A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry
Abstract
:1. Introduction
1.1. Characteristics of LAB
1.2. Source of LAB in Dairy Products
| LAB Source | Family | Genus | Gram | Shape | Acid- Resistant | Respiration | References |
|---|---|---|---|---|---|---|---|
| Dairy Product | Lactobacillaceae | Lactobacillus | + | Rod shaped | Changeable | Facultative anaerobic | [26] |
| Pediococcus | + | Spherical shaped | High acid resistant | Facultative anaerobic | [27] | ||
| Steptococcaceae | Streptococcus | + | Coccoid shaped | Low acid resistant | Facultative anaerobic | [28] | |
| Lactococcus | + | Coccoid | Changeable | Facultative anaerobic | [29] | ||
| Leuconostocaecae | Leuconostoc | + | Spherical, oval shaped | Changeable | Facultative anaerobic | [30] | |
| Bifidobacteriaceae | Bifidobacterium | + | Rod-branch-shaped | High acid resistant | Anaerobic | [31] | |
| Enterococcaceae | Enterococcus | + | Coccoid shape | Moderate acid resistant | Facultative anaerobic | [31] | |
| Propionibacteriaceae | Propionibacterium | + | Rod shaped | Low acid resistant | Anaerobic | [30] | |
| Non-diary | Aerococcaceae | Aerococcus | + | Coccoid shaped | Low acid resistant | Facultative anaerobic | [32] |
| Carbobacteriaceae | Carnobacterium | + | Rod shaped | Not available | Facultative anaerobic | [33] | |
| Leuconostocaecae | Oenococcus | + | Spherical shaped | Changeable | Facultative anaerobic | [34] | |
| Weissella | + | Coccoid or rod shaped | Changeable | Facultative anaerobic | [35] | ||
| Fructobacillus | + | Elongated and slightly cylindrical shaped | Not available | Facultative anaerobic | [36] | ||
| Enterococcaceae | Tetragenococcus | + | Coccoid shaped | Changeable | Facultative anaerobic | [37] | |
| Vagococcus | + | Coccoid shaped | Changeable | Facultative anaerobic | [38] |
1.3. Source of LAB in Fermented Food
Fermented Food
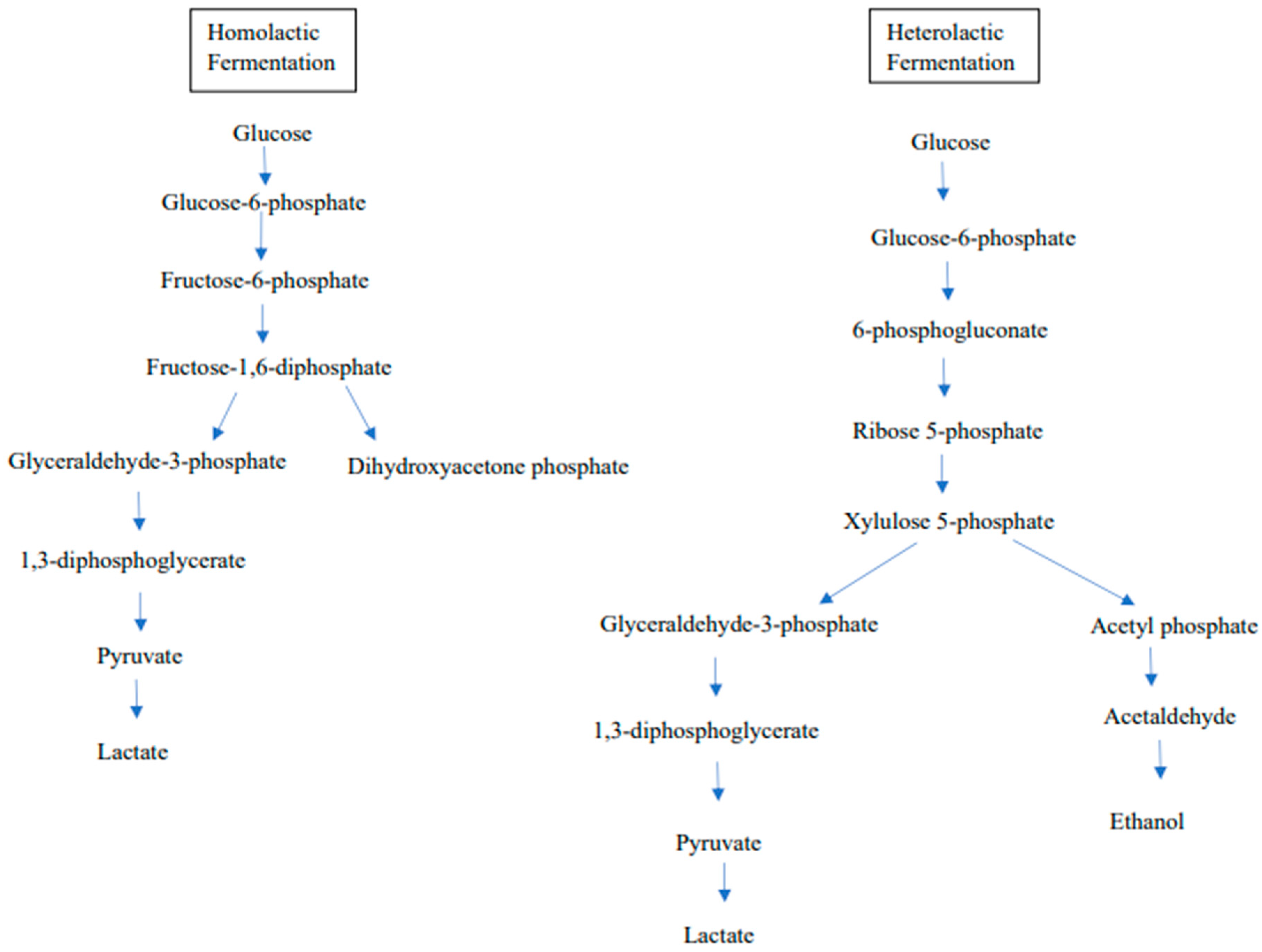
1.4. Metabolism Characteristics of LAB
1.5. Product Synthesized by LAB
1.5.1. Organic Acids
1.5.2. Bacteriocins
1.5.3. Vitamins
1.5.4. Exopolysaccharides (EPS)
1.5.5. Gamma-Aminobutyric Acid
1.5.6. Flavor Substances
1.6. Application of LAB in Clinical Nutrition
1.6.1. LAB in the Management of Lactose Intolerance
1.6.2. LAB in the Treatment of Diarrhea
1.6.3. Immunomodulatory Effects of LAB
1.6.4. LAB and Hepatoprotective Effects
1.6.5. LAB for Prevention and as a Potential Natural Anti-tumour Drug
1.6.6. LAB in the Management of Glycemic Control
1.7. Challenge of Lactic Acid Bacteria as a Food Nutrient
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.M.; Brown, A.C. Position of the American Dietetic Association: Functional Foods. J. Am. Diet. Assoc. 2009, 109, 735–746. [Google Scholar] [PubMed]
- EFSA. Call for Technical and Toxicological Data on Pullulan (E 1204) Authorised as a Food Additive in the EU; EFSA: Parma, Italy, 2017. [Google Scholar]
- Kruger, M.C.; Ha, P.C.; Todd, J.M.; Schollum, L.M.; Ma, J.; Qin, G.; Lau, E. High-Calcium, Vitamin D Fortified Milk Is Effective in Improving Bone Turnover Markers and Vitamin D Status in Healthy Postmenopausal Chinese Women. Eur. J. Clin. Nutr. 2012, 66, 856–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Alsuwaylihi, A.S.; McCullough, F. The safety and efficacy of probiotic supplementation for critically ill adult patients: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 322–332. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [Green Version]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yılmaz, B.; Şahin, T.Ö.; Güneşliol, B.E.; Ayten, Ş.; Russo, P.; Spano, G.; Rocha, J.M.; Bartkiene, E.; Özogul, F. Dairy Lactic Acid Bacteria and Their Potential Function in Dietetics: The Food–Gut-Health Axis. Foods 2021, 10, 3099. [Google Scholar] [CrossRef]
