/mL | 108 CFU/mL | Immersion | 15 °C | Single phages reduced biofilm cells by 3–4.5 log units and cocktail by 3.8–5.4 and log10 CFU/cm2. | [65] |
| Stainless steel, rubber, and MBEC biofilm devices | S. Enteritidis (ATCC13076) and S. Typhimurium (ATCC14028) | BP 1369 and BP 1370/Myoviridae and Podoviridae, respectively. | Single | 108 PFU/mL | Initial inoculum of 105 CFU/mL | Immersion | 10 and 30 °C | A reduction in biofilm cells by 3.0, 2.0, and 3.0 log CFU/cm2 on stainless steel, rubber, and an MBEC device. | [145] |
| Stainless steel chips, ceramic tile chips, and high-density polyethylene chips | Cocktail of O157:H7 (EK27, ATCC 43895, and 472) | BEC8 | Cocktail | 106 PFU/mL | 106, 105, and 104 CFU/chip | Spot inoculation | 4, 12, 23, and 37 °C | No biofilm survivors were detected (detection limit 10 CFU/chip) after 1 h of treatment at 12, 23, and 37 °C. | [146] |
| Polystyrene and stainless steel | S. Enteritidis | PVP-SE | Single | MOIs (0.1, 1, and 10) | 104 CFU/mL | Immersion | 4 and 22 °C | A 2–5 log10 CFU/cm2 reduction with a higher killing efficiency at room temperature. | [147] |
| Stainless steel | E. coli O113:H21 and O154:H10 | SA21RB | Single | 1013 PFU/mL | 106 and 105 CFU/mL, respectively | Immersion | 22 °C | A reduction in biofilm cells by 2.5 and 2.1 log10 CFU/cm2 for O113:H21 and O154:H10, respectively, for 24 h biofilm after 3 h of phage treatment. | [68] |
| Polystyrene microplate | S. Enteritidis | CW1, CW11, M4, and M10 | Cocktail | Not indicated | 102 CFU/mL for developing biofilm. Mature biofilm (48 h) number not indicated | Immersion | 37 °C | A reduction in cells in the developing biofilm and mature biofilm by 0.79 and 0.4 log10 CFU/cm2, respectively. | [148] |
6. Future Perspective and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Govender, N.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; Sekwadi, P.; Ntshoe, G. Outbreak of listeriosis in South Africa associated with processed meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef]
- Health Canada. Outbreak of E. coli Infections Linked to Romaine Lettuce. 2018. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2018/public-health-notice-outbreak-e-coli-infections-linked-romaine-lettuce.html (accessed on 1 April 2023).
- Xiang, Y.; Li, F.; Dong, N.; Tian, S.; Zhang, H.; Du, X.; Zhou, X.; Xu, X.; Yang, H.; Xie, J. Investigation of a salmonellosis outbreak caused by multidrug resistant Salmonella Typhimurium in China. Front. Microbiol. 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.; Inns, T.; Aird, H.; Swift, C.; Astbury, J.; Forester, E.; Decraene, V. Campylobacter outbreak associated with raw drinking milk, North West England, 2016. Epidemiol. Infect. 2020, 148, e13. [Google Scholar] [CrossRef] [Green Version]
- Acuff, G. Chemical decontamination strategies for meat. In Improving the Safety of Fresh Meat; Sofos, J.N., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 350–363. [Google Scholar]
- Anderson, N.M. Recent advances in low moisture food pasteurization. Curr. Opin. Food Sci. 2019, 29, 109–115. [Google Scholar] [CrossRef]
- FDA. Food additives permitted for direct addition to food for human consumption: Bacteriophage preparation. Fed. Regist. 2006, 71, 47729–47732. [Google Scholar]
- FDA. Intralytix Wins Regulatory Approval For Phage-Based Food Safety Product Effective Against Salmonella. 2013. Available online: http://www.intralytix.com (accessed on 17 April 2023).
- Alomari, M.M.M.; Dec, M.; Urban-Chmiel, R. Bacteriophages as an alternative method for control of zoonotic and foodborne pathogens. Viruses 2021, 13, 2348. [Google Scholar] [CrossRef]
- Matthews, L.; Low, J.; Gally, D.; Pearce, M.; Mellor, D.; Heesterbeek, J.; Chase-Topping, M.; Naylor, S.; Shaw, D.; Reid, S. Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proc. Natl. Acad. Sci. USA 2006, 103, 547–552. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Gould, L.H.; Mody, R.K.; Ong, K.L.; Clogher, P.; Cronquist, A.B.; Garman, K.N.; Lathrop, S.; Medus, C.; Spina, N.L.; Webb, T.H. Increased recognition of non-O157 Shiga toxin–producing Escherichia coli infections in the United States during 2000–2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 2013, 10, 453–460. [Google Scholar] [CrossRef]
- Desmarchelier, P.; Fegan, N. Enteropathogenic Escherichia coli. In Foodborne Microorganisms of Public Health Signiicance, 6th ed.; Hocking, A.D., Ed.; Australian Institute of Food Science and Technology (NSW Branch): Sydney, Australia, 2003; pp. 267–310. [Google Scholar]
- Kim, C.; Ndegwa, E. Influence of pH and temperature on growth characteristics of leading foodborne pathogens in a laboratory medium and select food beverages. Austin Food Sci. 2018, 3, 1031. [Google Scholar]
