Comparative Quantitation of Kokumi γ-Glutamyl Peptides in Spanish Dry-Cured Ham under Salt-Reduced Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Total Peptide Extraction and Ultrafiltration
2.2.1. Ethanolic Deproteinization-Based Method
2.2.2. Non-Ethanolic Deproteinization-Based Method
2.3. Amino Acids Determination
2.4. Tageted Quantitative Analysis of γ-Glutamyl Peptides
3. Results
3.1. Amino Acids Determination
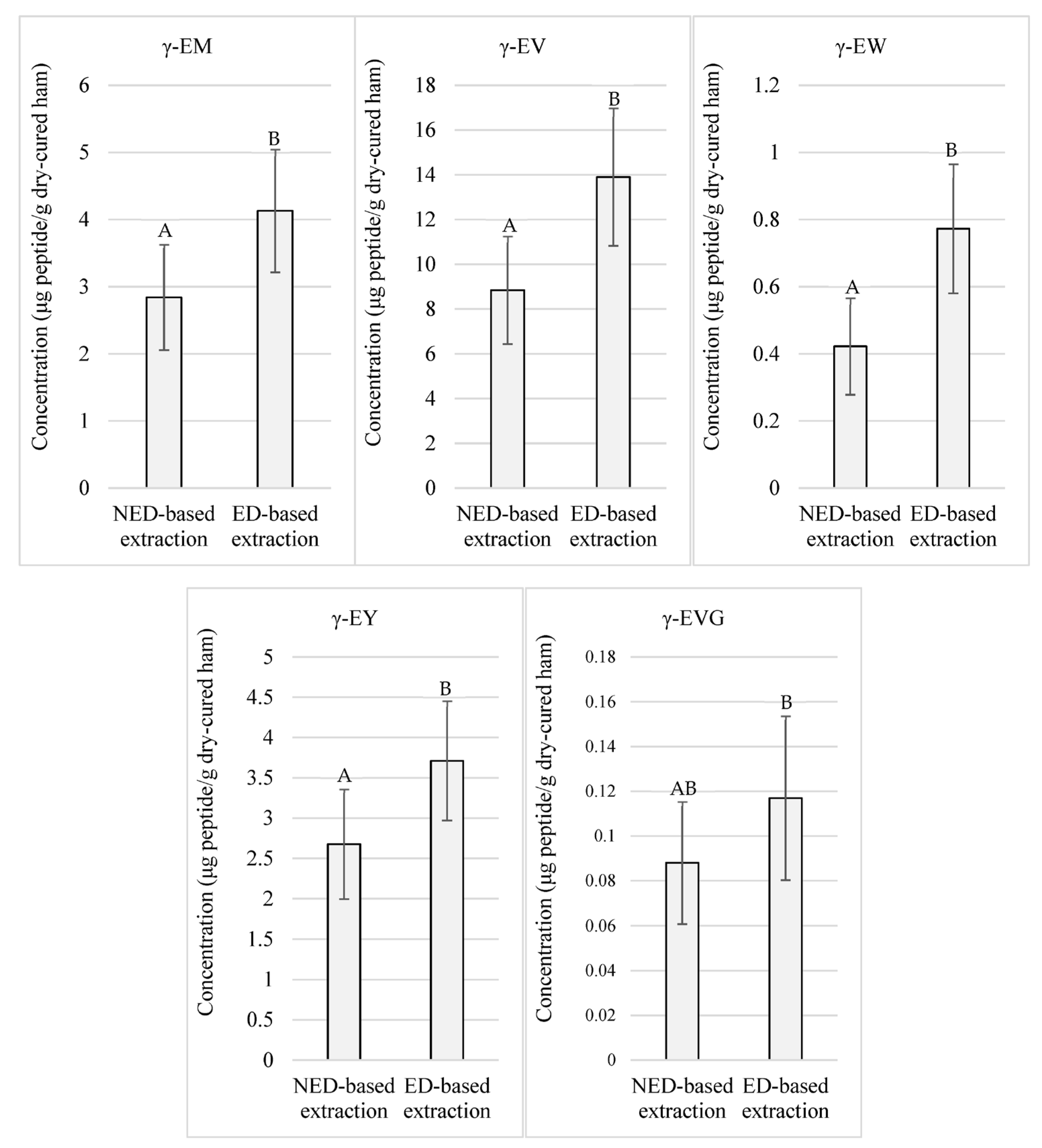
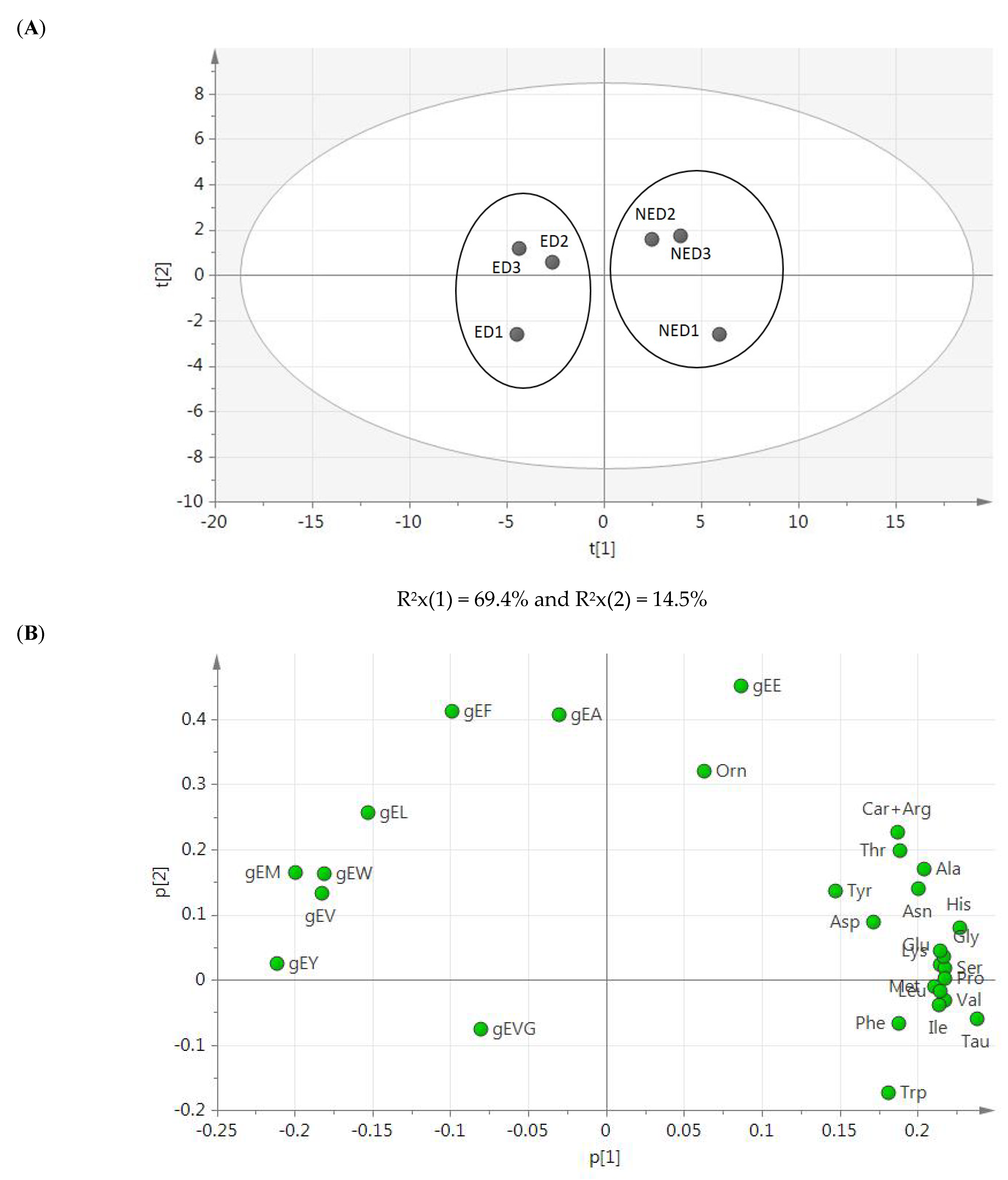
3.2. γ-Glutamyl Peptides-Targeted Quantitative Analysis
4. Discussion
4.1. Amino Acids Determination
4.2. γ-Glutamyl Peptides-Targeted Quantitative Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Toldrá, F.; Flores, M. The Role of Muscle Proteases and Lipases in Flavor Development during the Processing of Dry-Cured Ham. Crit. Rev. Food Sci. Nutr. 1998, 38, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Yokoyama, I.; Gallego, M.; Toldrá, F.; Arihara, K.; Mora, L. Impact of Oxidation on the Cardioprotective Properties of the Bioactive Dipeptide AW in Dry-Cured Ham. Food Res. Int. 2022, 162, 112128. [Google Scholar] [CrossRef] [PubMed]
- Sentandreu, M.A.; Toldrá, F. Dipeptidyl Peptidase Activities along the Processing of Serrano Dry-Cured Ham. Eur. Food Res. Technol. 2001, 213, 83–87. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Barat, J.M.; Toldrá, F. Biochemical Changes in Dry-Cured Loins Salted with Partial Replacements of NaCl by KCl. Food Chem. 2009, 117, 627–633. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.-C.; Barat, J.M.; Toldrá, F. Biochemical and Sensory Properties of Dry-Cured Loins as Affected by Partial Replacement of Sodium by Potassium, Calcium, and Magnesium. J. Agric. Food Chem. 2009, 57, 9699–9705. [Google Scholar] [CrossRef]
- Heres, A.; Gallego, M.; Mora, L.; Toldrá, F. Identification and Quantitation of Bioactive and Taste-Related Dipeptides in Low-Salt Dry-Cured Ham. Int. J. Mol. Sci. 2022, 23, 2507. [Google Scholar] [CrossRef]
- Wang, H.; Suo, R.; Liu, X.; Wang, Y.; Sun, J.; Liu, Y.; Wang, W.; Wang, J. Kokumi γ-Glutamyl Peptides: Some Insight into Their Evaluation and Detection, Biosynthetic Pathways, Contribution and Changes in Food Processing. Food Chem. Adv. 2022, 1, 100061. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Stoeva, S.; Aristoy, M.C.C.; Laib, K.; Voelter, W.; Toldra, E.; Toldrá, F.; Toldra, E. Identification of Small Peptides Generated in Spanish Dry-Cured Ham. J. Food Sci. 2003, 68, 64–69. [Google Scholar] [CrossRef]
