Laccase-Induced Gelation of Sugar Beet Pectin–Curcumin Nanocomplexes Enhanced by Genipin Crosslinking
Abstract
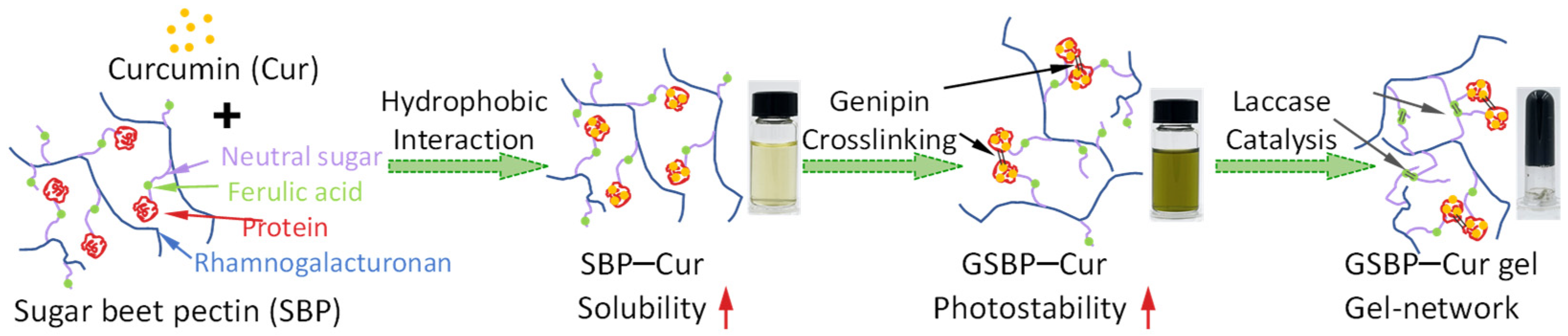
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Spectroscopy Determination
2.4. Texture Analysis
2.5. Rheological Measurements
2.6. Stability Analysis of Curcumin
2.7. Statistical Analysis
3. Results and Discussion
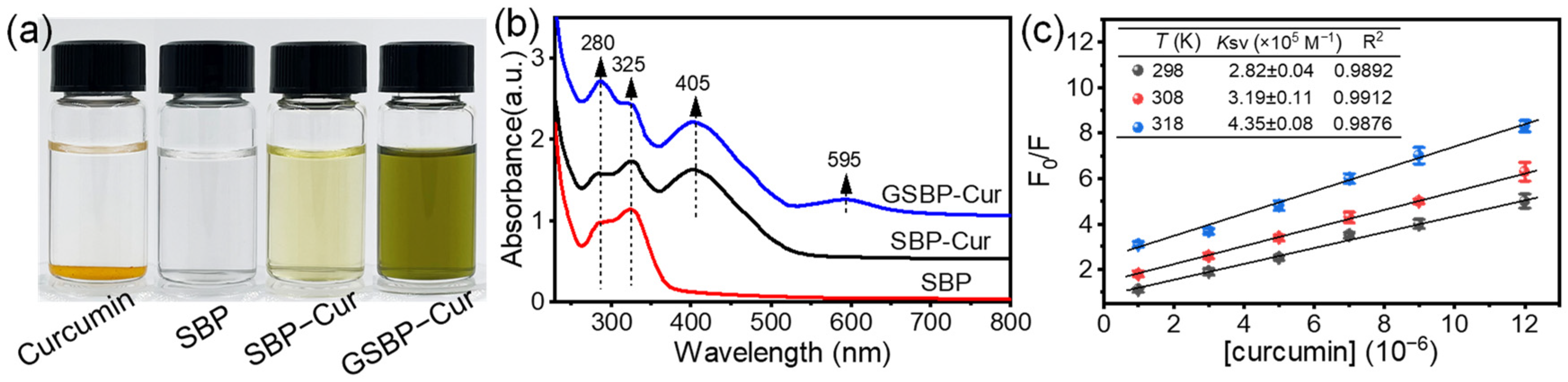
3.1. Characterization of the SBP-Cur and GSBP-Cur
3.2. The Interaction between SBP and Curcumin
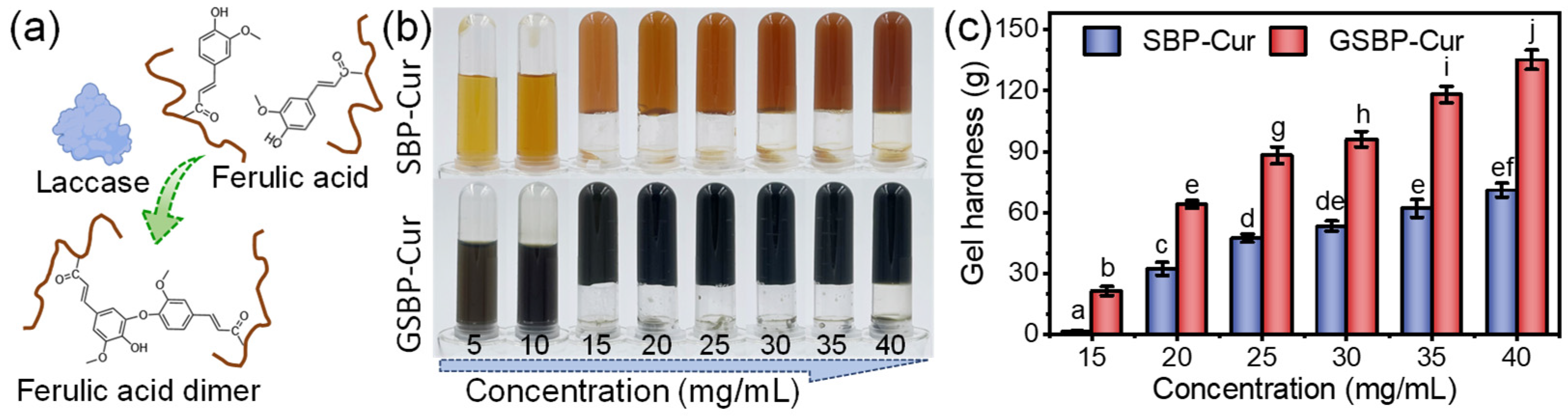
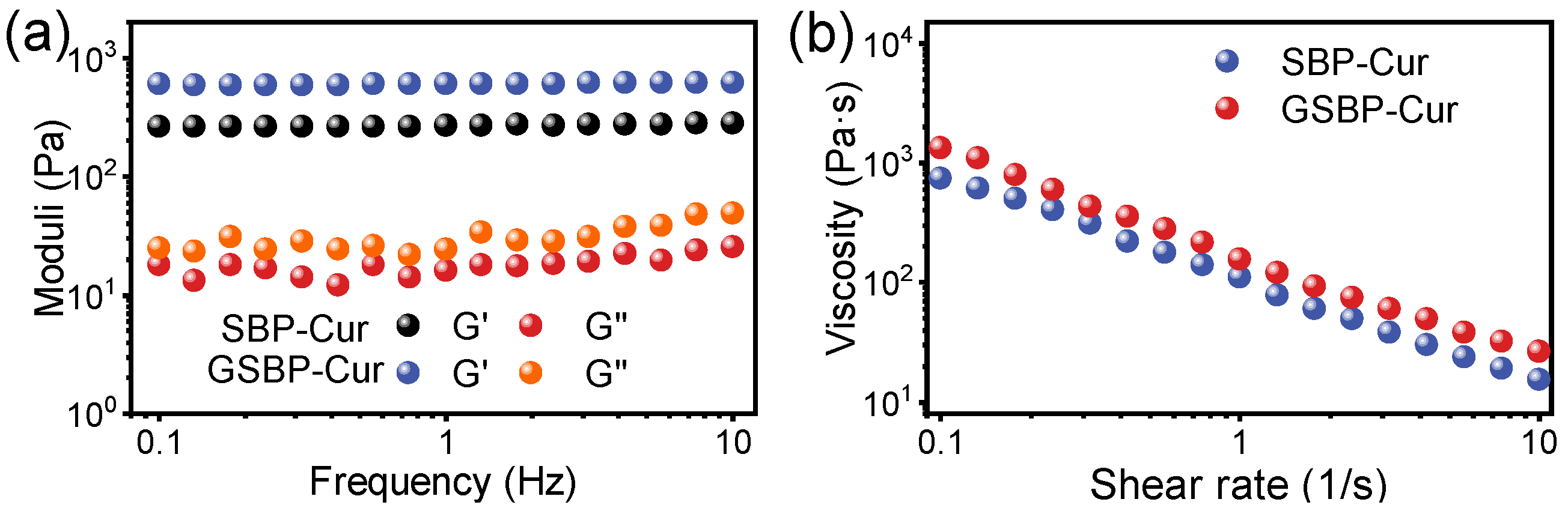
3.3. Gelation and Rheology Property Analysis
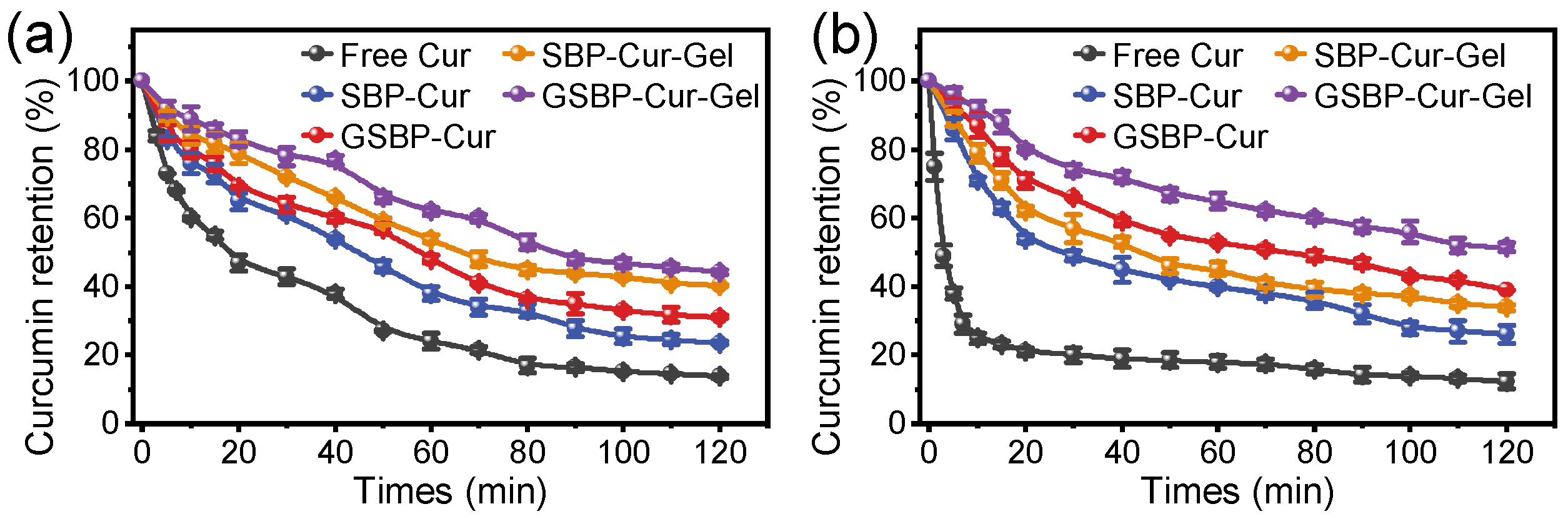
3.4. Protection of Curcumin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunwar, A.; Barik, A.; Pandey, R.; Priyadarsini, K.I. Transport of liposomal and albumin loaded curcumin to living cells: An absorption and fluorescence spectroscopic study. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2006, 1760, 1513–1520. [Google Scholar] [CrossRef]
- Wang, R.; Wen, Q.-H.; Zeng, X.-A.; Lin, J.-W.; Li, J.; Xu, F.-Y. Binding affinity of curcumin to bovine serum albumin enhanced by pulsed electric field pretreatment. Food Chem. 2022, 377, 131945. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Lin, J.; Meng, H.; Guo, X.; Yu, S. Enhancing the Emulsification and Photostability Properties of Pectin from Different Sources Using Genipin Crosslinking Technique. Foods 2022, 11, 2392. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Guo, X.; Yu, S.; Zhang, T.; Tang, X.; Yang, Z.; Meng, H. Tannic Acid-Aminated Sugar Beet Pectin Nanoparticles as a Stabilizer of High-Internal-Phase Pickering Emulsions. J. Agric. Food Chem. 2022, 70, 8052–8063. [Google Scholar] [CrossRef]
- Xu, F.-Y.; Lin, J.-W.; Wang, R.; Chen, B.-R.; Li, J.; Wen, Q.-H.; Zeng, X.-A. Succinylated whey protein isolate-chitosan core-shell composite particles as a novel carrier: Self-assembly mechanism and stability studies. Food Res. Int. 2022, 160, 111695. [Google Scholar] [CrossRef]