- Zhang, P.; Tran, F.; Stanford, K.; Yang, X. Are antimicrobial interventions associated with heat-resistant Escherichia coli on meat? Appl. Environ. Microbiol. 2020, 86, e00512–e00520. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bumunang, E.W.; Stanford, K.; Bie, X.; Niu, Y.D.; McAllister, T.A. Biofilm Formation by Shiga Toxin-Producing Escherichia coli on Stainless Steel Coupons as Affected by Temperature and Incubation Time. Microorganisms 2019, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Bumunang, E.; Ateba, C.; Stanford, K.; McAllister, T.A.; Niu, Y.D. Biofilm formation by South-African Shiga toxigenic non-O157 Escherichia coli on stainless steel coupons. Can. J. Microbiol. 2020, 66, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumunang, E.W.; McAllister, T.A.; King, R.; Ortega Polo, R.; Stanford, K.; Zaheer, R.; Niu, Y.D.; Ateba, C.N. Characterization of Non-O157 Escherichia coli from Cattle Faecal Samples in the North-West Province of South Africa. Microorganisms 2019, 7, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, J.M.; Peterkin, P. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Vivant, A.-L.; Garmyn, D.; Piveteau, P. Listeria monocytogenes, a down-to-earth pathogen. Front. Cell. Infect. Microbiol. 2013, 3, 87. [Google Scholar] [CrossRef] [Green Version]
- Sleator, R.D.; Watson, D.; Hill, C.; Gahan, C.G. The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology 2009, 155, 2463–2475. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Dygico, L.K.; Gahan, C.G.; Grogan, H.; Burgess, C.M. The ability of Listeria monocytogenes to form biofilm on surfaces relevant to the mushroom production environment. Int. J. Food Microbiol. 2020, 317, 108385. [Google Scholar] [CrossRef] [PubMed]
- Pouillot, R.; Klontz, K.C.; Chen, Y.; Burall, L.S.; Macarisin, D.; Doyle, M.; Bally, K.M.; Strain, E.; Datta, A.R.; Hammack, T.S. Infectious dose of Listeria monocytogenes in outbreak linked to ice cream, United States, 2015. Emerg. Infect. Dis. 2016, 22, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, R.L.; Gorris, L.G.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Goulet, V.; Hebert, M.; Hedberg, C.; Laurent, E.; Vaillant, V.; De Valk, H.; Desenclos, J.-C. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clin. Infect. Dis. 2012, 54, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [Green Version]
- Acheson, D.; Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Keener, K.; Bashor, M.; Curtis, P.; Sheldon, B.; Kathariou, S. Comprehensive review of Campylobacter and poultry processing. Compr. Rev. Food Sci. Food Saf. 2004, 3, 105–116. [Google Scholar] [CrossRef]
- Newell, D.; Fearnley, C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef] [Green Version]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Zhang, Q. Campylobacter in poultry: Ecology and potential interventions. Avian Dis. 2015, 59, 185–200. [Google Scholar] [CrossRef]
- Teh, A.H.T.; Lee, S.M.; Dykes, G.A. Does Campylobacter jejuni form biofilms in food-related environments? Appl. Environ. Microbiol. 2014, 80, 5154–5160. [Google Scholar] [CrossRef] [Green Version]
- Black, R.E.; Levine, M.M.; Clements, M.L.; Hughes, T.P.; Blaser, M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988, 157, 472–479. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.W.; Hall, H.K. Adaptive acidification tolerance response of Salmonella typhimurium. J. Bacteriol. 1990, 172, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, T. Public-health aspects of Salmonella infection. Salmonella Domest. Anim. 2000, 1, 245–263. [Google Scholar]
- Bonardi, S. Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol. Infect. 2017, 145, 1513–1526. [Google Scholar] [CrossRef] [Green Version]
- Besser, T.; Goldoft, M.; Pritchett, L.; Khakhria, R.; Hancock, D.; Rice, D.; Gay, J.; Johnson, W.; Gay, C. Multiresistant Salmonella Typhimurium DT104 infections of humans and domestic animals in the Pacific Northwest of the United States. Epidemiol. Infect. 2000, 124, 193–200. [Google Scholar] [CrossRef]
- Krishnasamy, V.P.; Marshall, K.; Dewey-Mattia, D.; Wise, M. Outbreak characteristics and epidemic curves for multistate outbreaks of Salmonella infections associated with produce: United States, 2009–2015. Foodborne Pathog. Dis. 2020, 17, 15–22. [Google Scholar] [CrossRef]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef]
- Niu, T.-X.; Wang, X.-N.; Wu, H.-Y.; Bi, J.-R.; Hao, H.-S.; Hou, H.-M.; Zhang, G.-L. Transcriptomic analysis, motility and biofilm formation characteristics of Salmonella Typhimurium exposed to benzyl isothiocyanate treatment. Int. J. Mol. Sci. 2020, 21, 1025. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Yang, Y.; Yuk, H. Biofilm formation of Salmonella Typhimurium on stainless steel and acrylic surfaces as affected by temperature and pH level. LWT-Food Sci. Technol. 2014, 55, 383–388. [Google Scholar] [CrossRef]
- Ma, Z.; Tang, X.; Stanford, K.; Chen, X.; McAllister, T.A.; Niu, Y.D. Single-and Dual-Species Biofilm Formation by Shiga Toxin-Producing Escherichia coli and Salmonella, and Their Susceptibility to an Engineered Peptide WK2. Microorganisms 2021, 9, 2510. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.F.; Kasuga, F.; Fazil, A.; Ogden, I.D.; Rotariu, O.; Strachan, N.J. Dose–response modeling of Salmonella using outbreak data. Int. J. Food Microbiol. 2010, 144, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Microbiol. 2015, 13, 497–508. [Google Scholar] [CrossRef]
- Abee, T.; Koomen, J.; Metselaar, K.; Zwietering, M.; Den Besten, H. Impact of pathogen population heterogeneity and stress-resistant variants on food safety. Annu. Rev. Food Sci. Technol. 2016, 7, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Weigel, W.; Dersch, P. Phenotypic heterogeneity: A bacterial virulence strategy. Microbes Infect. 2018, 20, 570–577. [Google Scholar] [CrossRef]