- Rico, A.G.; Braun, J.P.; Benard, P.; Thouvenot, J.P. Tissue and Blood Gamma-Glutamyl Transferase Distribution in the Pig. Res. Vet. Sci. 1977, 23, 395–396. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M.; Sanz, Y. Dry-Cured Ham Flavour: Enzymatic Generation and Process Influence. Food Chem. 1997, 59, 523–530. [Google Scholar] [CrossRef]
- Yang, J.; Bai, W.; Zeng, X.; Cui, C. Gamma Glutamyl Peptides: The Food Source, Enzymatic Synthesis, Kokumi-Active and the Potential Functional Properties—A Review. Trends Food Sci. Technol. 2019, 91, 339–346. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Sánchez, C.; Nuñez, M.; Picon, A. Microbiota of Iberian Dry-Cured Ham as Influenced by Chemical Composition, High Pressure Processing and Prolonged Refrigerated Storage. Food Microbiol. 2019, 80, 62–69. [Google Scholar] [CrossRef]
- Blesa, E.; Aliño, M.; Barat, J.M.; Grau, R.; Toldrá, F.; Pagán, M.J. Microbiology and Physico-Chemical Changes of Dry-Cured Ham during the Post-Salting Stage as Affected by Partial Replacement of NaCl by Other Salts. Meat Sci. 2008, 78, 135–142. [Google Scholar] [CrossRef]
- Toldrá, F.; Cerveró, M.C.; Part, C. Porcine Aminopeptidase Activity as Affected by Curing Agents. J. Food Sci. 1993, 58, 724–726. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, T.; Mu, W.; Jiang, B. Purification and Characterization of γ-Glutamyltranspeptidase from Bacillus subtilis SK11.004. J. Agric. Food Chem. 2011, 59, 6233–6238. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; De Gobba, C.; Zhang, L.; Bredie, W.L.P.; Lametsch, R. Production of Taste Enhancers from Protein Hydrolysates of Porcine Hemoglobin and Meat Using Bacillus amyloliquefaciens γ-Glutamyltranspeptidase. J. Agric. Food Chem. 2020, 68, 11782–11789. [Google Scholar] [CrossRef]
- Flores, M.; Aristoy, M.-C.; Spanier, A.M.; Toldrá, F. Non-Volatile Components Effects on Quality of “Serrano” Dry-Cured Ham as Related to Processing Time. J. Food Sci. 1997, 62, 1235–1239. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Reig, M.; Toldrá, F. Challenges in the Quantitation of Naturally Generated Bioactive Peptides in Processed Meats. Trends Food Sci. Technol. 2017, 69, 306–314. [Google Scholar] [CrossRef]