- Shehata, M.G.; El Sohaimy, S.A.; El-Sahn, M.A.; Youssef, M.M. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016, 61, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.O.; Leveau, J.H.J.; Marco, M.L. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environ. Microbiol. Rep. 2020, 12, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Mulaw, G.; Sisay Tessema, T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int. J. Microbiol. 2019, 2019, 7179514–7179521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoren, M.J.; Liu, J.; Castellanos, J.; Rodríguez-Malavé, N.I.; Schiestl, R.H. A novel probiotic, Lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period. Gut Microbes 2019, 10, 458–480. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [Green Version]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.; Linder, J.; Shane, A.; Merenstein, D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 2019, 29, 494–499. [Google Scholar] [CrossRef]
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T.; et al. In Vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, Y.; Cui, H.; Li, Y.; Sun, Y.; Qiu, H.-J. Characterization of Lactic Acid Bacteria Isolated From the Gastrointestinal Tract of a Wild Boar as Potential Probiotics. Front. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Khedid, K.; Faid, M.; Mokhtari, A.; Soulaymani, A.; Zinedine, A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol. Res. 2009, 164, 81–91. [Google Scholar] [CrossRef]
- Khan, U.; Selamoglu, Z. Use of Enzymes in Dairy Industry: A Review of Current Progress. Arch. Razi Inst. 2020, 75, 131–136. [Google Scholar]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, A.R.U.; Jahid, I.K. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Marcial-Coba, M.S.; Knøchel, S.; Nielsen, D.S. Low-moisture food matrices as probiotic carriers. FEMS Microbiol. Lett. 2019, 366, fnz006. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Mokoena, M.P. Lactic acid bacteria and their Bacteriocins: Classification, biosynthesis and applications against Uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, J. Advances in microbiological quality control. In Managing Wine Quality; Woodhead Publishing: Sawston, UK, 2022; pp. 207–241. [Google Scholar]
- Hossain, Z. Bacteria: Streptococcus. In Encyclopedia of Food Safety; Academic Press: Cambridge, MA, USA, 2014; pp. 535–545. [Google Scholar]
- Cai, Y.; Yang, J.; Pang, H.; Kitahara, M. Lactococcus fujiensis sp. Nov., a lactic acid bacterium isolated from vegetable matter. Int. J. Syst. Evol. Microbiol. 2011, 61, 1590–1594. [Google Scholar] [CrossRef]
- Onyeaka, H.N.; Nwabor, O.F. Lactic acid bacteria and bacteriocins as biopreservatives. In Food Preservation and Safety of Natural Products; Academic Press: Cambridge, MA, USA, 2022; pp. 147–162. [Google Scholar]
- Kajihara, T.; Nakamura, S.; Iwanaga, N.; Oshima, K.; Takazono, T.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Kohno, N.; Kohno, S. Clinical characteristics and risk factors of enterococcal infections in Nagasaki, Japan: A retrospective study. BMC Infect. Dis. 2015, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- Ezechukwu, I.; Singal, M.; Igbinosa, O. Aerococcus Viridans: Case report, microbiology, and literature review. Am. J. Case Rep. 2019, 20, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main groups of microorganisms of relevance for food safety and stability. In Innovative Technologies for Food Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 53–107. [Google Scholar]