- Wakamoto, Y.; Dhar, N.; Chait, R.; Schneider, K.; Signorino-Gelo, F.; Leibler, S.; McKinney, J.D. Dynamic persistence of antibiotic-stressed mycobacteria. Science 2013, 339, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Arnoldini, M.; Vizcarra, I.A.; Peña-Miller, R.; Stocker, N.; Diard, M.; Vogel, V.; Beardmore, R.E.; Hardt, W.-D.; Ackermann, M. Bistable expression of virulence genes in Salmonella leads to the formation of an antibiotic-tolerant subpopulation. PLoS Biol. 2014, 12, e1001928. [Google Scholar] [CrossRef]
- Dubnau, D.; Losick, R. Bistability in bacteria. Mol. Microbiol. 2006, 61, 564–572. [Google Scholar] [CrossRef]
- Veening, J.-W.; Smits, W.K.; Kuipers, O.P. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 2008, 62, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.; Ward, S.; Hyman, P. More is better: Selecting for broad host range bacteriophages. Front Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.R.; Dobias, D.T.; Medina, S.J.; Servilio, L.; Gupta, A.; Lenski, R.E. Ecological speciation of bacteriophage lambda in allopatry and sympatry. Science 2016, 354, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.; Dutilh, B.E. Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Suárez, V.; Quiberoni, A.; Binetti, A.; Reinheimer, J. Thermophilic lactic acid bacteria phages isolated from Argentinian dairy industries. J. Food Prot. 2002, 65, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, M.K.; Maurice, C.F. Ménage à trois in the human gut: Interactions between host, bacteria and phages. Nat. Rev. Microbiol. 2017, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, M.; Rastogi, S.; Singh, D.; Shukla, A.; Dhameja, N.; Kumar, D.; Kumar, R.; Nath, G. Study on the effect of oral administration of bacteriophages in charles foster rats with special reference to immunological and adverse effects. Front. Pharmacol. 2021, 12, 615445. [Google Scholar] [CrossRef]
- Hayashi, T.; Makino, K.; Ohnishi, M.; Kurokawa, K.; Ishii, K.; Yokoyama, K.; Han, C.-G.; Ohtsubo, E.; Nakayama, K.; Murata, T. Complete genome sequence of enterohemorrhagic Eschelichia coli O157: H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001, 8, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Mauro, S.A.; Koudelka, G.B. Shiga toxin: Expression, distribution, and its role in the environment. Toxins 2011, 3, 608–625. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Gill, P.; Hassan, Z.; Jahromi, S.R.M.; Roohvand, F. Recombinant λ-phage nanobioparticles for tumor therapy in mice models. Genet. Vaccines Ther. 2010, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.R.; Bartley, K.; Jepson, C.D.; Craik, V.; March, J.B. Comparison of a bacteriophage-delivered DNA vaccine and a commercially available recombinant protein vaccine against hepatitis B. FEMS Immunol. Med. Microbiol. 2011, 61, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Arachchi, G.J.G.; Cridge, A.G.; Dias-Wanigasekera, B.M.; Cruz, C.D.; McIntyre, L.; Liu, R.; Flint, S.H.; Mutukumira, A.N. Effectiveness of phages in the decontamination of Listeria monocytogenes adhered to clean stainless steel, stainless steel coated with fish protein, and as a biofilm. J. Ind. Microbiol. Biotechnol. 2013, 40, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.; Purushothaman, P.; Gupta, N.; Ragnone, M.; Verma, S.; De Mello, A. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017, 127, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zampara, A.; Sørensen, M.C.H.; Elsser-Gravesen, A.; Brøndsted, L. Significance of phage-host interactions for biocontrol of Campylobacter jejuni in food. Food Control 2017, 73, 1169–1175. [Google Scholar] [CrossRef]
- Bumunang, E.W.; Ateba, C.N.; Stanford, K.; Niu, Y.D.; Wang, Y.; McAllister, T.A. Activity of bacteriophage and complex tannins against biofilm-forming shiga toxin-producing Escherichia coli from Canada and South Africa. Antibiotics 2020, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.M.; Kuhl, S.J.; Abedon, S.T. Re-establishing a place for phage therapy in western medicine. Future Microbiol. 2015, 10, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues. Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current state of compassionate phage therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Sacher, J.C.; Zheng, J. Phage therapy collaboration and compassionate use. In Bacteriophages: Biology, Technology, Therapy; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M., Eds.; Springer Nature Switzerland AG: New York, NY, USA, 2021; pp. 1069–1098. [Google Scholar]
- Niu, Y.D.; McAllister, T.A.; Nash, J.H.; Kropinski, A.M.; Stanford, K. Four Escherichia coli O157: H7 phages: A new bacteriophage genus and taxonomic classification of T1-like phages. PLoS ONE 2014, 9, e100426. [Google Scholar] [CrossRef] [Green Version]
- Korf, I.H.; Meier-Kolthoff, J.P.; Adriaenssens, E.M.; Kropinski, A.M.; Nimtz, M.; Rohde, M.; van Raaij, M.J.; Wittmann, J. Still Something to Discover: Novel Insights into Escherichia coli Phage Diversity and Taxonomy. Viruses 2019, 11, 454. [Google Scholar] [CrossRef] [Green Version]
- Bumunang, E.W.; McAllister, T.A.; Polo, R.O.; Ateba, C.N.; Stanford, K.; Schlechte, J.; Walker, M.; MacLean, K.; Niu, Y.D. Genomic Profiling of Non-O157 Shiga Toxigenic Escherichia coli-Infecting Bacteriophages from South Africa. PHAGE Ther. Appl. Res. 2022, 3, 221–230. [Google Scholar] [CrossRef]