- Cerrato, A.; Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Laganà, A. Investigating the Short Peptidome Profile of Italian Dry-Cured Ham at Different Processing Times by High-Resolution Mass Spectrometry and Chemometrics. Int. J. Mol. Sci. 2022, 23, 3193. [Google Scholar] [CrossRef]
- Degnes, K.F.; Kvitvang, H.F.N.; Haslene-Hox, H.; Aasen, I.M. Changes in the Profiles of Metabolites Originating from Protein Degradation During Ripening of Dry Cured Ham. Food Bioprocess Technol. 2017, 10, 1122–1130. [Google Scholar] [CrossRef]
- Paolella, S.; Prandi, B.; Falavigna, C.; Buhler, S.; Dossena, A.; Sforza, S.; Galaverna, G. Occurrence of Non-Proteolytic Amino Acyl Derivatives in Dry-Cured Ham. Food Res. Int. 2018, 114, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Gallego, M.; Toldrá, F. Degradation of Myosin Heavy Chain and Its Potential as a Source of Natural Bioactive Peptides in Dry-Cured Ham. Food Biosci. 2019, 30, 100416. [Google Scholar] [CrossRef]
- Mora, L.; Sentandreu, M.Á.; Koistinen, K.M.; Fraser, P.D.; Toldrá, F.; Bramley, P.M. Naturally Generated Small Peptides Derived from Myofibrillar Proteins in Serrano Dry-Cured Ham. J. Agric. Food Chem. 2009, 57, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.-C.; Mora, L. Bioactive Peptides Generated in the Processing of Dry-Cured Ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef]
- Malva, A.D.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M.; Marino, R. Methods for Extraction of Muscle Proteins from Meat and Fish Using Denaturing and Nondenaturing Solutions. J. Food Qual. 2018, 2018, 8478471. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Lametsch, R. Current Progress in Kokumi-Active Peptides, Evaluation and Preparation Methods: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1230–1241. [Google Scholar] [CrossRef]
- Mora, L.; Sentandreu, M.Á.; Fraser, P.D.; Toldrá, F.; Bramley, P.M. Oligopeptides Arising from the Degradation of Creatine Kinase in Spanish Dry-Cured Ham. J. Agric. Food Chem. 2009, 57, 8982–8988. [Google Scholar] [CrossRef]
- Harrison, A.G. Characterization of α- Andγ-Glutamyl Dipeptides by Negative Ion Collision-Induced Dissociation. J. Mass Spectrom. 2004, 39, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Metwally, H.; McAllister, R.G.; Konermann, L. Exploring the Mechanism of Salt-Induced Signal Suppression in Protein Electrospray Mass Spectrometry Using Experiments and Molecular Dynamics Simulations. Anal. Chem. 2015, 87, 2434–2442. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Cittadini, A.; Bermúdez, R.; Munekata, P.E.; Domínguez, R. Influence of Partial Replacement of NaCl with KCl, CaCl2 and MgCl2 on Proteolysis, Lipolysis and Sensory Properties during the Manufacture of Dry-Cured Lacón. Food Control 2015, 55, 90–96. [Google Scholar] [CrossRef]