- Bartowsky, E.J. Wines. Malolactic Fermentation. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 800–804. [Google Scholar]
- Fusco, V.; Quero, G.M.; Cho, G.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Maeno, S.; Tanizawa, Y.; Kneifel, W.; Arita, M.; Dicks, L.; Salminen, S. Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl. Environ. Microbiol. 2018, 84, e01290-18. [Google Scholar] [CrossRef] [Green Version]
- Guindo, C.O.; Morsli, M.; Bellali, S.; Drancourt, M.; Grine, G. A Tetragenococcus halophilus human gut isolate. Curr. Res. Microb. Sci. 2022, 3, 100112. [Google Scholar] [CrossRef]
- Fu, S.; Xia, W.; Wang, Q.; Rahman, M.M.; Hao, J.; Ye, S.; Liu, Y.; Li, R. Genomic characterization and pathogenicity analysis of the probiotic Vagococcus lutrae strain VL-18 causing severe skin lesions in warm-blooded animals. Aquaculture 2020, 523, 735166. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.; Jung, S.; Chae, S. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [Green Version]
- Touret, T.; Oliveira, M.; Semedo-Lemsaddek, T. Putative probiotic lactic acid bacteria isolated from sauerkraut fermentations. PLoS ONE 2018, 13, e0203501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented foods: Their health-promoting components and potential effects on gut microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, A.; Rad, A.H.; Ghasempour, Z.; Sabahi, S.; Kafil, H.S.; Hasannezhad, P.; Rahbar Saadat, Y.; Shahbazi, N. The biological activities of postbiotics in gastrointestinal disorders. Crit. Rev. Food Sci. Nutr. 2022, 62, 5983–6004. [Google Scholar] [CrossRef]
- Rad, A.H.; Abbasi, A.; Kafil, H.S.; Ganbarov, K. Potential Pharmaceutical and Food Applications of Postbiotics: A Review. Curr. Pharm. Biotechnol. 2020, 21, 1576–1587. [Google Scholar] [CrossRef]
- Sahadeva, R.P.K.; Leong, S.F.; Chua, K.H.; Tan, C.H.; Chan, H.Y.; Tong, E.V.; Wong, S.Y.W.; Chan, H.K. Survival of commercial probiotic strains to pH and bile. Int. Food Res. J. 2011, 18, 1515–1522. [Google Scholar]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current trends in food and pharmaceutical industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Nout, M.J.R. Food Technologies: Fermentation. In Encyclopedia of Food Safety; Foods, Materials, Technologies and Risks; Holt, S., Phadnis, R., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 3, pp. 168–177. [Google Scholar]
- Sharma, R.; Bagrodia, D.; Pandey, M.; Sanodiya, B.S.; Thakur, G.S. Efficacy and potential of lactic acid bacteria modulating human health. Int. J. Pharma Bio Sci. 2012, 3, 935–948. [Google Scholar]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavor: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef] [Green Version]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Eiteman, M.A.; Ramalingam, S. Microbial production of lactic acid. Biotechnol. Lett. 2015, 37, 955–972. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Xu, Q.; Zang, Y.; Zhou, J.; Liu, P.; Li, X.; Yong, Q.; Ouyang, J. Highly efficient production of D-lactic acid from chicory-derived inulin by lactobacillus bulgaricus. Bioprocess Biosyst. Eng. 2016, 39, 1749–1757. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from lactic acid bacteria. A powerful alternative as antimicrobials, probiotics, and Immunomodulators in veterinary medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D. Bacteriocins from lactobacillus plantarum production, genetic organization and mode of action: Produção, organização genética E modo de ação. Braz. J. Microb. 2009, 40, 209–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumariya, R.; Garsa, A.; Rajput, Y.; Sood, S.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microbial. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Gwak, J.; Kamarajan, P.; Fenno, J.; Rickard, A.; Kapila, Y. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [Green Version]