- Iriarte, F.; Balogh, B.; Momol, M.; Smith, L.; Wilson, M.; Jones, J. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl. Environ. Microbiol. 2007, 73, 1704–1711. [Google Scholar] [CrossRef] [Green Version]
- Sinton, L.W.; Hall, C.H.; Lynch, P.A.; Davies-Colley, R.J. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 2002, 68, 1122–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.J.; Costa, L.; Pereira, C.; Cunha, Â.; Calado, R.; Gomes, N.C.; Almeida, A. Influence of environmental variables in the efficiency of phage therapy in aquaculture. Microb. Biotechnol. 2014, 7, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Branston, S.D.; Stanley, E.C.; Ward, J.M.; Keshavarz-Moore, E. Determination of the survival of bacteriophage M13 from chemical and physical challenges to assist in its sustainable bioprocessing. Biotechnol. Bioprocess Eng. 2013, 18, 560–566. [Google Scholar] [CrossRef]
- Yamaki, S.; Omachi, T.; Kawai, Y.; Yamazaki, K. Characterization of a novel Morganella morganii bacteriophage FSP1 isolated from river water. FEMS Microbiol. Lett. 2014, 359, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Jepson, C.D.; March, J.B. Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine 2004, 22, 2413–2419. [Google Scholar] [CrossRef]
- Cui, Z.; Feng, T.; Gu, F.; Li, Q.; Dong, K.; Zhang, Y.; Zhu, Y.; Han, L.; Qin, J.; Guo, X. Characterization and complete genome of the virulent Myoviridae phage JD007 active against a variety of Staphylococcus aureus isolates from different hospitals in Shanghai, China. Virol. J. 2017, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Breitbart, M.; Wegley, L.; Leeds, S.; Schoenfeld, T.; Rohwer, F. Phage community dynamics in hot springs. Appl. Environ. Microbiol. 2004, 70, 1633–1640. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, H.-W.; Tremblay, D.; Moineau, S. Long-term bacteriophage preservation. WFCC Newsl. 2004, 38, 35–40. [Google Scholar]
- Fister, S.; Stessl, B.; Fuchs, S.; Schoder, D.; Wagner, M.; Rossmanith, P. Screening and characterisation of bacteriophage P100 insensitive Listeria monocytogenes isolates in Austrian dairy plants. Food Control 2016, 59, 108–117. [Google Scholar] [CrossRef]
- Metselaar, K.I.; den Besten, H.M.; Abee, T.; Moezelaar, R.; Zwietering, M.H. Isolation and quantification of highly acid resistant variants of Listeria monocytogenes. Int. J. Food Microbiol. 2013, 166, 508–514. [Google Scholar] [CrossRef]
- Hatchette, T.F.; Farina, D. Infectious diarrhea: When to test and when to treat. Can. Med Assoc. J. 2011, 183, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuPont, H.L. Persistent diarrhea: A clinical review. JAMA 2016, 315, 2712–2723. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.; Garton, N.J.; Stapley, A.G.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.D.; Liu, H.; Du, H.; Meng, R.; Sayed Mahmoud, E.; Wang, G.; McAllister, T.A.; Stanford, K. Efficacy of individual bacteriophages does not predict efficacy of bacteriophage cocktails for control of Escherichia coli O157. Front. Microbiol. 2021, 12, 616712. [Google Scholar] [CrossRef]
- Abedon, S.T.; Herschler, T.D.; Stopar, D. Bacteriophage latent-period evolution as a response to resource availability. Appl. Environ. Microbiol. 2001, 67, 4233–4241. [Google Scholar] [CrossRef] [Green Version]
- Newell, D.; Elvers, K.; Dopfer, D.; Hansson, I.; Jones, P.; James, S.; Gittins, J.; Stern, N.; Davies, R.; Connerton, I. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 2011, 77, 8605–8614. [Google Scholar] [CrossRef] [Green Version]
- Richards, P.J.; Connerton, P.L.; Connerton, I.F. Phage biocontrol of Campylobacter jejuni in chickens does not produce collateral effects on the gut microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Chen, S.; Sanderson, M.; Lanzas, C. Investigating effects of between-and within-host variability on Escherichia coli O157 shedding pattern and transmission. Prev. Vet. Med. 2013, 109, 47–57. [Google Scholar] [CrossRef]
- Williams, K.; Ward, M.; Dhungyel, O.; Hall, E.; Van Breda, L. A longitudinal study of the prevalence and super-shedding of Escherichia coli O157 in dairy heifers. Vet. Microbiol. 2014, 173, 101–109. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M.B.; Shaw, K.M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. Microbiology 1987, 133, 1127–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, S.W.; Low, J.C.; Besser, T.E.; Mahajan, A.; Gunn, G.J.; Pearce, M.C.; McKendrick, I.J.; Smith, D.G.; Gally, D.L. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157: H7 in the bovine host. Infect. Immun. 2003, 71, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.; McAllister, T.; Niu, Y.; Stephens, T.; Mazzocco, A.; Waddell, T.; Johnson, R. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157: H7 in feedlot cattle. J. Food Prot. 2010, 73, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, H.; Zou, G.; Song, Z.; Zhou, Y.; Li, H.; Tan, C.; Chen, H.; Fischetti, V.A.; Li, J. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Control. Release 2023, 353, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Fischer, S.; Abdulmawjood, A.; Glünder, G.; Klein, G. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 2013, 79, 7525–7533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef]