- Muñoz-Rosique, B.; Salazar, E.; Tapiador, J.; Peinado, B.; Tejada, L. Effect of Salt Reduction on the Quality of Boneless Dry-Cured Ham from Iberian and White Commercially Crossed Pigs. Foods 2022, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Saldaña, C.; Toldrá, F.; Mora, L. Identification of Dipeptides by MALDI-ToF Mass Spectrometry in Long-Processing Spanish Dry-Cured Ham. Food Chem. Mol. Sci. 2021, 3, 100048. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sugawara, T.; Obiya, S.; Enomoto, A.; Kaneko, M.; Ota, S.; Soga, T.; Tomita, M. Sensory Properties and Metabolomic Profiles of Dry-Cured Ham during the Ripening Process. Food Res. Int. 2020, 129, 108850. [Google Scholar] [CrossRef]
- Gallego, M.; Toldrá, F.; Mora, L. Quantification and in Silico Analysis of Taste Dipeptides Generated during Dry-Cured Ham Processing. Food Chem. 2022, 370, 130977. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Tian, W.; Li, M.-Y.; Liu, Y.-X.; Zhao, G.-M. Separation and Identification of Peptides from Dry-Cured Jinhua Ham. Int. J. Food Prop. 2017, 20 (Suppl. 3), S2980–S2989. [Google Scholar] [CrossRef]
- Sforza, S.; Galaverna, G.; Schivazappa, C.; Marchelli, R.; Dossena, A.; Virgili, R. Effect of Extended Aging of Parma Dry-Cured Ham on the Content of Oligopeptides and Free Amino Acids. J. Agric. Food Chem. 2006, 54, 9422–9429. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Yokoyama, I.; Gallego, M.; Toldrá, F.; Arihara, K.; Mora, L. Antihypertensive Potential of Sweet Ala-Ala Dipeptide and Its Quantitation in Dry-Cured Ham at Different Processing Conditions. J. Funct. Foods 2021, 87, 104818. [Google Scholar] [CrossRef]
- Marušić, N.; Aristoy, M.-C.; Toldrá, F. Nutritional Pork Meat Compounds as Affected by Ham Dry-Curing. Meat Sci. 2013, 93, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-B.; Ahn, J.-H.; Yang, H.-G.; Lee, J.; Park, W.-J.; Kim, Y.-W. Effects of PH and NaCl on Hydrolysis and Transpeptidation Activities of a Salt-Tolerant γ-Glutamyltranspeptidase from Bacillus Amyloliquefaciens S0904. Food Sci. Biotechnol. 2021, 30, 853–860. [Google Scholar] [CrossRef]