- Chen, F.-P.; Ou, S.-Y.; Tang, C.-H. Core–Shell Soy Protein–Soy Polysaccharide Complex (Nano)particles as Carriers for Improved Stability and Sustained Release of Curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Solghi, S.; Emam-Djomeh, Z.; Fathi, M.; Farahani, F. The encapsulation of curcumin by whey protein: Assessment of the stability and bioactivity. J. Food Process Eng. 2020, 43, e13403. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Z.; Meng, H.; Guo, X. Genipin crosslinked gum arabic: Synthesis, characterization, and emulsification properties. Carbohydr. Polym. 2021, 261, 117880. [Google Scholar] [CrossRef]
- Lin, J.; Lei, X.; Chen, J.; Lin, Q.; Zhang, Y.; Tang, Z.-S. Influences of the different chemical components in soybean soluble polysaccharide on the emulsifying performance of its conjugates formed with soybean protein isolate. Int. J. Food Sci. Technol. 2023, 58, 656–664. [Google Scholar] [CrossRef]
- Lin, J.; Yu, S.; Ai, C.; Zhang, T.; Guo, X. Emulsion stability of sugar beet pectin increased by genipin crosslinking. Food Hydrocoll. 2020, 101, 105459. [Google Scholar] [CrossRef]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
- Nakamura, A.; Furuta, H.; Kato, M.; Maeda, H.; Nagamatsu, Y. Effect of soybean soluble polysaccharides on the stability of milk protein under acidic conditions. Food Hydrocoll. 2003, 17, 333–343. [Google Scholar] [CrossRef]
- Chen, F.P.; Ou, S.Y.; Chen, Z.; Tang, C.H. Soy Soluble Polysaccharide as a Nanocarrier for Curcumin. J. Agric. Food Chem. 2017, 65, 1707–1714. [Google Scholar] [CrossRef]
- Zagury, Y.; David, S.; Edelman, R.; Hazan Brill, R.; Livney, Y.D. Sugar beet pectin as a natural carrier for curcumin, a water-insoluble bioactive for food and beverage enrichment: Formation and characterization. Innov. Food Sci. Emerg. Technol. 2021, 74, 102858. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.; Wang, Y.; Ye, X.; Chen, S.; Pan, H.; Chen, J. Fabrication of rhamnogalacturonan-I enriched pectin-based emulsion gels for protection and sustained release of curcumin. Food Hydrocoll. 2022, 128, 107592. [Google Scholar] [CrossRef]