- Arthur, T.M.; Kalchayanand, N.; Agga, G.E.; Wheeler, T.L.; Koohmaraie, M. Evaluation of bacteriophage application to cattle in lairage at beef processing plants to reduce Escherichia coli O157: H7 prevalence on hides and carcasses. Foodborne Pathog. Dis. 2017, 14, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Grant, A.Q.; Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017, 96, 2845–2852. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Eriksen, R.S.; Svenningsen, S.L.; Sneppen, K.; Mitarai, N. A growing microcolony can survive and support persistent propagation of virulent phages. Proc. Natl. Acad. Sci. USA 2018, 115, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigent, M.; Leroy, M.; Confalonieri, F.; Dutertre, M.; DuBow, M.S. A diversity of bacteriophage forms and genomes can be isolated from the surface sands of the Sahara Desert. Extremophiles 2005, 9, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.; Schukken, Y.; Nightingale, C.; Fortes, E.; Ho, A.; Her, Z.; Grohn, Y.; McDonough, P.; Wiedmann, M. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 2004, 70, 4458–4467. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Kittler, S.; Klein, G.; Glünder, G. Impact of a single phage and a phage cocktail application in broilers on reduction of Campylobacter jejuni and development of resistance. PLoS ONE 2013, 8, e78543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shibiny, A.; Scott, A.; Timms, A.; Metawea, Y.; Connerton, P.; Connerton, I. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 2009, 72, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Raya, R.R.; Oot, R.A.; Moore-Maley, B.; Wieland, S.; Callaway, T.R.; Kutter, E.M.; Brabban, A.D. Naturally resident and exogenously applied T4-like and T5-like bacteriophages can reduce Escherichia coli O157: H7 levels in sheep guts. Bacteriophage 2011, 1, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, C.M.; Gannon, B.W.; Halfhide, D.E.; Santos, S.B.; Hayes, C.M.; Roe, J.M.; Azeredo, J. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 2010, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Rivas, L.; Coffey, B.; McAuliffe, O.; McDonnell, M.J.; Burgess, C.M.; Coffey, A.; Ross, R.P.; Duffy, G. In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157: H7. Appl. Environ. Microbiol. 2010, 76, 7210–7216. [Google Scholar] [CrossRef] [Green Version]
- Rozema, E.A.; Stephens, T.P.; Bach, S.J.; Okine, E.K.; Johnson, R.P.; Stanford, K.; McAllister, T.A. Oral and rectal administration of bacteriophages for control of Escherichia coli O157: H7 in feedlot cattle. J. Food Prot. 2009, 72, 241–250. [Google Scholar] [CrossRef]
- Sheng, H.; Knecht, H.J.; Kudva, I.T.; Hovde, C.J. Application of bacteriophages to control intestinal Escherichia coli O157: H7 levels in ruminants. Appl. Environ. Microbiol. 2006, 72, 5359–5366. [Google Scholar] [CrossRef] [Green Version]
- Atterbury, R.J.; Van Bergen, M.; Ortiz, F.; Lovell, M.; Harris, J.; De Boer, A.; Wagenaar, J.; Allen, V.; Barrow, P. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.L.; Sieo, C.C.; Tan, W.S.; Abdullah, N.; Hair-Bejo, M.; Abu, J.; Ho, Y.W. Evaluation of a lytic bacteriophage, Φ st1, for biocontrol of Salmonella enterica serovar Typhimurium in chickens. Int. J. Food Microbiol. 2014, 172, 92–101. [Google Scholar] [CrossRef]
- Fiorentin, L.; Vieira, N.D.; Barioni, W., Jr. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 2005, 34, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Lindén, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E. The hurdle approach–A holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy green vegetables. A review. Front Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef] [Green Version]
- Heiman, K.E.; Mody, R.K.; Johnson, S.D.; Griffin, P.M.; Gould, L.H. Escherichia coli O157 outbreaks in the United States, 2003–2012. Emerg. Infect. Dis. 2015, 21, 1293. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Sodha, S.; Ayers, T.; Lynch, M.; Gould, L.; Tauxe, R. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Vestrheim, D.; Lange, H.; Nygård, K.; Borgen, K.; Wester, A.; Kvarme, M.; Vold, L. Are ready-to-eat salads ready to eat? An outbreak of Salmonella Coeln linked to imported, mixed, pre-washed and bagged salad, Norway, November 2013. Epidemiol. Infect. 2016, 144, 1756–1760. [Google Scholar] [CrossRef] [Green Version]
- Self, J.L.; Conrad, A.; Stroika, S.; Jackson, A.; Burnworth, L.; Beal, J.; Wellman, A.; Jackson, K.A.; Bidol, S.; Gerhardt, T. Outbreak of listeriosis associated with consumption of packaged salad—United States and Canada, 2015–2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 879–881. [Google Scholar] [CrossRef] [Green Version]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Gardner, T.J.; Fitzgerald, C.; Xavier, C.; Klein, R.; Pruckler, J.; Stroika, S.; McLaughlin, J.B. Outbreak of campylobacteriosis associated with consumption of raw peas. Clin. Infect. Dis. 2011, 53, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hofstra, N.; Franz, E. Impacts of climate change on the microbial safety of pre-harvest leafy green vegetables as indicated by Escherichia coli O157 and Salmonella spp. Int. J. Food Microbiol. 2013, 163, 119–128. [Google Scholar] [CrossRef]