- Fukao, T.; Suzuki, H. Enzymatic Synthesis of γ-Glutamylvalylglycine Using Bacterial γ-Glutamyltranspeptidase. J. Agric. Food Chem. 2021, 69, 7675–7679. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Legler, P.M.; Khavrutskii, I.; Scorpio, A.; Compton, J.R.; Robertson, K.L.; Friedlander, A.M.; Wallqvist, A. Probing the Donor and Acceptor Substrate Specificity of the γ-Glutamyl Transpeptidase. Biochemistry 2012, 51, 1199–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ED-Based Method | NED-Based Method | |||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| Asp | 1687.91 a | 211.78 | 2377.84 b | 329.34 |
| Glu | 3347.55 a | 425.62 | 5502.06 b | 567.84 |
| Ser | 1569.98 a | 188.38 | 2893.25 b | 380.54 |
| Asn | 149.88 a | 63.73 | 304.25 a | 105.75 |
| Gly | 1342.80 a | 183.80 | 2439.31 b | 309.56 |
| Gln | NQ | NQ | 53.33 | 3.48 |
| His | 996.27 a | 96.61 | 1427.74 b | 597.85 |
| Thr | 1573.51 a | 199.89 | 2562.46 b | 262.47 |
| Ala | 2668.40 a | 369.60 | 3855.85 b | 290.97 |
| Pro | 1840.77 a | 96.24 | 3020.00 b | 360.21 |
| Tyr | 371.89 a | 28.64 | 597.08 b | 133.21 |
| Val | 2319.63 a | 258.43 | 4030.81 b | 661.33 |
| Met | 970.91 a | 146.31 | 1593.6 b | 294.79 |
| Ile | 1792.02 a | 286.36 | 3115.8 b | 602.42 |
| Leu | 3028.03 a | 517.82 | 5258.94 b | 945.43 |
| Phe | 1364.47 a | 54.12 | 2094.46 a | 975.06 |
| Trp | 328.76 a | 57.85 | 411.68 a | 171.72 |
| Lys | 7514.67 a | 1514.19 | 11,910.47 b | 1196.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heres, A.; Li, Q.; Toldrá, F.; Lametsch, R.; Mora, L. Comparative Quantitation of Kokumi γ-Glutamyl Peptides in Spanish Dry-Cured Ham under Salt-Reduced Production. Foods 2023, 12, 2814. https://doi.org/10.3390/foods12142814
Heres A, Li Q, Toldrá F, Lametsch R, Mora L. Comparative Quantitation of Kokumi γ-Glutamyl Peptides in Spanish Dry-Cured Ham under Salt-Reduced Production. Foods. 2023; 12(14):2814. https://doi.org/10.3390/foods12142814
Chicago/Turabian StyleHeres, Alejandro, Qian Li, Fidel Toldrá, René Lametsch, and Leticia Mora. 2023. "Comparative Quantitation of Kokumi γ-Glutamyl Peptides in Spanish Dry-Cured Ham under Salt-Reduced Production" Foods 12, no. 14: 2814. https://doi.org/10.3390/foods12142814
APA StyleHeres, A., Li, Q., Toldrá, F., Lametsch, R., & Mora, L. (2023). Comparative Quantitation of Kokumi γ-Glutamyl Peptides in Spanish Dry-Cured Ham under Salt-Reduced Production. Foods, 12(14), 2814. https://doi.org/10.3390/foods12142814