- Pan, Y.; Li, X.-M.; Meng, R.; Xu, B.-C.; Zhang, B. Investigation of the Formation Mechanism and Curcumin Bioaccessibility of Emulsion Gels Based on Sugar Beet Pectin and Laccase Catalysis. J. Agric. Food Chem. 2021, 69, 2557–2563. [Google Scholar] [CrossRef]
- Cai, R.; Pan, S.; Li, R.; Xu, X.; Pan, S.; Liu, F. Curcumin loading and colon release of pectin gel beads: Effect of different de-esterification method. Food Chem. 2022, 389, 133130. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Wen, H.; Chen, Y.; Yu, Q.; Shen, M.; Xie, J. Curcumin-Loaded pH-Sensitive Biopolymer Hydrogels: Fabrication, Characterization, and Release Properties. ACS Food Sci. Technol. 2022, 2, 512–520. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids acting as emulsifying agents—How do they do it? Food Hydrocoll. 2018, 78, 2–14. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.-S.; Chandrapala, J.; Brennan, C.S.; Han, Z.; Zeng, X.-A. Elucidation of the cellulose nanocrystal-sugar beet pectin interactions for emulsification enhancement. Food Hydrocoll. 2023, 135, 108198. [Google Scholar] [CrossRef]
- Lin, J.; Guo, X.; Ai, C.; Zhang, T.; Yu, S. Genipin crosslinked sugar beet pectin-whey protein isolate/bovine serum albumin conjugates with enhanced emulsifying properties. Food Hydrocoll. 2020, 105, 105802. [Google Scholar] [CrossRef]
- Liang, B.; Shu, Y.; Wan, P.; Zhao, H.; Dong, S.; Hao, W.; Yin, P. Genipin-enhanced nacre-inspired montmorillonite-chitosan film with superior mechanical and UV-blocking properties. Compos. Sci. Technol. 2019, 182, 107747. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Pessoa, M.G.; Paulino, B.N.; Pastore, G.M. Genipin: A natural blue pigment for food and health purposes. Trends Food Sci. Technol. 2017, 67, 271–279. [Google Scholar] [CrossRef]