- Garcia, B.C.B.; Dimasupil, M.A.Z.; Vital, P.G.; Widmer, K.W.; Rivera, W.L. Fecal contamination in irrigation water and microbial quality of vegetable primary production in urban farms of Metro Manila, Philippines. J. Environ. Sci. Health Part B 2015, 50, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.; Bach, S.; Dinu, L.-D. Behavior of Escherichia coli O157: H7 in leafy vegetables. J. Food Prot. 2007, 70, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, H.; Nielsen, N.L.; Sommer, H.M.; Nørrung, B.; Christensen, B.B. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 2003, 83, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, R.G.; Kalinowski, R.M.; Bodnaruk, P.W.; Eifert, J.D.; Boyer, R.R.; Duncan, S.E.; Bailey, R.H. Practical application of bacteriophage in food manufacturing facilities for the control of Listeria sp. J. Food Saf. 2020, 43, e12871. [Google Scholar] [CrossRef]
- Brown, K.; Wray, S. Control of airborne contamination in food processing. In Hygiene in Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 174–202. [Google Scholar]
- Fauvart, M.; De Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef]
- Raghupathi, P.K.; Liu, W.; Sabbe, K.; Houf, K.; Burmølle, M.; Sørensen, S.J. Synergistic interactions within a multispecies biofilm enhance individual species protection against grazing by a pelagic protozoan. Front. Microbiol. 2018, 8, 2649. [Google Scholar] [CrossRef] [Green Version]
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003, 48, 1511–1524. [Google Scholar] [CrossRef]
- Wang, R.; Bono, J.L.; Kalchayanand, N.; Shackelford, S.; Harhay, D.M. Biofilm formation by Shiga toxin–producing Escherichia coli O157: H7 and Non-O157 strains and their tolerance to sanitizers commonly used in the food processing environment. J. Food Prot. 2012, 75, 1418–1428. [Google Scholar] [CrossRef] [Green Version]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [Green Version]
- Carson, L.; Gorman, S.P.; Gilmore, B.F. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med. Microbiol. 2010, 59, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the pre-neck appendage protein (Dpo7) from phage vB_SepiS-phiIPLA7 as an anti-biofilm agent in Staphylococcal species. Front. Microbiol. 2015, 6, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, I.-N.; Dykhuizen, D.E.; Slobodkin, L.B. The evolution of phage lysis timing. Evol. Ecol. 1996, 10, 545–558. [Google Scholar] [CrossRef]
- Harper, D.R.; Parracho, H.M.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; Sutherland, I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 2008, 9, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Siringan, P.; Connerton, P.L.; Payne, R.J.; Connerton, I.F. Bacteriophage-mediated dispersal of Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 2011, 77, 3320–3326. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, R.G.; Kalinowski, R.M.; Bodnaruk, P.W.; Eifert, J.D.; Boyer, R.R.; Duncan, S.E.; Bailey, R.H. Fate of Listeria on various food contact and noncontact surfaces when treated with bacteriophage. J. Food Saf. 2020, 40, e12775. [Google Scholar] [CrossRef]
- Patel, J.; Sharma, M.; Millner, P.; Calaway, T.; Singh, M. Inactivation of Escherichia coli O157: H7 attached to spinach harvester blade using bacteriophage. Foodborne Pathog. Dis. 2011, 8, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Sadekuzzaman, M.; Mizan, M.F.R.; Yang, S.; Kim, H.-S.; Ha, S.-D. Application of bacteriophages for the inactivation of Salmonella spp. in biofilms. Food Sci. Technol. Int. 2018, 24, 424–433. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of Escherichia coli O157: H7 viability on hard surfaces by treatment with a bacteriophage mixture. Int. J. Food Microbiol. 2011, 145, 37–42. [Google Scholar] [CrossRef]
- Milho, C.; Silva, M.D.; Melo, L.; Santos, S.; Azeredo, J.; Sillankorva, S. Control of Salmonella Enteritidis on food contact surfaces with bacteriophage PVP-SE2. Biofouling 2018, 34, 753–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, Z.; Zhang, L.; Cai, L.; Xu, X.; Chen, Y.; Wang, H. Biofilm removal mediated by Salmonella phages from chicken-related sources. Food Sci. Hum. Wellness 2023, 12, 1799–1808. [Google Scholar] [CrossRef]
- Ding, Y.; Nan, Y.; Qiu, Y.; Niu, D.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. Use of a phage cocktail to reduce the numbers of seven Escherichia coli strains belonging to different STEC serogroups applied to fresh produce and seeds. J. Food Saf. 2023; early view. [Google Scholar] [CrossRef]
- Sillankorva, S.; Neubauer, P.; Azeredo, J. Pseudomonas fluorescens biofilms subjected to phage phiIBB-PF7A. BMC Biotechnol. 2008, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Jassim, S.; Abdulamir, A.; Abu Bakar, F. Novel phage-based bio-processing of pathogenic Escherichia coli and its biofilms. World J. Microbiol. Biotechnol. 2012, 28, 47–60. [Google Scholar] [CrossRef]
- Hong, Y.; Schmidt, K.; Marks, D.; Hatter, S.; Marshall, A.; Albino, L.; Ebner, P. Treatment of Salmonella-contaminated eggs and pork with a broad-spectrum, single bacteriophage: Assessment of efficacy and resistance development. Foodborne Pathog. Dis. 2016, 13, 679–688. [Google Scholar] [CrossRef]