- Mauriello, G. Control of microbial activity using antimicrobial packaging. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 141–152. [Google Scholar]
- Negash, A.W.; Tsehai, B.A. Current applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Geitenes, S.; Veit, M.R.; Sarmento, C.M.; Colla, E. Growth inhibition of lactic acid bacteria in ham by nisin: A model approach. Meat Sci. 2014, 98, 744–752. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ben Slima, S.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Ben Salah, R. Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Cao, P.; Jin, Y.; Pan, D.; Zeng, X.; Guo, Y. Characterization of probiotic bacteria involved in fermented milk processing enriched with folic acid. J. Dairy Sci. 2017, 100, 4223–4229. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Kariluoto, S.; Aittamaa, M.; Korhola, M.; Salovaara, H.; Vahteristo, L.; Piironen, V. Effects of yeasts and bacteria on the levels of folates in rye sourdoughs. Int. J. Food Microbiol. 2006, 106, 137–143. [Google Scholar] [CrossRef]
- Greppi, A.; Hemery, Y.; Berrazaga, I.; Almaksour, Z.; Humblot, C. Ability of lactobacilli isolated from traditional cereal-based fermented food to produce folate in culture media under different growth conditions. LWT 2017, 86, 277–284. [Google Scholar] [CrossRef]
- Khalili, M.; Rad, A.; Khosroushahi, A.; Khosravi, H.; Jafarzadeh, S. Application of probiotics in folate bio-fortification of yoghurt. Probiotics Antimicrob. 2020, 12, 756–763. [Google Scholar] [CrossRef]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of vitamin B2 (Riboflavin) by microorganisms: An overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-Date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanalibaba, P.; Cakmak, G.A. Exopolysaccharides production by lactic acid bacteria. Appl. Microbiol. 2016, 2, 1000115. [Google Scholar] [CrossRef]
- Singh, P.; Saini, P. Food and health potentials of Exopolysaccharides derived from lactobacilli. Microbiol. Res. J. Int. 2017, 22, 1–14. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Lu, L.; Liu, Y.; Wang, F.; Xiao, M. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Biores. Technol. 2010, 101, 5528–5533. [Google Scholar] [CrossRef]
- Flemming, H. EPS—Then and now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Majee, S.; Avlani, D.; Gopa, R.; Biswas, G. Rheological behaviour and pharmaceutical applications of bacterial exopolysaccharides. J. App. Pharm. Sci. 2017, 7, 224–232. [Google Scholar]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Baruah, R.; Das, D. Heteropolysaccharides from lactic acid bacteria: Current trends and applications. J. Prob. Health 2016, 4, 141. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic acid bacteria Exopolysaccharides producers: A sustainable tool for functional foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Improvement of the texture of yogurt by use of Exopolysaccharide producing lactic acid bacteria. BioMed Res. Int. 2016, 2016, 7945675. [Google Scholar] [CrossRef] [Green Version]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Jooyandeh, H.; Falah, F.; Vasiee, A. Gamma-aminobutyric acid production by Lactobacillus brevis A3: Optimization of production, antioxidant potential, cell toxicity, and antimicrobial activity. Food Sci. Nutr. 2020, 8, 5330–5339. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [Green Version]