- Zaidel, D.N.A.; Chronakis, I.S.; Meyer, A.S. Enzyme catalyzed oxidative gelation of sugar beet pectin: Kinetics and rheology. Food Hydrocoll. 2012, 28, 130–140. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.-S.; Brennan, C.S.; Chandrapala, J.; Gao, W.; Han, Z.; Zeng, X.-A. Valorizing protein-polysaccharide conjugates from sugar beet pulp as an emulsifier. Int. J. Biol. Macromol. 2023, 226, 679–689. [Google Scholar] [CrossRef]
- Liu, L.-L.; Liu, P.-Z.; Li, X.-T.; Zhang, N.; Tang, C.-H. Novel Soy β-Conglycinin Core–Shell Nanoparticles As Outstanding Ecofriendly Nanocarriers for Curcumin. J. Agric. Food Chem. 2019, 67, 6292–6301. [Google Scholar] [CrossRef]
- Lin, J.; Meng, H.; Yu, S.; Wang, Z.; Ai, C.; Zhang, T.; Guo, X. Genipin-crosslinked sugar beet pectin-bovine serum albumin nanoparticles as novel pickering stabilizer. Food Hydrocoll. 2021, 112, 106306. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.-S.; Brennan, C.S.; Zeng, X.-A. Thermomechanically micronized sugar beet pulp: Dissociation mechanism, physicochemical characteristics, and emulsifying properties. Food Res. Int. 2022, 160, 111675. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z. Fabrication of curcumin-loaded whey protein microgels: Structural properties, antioxidant activity, and in vitro release behavior. LWT—Food Sci. Technol. 2019, 103, 94–100. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Chen, F.-P.; Li, B.-S.; Tang, C.-H. Nanocomplexation of soy protein isolate with curcumin: Influence of ultrasonic treatment. Food Res. Int. 2015, 75, 157–165. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.M.; Yu, S.J.; Guo, X.M.; Ai, C.; Tang, X.Y.; Chen, H.L.; Lin, J.W.; Zhang, X.; Meng, H.C. Effects of pH and temperature on the structure, rheological and gel-forming properties of sugar beet pectins. Food Hydrocoll. 2021, 116, 106646. [Google Scholar] [CrossRef]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering Emulsion Gels as Functional Colloids Emphasizing Food Applications: A Review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef]