- Abhisingha, M.; Dumnil, J.; Pitaksutheepong, C. Efficiency of phage cocktail to reduce Salmonella Typhimurium on chicken meat during low temperature storage. LWT-Food Sci. Technol. 2020, 129, 109580. [Google Scholar] [CrossRef]
- Witte, S.; Huijboom, L.; Klamert, S.; van de Straat, L.; Hagens, S.; Fieseler, L.; de Vegt, B.T.; van Mierlo, J.T. Application of bacteriophages EP75 and EP335 efficiently reduces viable cell counts of Escherichia coli O157 on beef and vegetables. Food Microbiol. 2022, 104, 103978. [Google Scholar] [CrossRef] [PubMed]
- Gencay, Y.E.; Ayaz, N.D.; Copuroglu, G.; Erol, I. Biocontrol of Shiga Toxigenic Escherichia coli O157: H7 in Turkish raw meatball by bacteriophage. J. Food Saf. 2016, 36, 120–131. [Google Scholar] [CrossRef]
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157: H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Duc, H.M.; Son, H.M.; Yi, H.P.S.; Sato, J.; Ngan, P.H.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157: H7 in different food matrices. Food Res. Int. 2020, 131, 108977. [Google Scholar] [CrossRef]
- Shebs-Maurine, E.; Giotto, F.; Laidler, S.; de Mello, A. Effects of bacteriophages and peroxyacetic acid applications on beef contaminated with Salmonella during different grinding stages. Meat Sci. 2021, 173, 108407. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; ur Rahman, H.U. Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. LWT-Food Sci. Technol. 2020, 132, 109784. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, M.; Wang, Y.; Yang, Z.; Ye, M.; Wu, L.; Bao, H.; Pang, M.; Zhou, Y.; Wang, R. Broad host range phage vB-LmoM-SH3-3 reduces the risk of Listeria contamination in two types of ready-to-eat food. Food Control 2020, 108, 106830. [Google Scholar] [CrossRef]
- Yang, S.; Sadekuzzaman, M.; Ha, S.-D. Reduction of Listeria monocytogenes on chicken breasts by combined treatment with UV-C light and bacteriophage ListShield. LWT-Food Sci. Technol. 2017, 86, 193–200. [Google Scholar] [CrossRef]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX™ P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Orquera, S.; Gölz, G.; Hertwig, S.; Hammerl, J.; Sparborth, D.; Joldic, A.; Alter, T. Control of Campylobacter spp. and Yersinia enterocolitica by virulent bacteriophages. J. Mol. Genet. Med. 2012, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Bigwood, T.; Hudson, J.; Billington, C.; Carey-Smith, G.; Heinemann, J. Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiol. 2008, 25, 400–406. [Google Scholar] [CrossRef]
- Firlieyanti, A.S.; Connerton, P.L.; Connerton, I.F. Campylobacters and their bacteriophages from chicken liver: The prospect for phage biocontrol. Int. J. Food Microbiol. 2016, 237, 121–127. [Google Scholar] [CrossRef]
- Lu, Y.T.; Ma, Y.; Wong, C.W.; Wang, S. Characterization and application of bacteriophages for the biocontrol of Shiga-toxin producing Escherichia coli in Romaine lettuce. Food Control 2022, 140, 109109. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barceló, C. Phage therapy faces evolutionary challenges. Viruses 2018, 10, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Food Type | Target Bacteria | Phage/Family | Phage/Mixture | Phage Dose | Bacteria Dose | Mode of Application | Temperature Condition | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Beef | E. coli O157 | EP75 and EP335 | Cocktail | 107 or 108 PFU/cm2 | 105 CFU/cm2 | Spot inoculation | 4 °C | Reductions of 0.8–1.1 log10 CFU/cm2 and 0.9–1.3 log10 CFU/cm2, respectively. | [154] |
| Raw meatball | E. coli O157:H7 | M8AEC16 | Single | 1010 PFU/mL | 102, 104 and 106 CFU/g | Immersion | 4 °C | A reduction of 0.69–2.09 log10 CFU/g after 5 h of application. | [155] |
| Beef and lettuce | E. coli O157:H7 | EcoShield™ | Cocktail | 109 PFU/mL | 103 CFU/g | Spray | 4 °C | Reduced the level of bacteria by ≥94% and 87% after 5 min contact time in meet and lettuce, respectively. | [156] |
| Beef | E. coli O157:H7 | PS5/Myoviridae | Single | 1010 PFU/mL | 107 CFU/mL | Spot inoculation | 4 and 24 °C | A 2.4 log10 CFU/piece after 24 h post application at 4 °C, whereas a 3.5 log10 CFU/piece after 6 h post application at 24 °C. | [157] |
| Chicken | S. Typhimurium | PS5/Myoviridae | Single | 1010 PFU/mL | 107 CFU/mL | Spot inoculation | 4 and 24 °C | A 1.2 log10 CFU/piece after 24 h post application at 4 °C and a 1.6 log10 CFU/piece after 6 h post application at 24 °C. | [157] |
| Beef (coarse and fine ground) | S. enterica (ATCC 51741), S. Heidelberg (ATCC 8326), S. Newport (ATCC 27869), and S. Enteritidis C (Se 13) | Salmonelex™ (S16 and the FO1a)/Myoviridae | Cocktail | 108 and 109 | 104 CFU/g | Spot inoculation | 5 °C | Overall, a reduction of 1.6 log10 CFU/g was observed after the application of 109 phage. | [158] |
| Ground red meat trim and poultry | S. Infantis (ATCC 51741), S. Heidelberg (ATCC 8326), S. Newport (ATCC 27869), and S. Enteritidis (SE13) | Salmonelex™ (S16 and the FO1a)/Myoviridae | Cocktail | 107 and 108 | 107 CFU/g | Tumbling | 4 °C | Overall, phage application on trim reduced 0.8 and 1 log10 CFU/g of Salmonella in ground pork and beef, respectively, whereas a reduction of 0.9 and 1.1 log10 CFU/g occurred in ground turkey and chicken, respectively. | [66] |