- Bu, X.; Guan, M.; Dai, L.; Ji, N.; Qin, Y.; Xu, X.; Xiong, L.; Shi, R.; Sun, Q. Fabrication of starch-based emulsion gel beads by an inverse gelation technique for loading proanthocyanidin and curcumin. Food Hydrocoll. 2023, 137, 108336. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Yuliarti, O.; Hoon, A.L.S.; Chong, S.Y. Influence of pH, pectin and Ca concentration on gelation properties of low-methoxyl pectin extracted from Cyclea barbata Miers. Food Struct. 2017, 11, 16–23. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. Theoretical study on physicochemical properties of curcumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 619–623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-W.; Jiang, G.-L.; Liang, C.-X.; Li, Y.-M.; Chen, X.-Y.; Zhang, X.-T.; Tang, Z.-S. Laccase-Induced Gelation of Sugar Beet Pectin–Curcumin Nanocomplexes Enhanced by Genipin Crosslinking. Foods 2023, 12, 2771. https://doi.org/10.3390/foods12142771
Lin J-W, Jiang G-L, Liang C-X, Li Y-M, Chen X-Y, Zhang X-T, Tang Z-S. Laccase-Induced Gelation of Sugar Beet Pectin–Curcumin Nanocomplexes Enhanced by Genipin Crosslinking. Foods. 2023; 12(14):2771. https://doi.org/10.3390/foods12142771
Chicago/Turabian StyleLin, Jia-Wei, Gui-Li Jiang, Cui-Xin Liang, Ye-Meng Li, Xing-Yi Chen, Xiao-Tong Zhang, and Zhong-Sheng Tang. 2023. "Laccase-Induced Gelation of Sugar Beet Pectin–Curcumin Nanocomplexes Enhanced by Genipin Crosslinking" Foods 12, no. 14: 2771. https://doi.org/10.3390/foods12142771
APA StyleLin, J.-W., Jiang, G.-L., Liang, C.-X., Li, Y.-M., Chen, X.-Y., Zhang, X.-T., & Tang, Z.-S. (2023). Laccase-Induced Gelation of Sugar Beet Pectin–Curcumin Nanocomplexes Enhanced by Genipin Crosslinking. Foods, 12(14), 2771. https://doi.org/10.3390/foods12142771