| Chicken skin | Cocktail of S. Typhimurium, S. Heidelberg, and S. Enteritidis | SalmoFresh™ | Cocktail | 109 PFU/mL | 103 CFU/g | Immersion in water followed by spot inoculation and in chlorine (30 ppm) followed by phage treatment | 4 °C | A reduction of 0.9–1 log10 CFU/cm2 with phage only. Whereas a greater reduction of 1.6 and 1.8 log10 CFU/cm2 after 2 and 24 h. after chlorine and phage treatment. | [159] |
| Chicken | S. Typhimurium, S. Newport, S., and Thompson | Salmonelex™ | Cocktail | 107 PFU/cm2 | 104 CFU/cm2 | Spread in sterile filtered water or sterile tap water | 4 °C | A reduction of 0.39 log10 CFU/cm2 and 0.67 log10 CFU/cm2 after 30 min and 8 h post-inoculation, respectively. | [104] |
| Meat | L. monocytogenes | Halal-certified List-shield | 109 PFU/mL | Concentration not indicated | Spot inoculation | 4 °C | A reduction of 2.3 log10 was recorded in phage-treated beef samples during the storage period of 15 days. | [160] | |
| Fresh salmon meat | L. monocytogenes | SH3-3/Myoviridae | Single | 108 CFU/mL | 105 CFU/g | Spot inoculation | 4 °C | A reduction of 2.67, 4.14, and 4.54 log10 after 24, 48, and 72 h of phage addition, respectively. | [161] |
| Chicken | Cocktail of L. monocytogenes strains ATCC 19113, ATCC19115, and ATCC 13932 | ListShield | Cocktail | 108 log CFU/g | 104 CFU/g | Spraying | 4 °C | A mean reduction of 0.56, 0.84, 0.46, and 0.10 log cycles in viable counts was observed at 0, 24, 48, and 72 h after phage treatment, respectively. | [162] |
| Cooked turkey and roast beef | A cocktail of L. monocytogenes (serotypes; 1/2a, 1/2b, and 4b) | LISTEX™P100 | Cocktail | 107 PFU/cm2 | 103 CFU/cm2 | Smearing | 4 and 10 °C | An initial reduction of 2.1 and 1.7 log10 CFU/cm2, respectively, for cooked turkey and roast beef at 4 °C, while an initial reduction of 1.5 and 1.7 log10 CFU/cm2, at 10 °C. | [163] |
| Raw chicken and pork meat | C. jejuni (NCTC 11168) and C. coli (NCTC 12668) | NCTC group II phage 12684 or CP81 | Single | MOI of 10 or 100 | 106 CFU/mL | Spot inoculation | 4 and 37 °C | No reduction at 4 °C after 7 days of inoculation. | [164] |
| Raw and cooked beef | C. jejuni | Cj6/Myoviridae | Single | MOI of 10 or 10,000 | Low cell density of <100/cm2 or high cell density of 104 CFU/cm2 | Spot inoculation | 5 and 24 °C | No reduction at 5 °C compared to control with low MOI. However, a 2 log10 CFU/cm2 reduction on raw and cooked meat at high host density and a high MOI of 10,000. | [165] |
| Chicken | C. jejuni (NCTC12662 or RM1221) | F356 and F357 | Cocktail | 107 PFU | 104 CFU/cm2 | Spot inoculation | 5 °C | A 0.73 log10 reduction at 5 °C after 24 h post-treatment. | [67] |
| Chicken liver | C. jejuni (HPC5 and 81–176) | Phages ϕ3 or ϕ15/Myoviridae | Single | 108 PFU/g | 103 or 105 CFU/g | Phage added to liver stomachates containing C. jejuni | 4 °C | A 0.2 to 0.7 log10 CFU/g reduction 48 h post-treatment. | [166] |
| Lettuce | Salmonella ser. Enteritidis (ATCC13076) and Salmonella ser. Typhimurium (ATCC14028) | BP 1369 and BP 1370/Myoviridae and Podoviridae, respectively | Single | 108 PFU/mL | 106 CFU/mL | Immersion | 10, 20, and 30 °C | A reduction of >1.0 log10 CFU/cm2 after 2 h of post-treatment. | [145] |
| Romaine lettuce | Individual strains of STEC (EDL933; O157:H7, SN061; O26: H11, SN576; O111:NM and SN608; and O103:H2) | VE04, VE05, and VE07 | Single | 108 PFU/mL | 107 CFU/mL | Spot inoculation and spreading with pipet | 10 °C | A reduction of 2.6–6 log10 CFU/cm2 after 3 days of storage at a temperature of 10 °C. | [167] |
| Romaine lettuce, mung bean sprouts, and seeds | Cocktail of Salmonella strains (Newport, Braenderup, Typhimurium, Kentucky, and Heidelberg | SalmoFresh™/Myoviridae | Cocktail | 108 PFU/mL | 105 CFU/mL | Spraying or immersion | 2, 10, and 25 °C | Overall reduction by spraying SalmoFresh™ onto lettuce and sprouts reduced Salmonella by 0.76 and 0.83 log10 CFU/g, respectively, whereas a reduction of 2.43 and 2.16 log10 CFU/g by immersion was observed on lettuce and sprouts, respectively. | [168] |
| Romaine and iceberg lettuce | E. coli O157:H7 | AYO26, AXO111, AXO121, AYO145A/Myoviridae, AXO103, AKFV33/Siphoviridae, and AXO45B | Cocktail | >108 PFU/mL | High (105 CFU/g) and low (103 CFU/g) | Immersion | 2 °C | A reduction of 2.6–3.2 and 1.7–2.3 log10 CFU/g for low and high contamination, respectively. | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumunang, E.W.; Zaheer, R.; Niu, D.; Narvaez-Bravo, C.; Alexander, T.; McAllister, T.A.; Stanford, K. Bacteriophages for the Targeted Control of Foodborne Pathogens. Foods 2023, 12, 2734. https://doi.org/10.3390/foods12142734
Bumunang EW, Zaheer R, Niu D, Narvaez-Bravo C, Alexander T, McAllister TA, Stanford K. Bacteriophages for the Targeted Control of Foodborne Pathogens. Foods. 2023; 12(14):2734. https://doi.org/10.3390/foods12142734
Chicago/Turabian StyleBumunang, Emmanuel W., Rahat Zaheer, Dongyan Niu, Claudia Narvaez-Bravo, Trevor Alexander, Tim A. McAllister, and Kim Stanford. 2023. "Bacteriophages for the Targeted Control of Foodborne Pathogens" Foods 12, no. 14: 2734. https://doi.org/10.3390/foods12142734
APA StyleBumunang, E. W., Zaheer, R., Niu, D., Narvaez-Bravo, C., Alexander, T., McAllister, T. A., & Stanford, K. (2023). Bacteriophages for the Targeted Control of Foodborne Pathogens. Foods, 12(14), 2734. https://doi.org/10.3390/foods12142734