- Lyu, C.; Liu, L.; Huang, J.; Zhao, W.; Hu, S.; Mei, L.; Yao, S. Biosynthesis of γ-aminobutyrate by engineered lactobacillus brevis cells immobilized in gellan gum gel beads. J. Biosci. Bioeng. 2019, 128, 123–128. [Google Scholar] [CrossRef]
- Sahab, N.R.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.; Poluektova, E.; Dyachkova, M.; Klimina, K.; Kovtun, A.; Averina, O.; Orlova, V.; Danilenko, V. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef]
- Coolbear, T.; Weimer, B.; Wilkinson, M.G. Lactic acid bacteria | lactic acid bacteria in flavor development. Encyclopedia. Dairy Sci. 2011, 73, 160–165. [Google Scholar]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [Green Version]
- Cano-Contreras, A.D.; Minero Alfaro, I.J.; Medina López, V.M.; Amieva Balmori, M.; Remes Troche, J.M.; Espadaler Mazo, J.; Perez Lopez, N. Efficacy of i3.1 probiotic on improvement of lactose intolerance symptoms. J. Clin. Gastroenterol. 2020, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sen, M. Role of probiotics in health and disease—A review. Int. J. Adv. Life Sci. Res. 2019, 2, 1–11. [Google Scholar]
- Gyawali, R.; Oyeniran, A.; Zimmerman, T.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. A comparative study of extraction techniques for maximum recovery of β-galactosidase from the yogurt bacterium Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Res. 2020, 87, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Pakdaman, M.N.; Udani, J.K.; Molina, J.P.; Shahani, M. The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance-a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 2015, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Roškar, I.; Švigelj, K.; Štempelj, M.; Volfand, J.; Štabuc, B.; Malovrh, Š.; Rogelj, I. Effects of a probiotic product containing Bifidobacterium animalis subsp. animalis IM386 and Lactobacillus plantarum MP2026 in lactose intolerant individuals: Randomized, placebo-controlled clinical trial. J. Funct. Foods 2017, 35, 1–8. [Google Scholar] [CrossRef]
- Ahn, S.I.; Kim, M.S.; Park, D.G.; Han, B.K.; Kim, Y.J. Effects of probiotics administration on lactose intolerance in adulthood: A meta-analysis. J. Dairy Sci. 2023, 106, 4489–4501. [Google Scholar] [CrossRef]
- Zhou, P.; Bian, W.; Li, Y.; Chang, L.; Wang, J.; Feng, X. Prevalence of malnutrition based on GLIM criteria for completeness of diagnosis and future risk of malnutrition based on current malnutrition diagnosis: Systematic review and meta-analysis. Front. Nutr. 2023, 10, 1174945. [Google Scholar]
- Wang, J.; Ke, H.; Liu, K.X.; Qu, J.M. Effects of exogenous probiotics on the gut microbiota and clinical outcomes in critically ill patients: A randomized controlled trial. Ann. Palliat. Med. 2021, 10, 1180–1190. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Batool, M.; Kowalczewski, P.L.; Shehzad, Q.; Usman, M.; Manzoor, M.F.; Zahra, S.M.; Yacub, A.; Aadil, R.M. Nutritional and health potential of probiotics: A review. Appl. Sci. 2021, 11, 11204. [Google Scholar] [CrossRef]
- Sanjukta, M.; Swastik, A. A brief overview on probiotics: The health friendly microbes. Biomed Pharmacol. J. 2021, 14, 1869–1880. [Google Scholar]
- Liu, X.; Zhang, Y.; Chu, J. Effect of probiotics on the nutritional status of severe stroke patients with nasal feeding that receive enteral nutrition: A protocol for systematic review and meta-analysis of randomized controlled trials. Medicine 2021, 100, e25657. [Google Scholar] [CrossRef] [PubMed]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbinski, M.; Gantzel, J.; Cukrowska, B. The effectiveness of synbiotic preparation containing lactobacillus and bifidobacterium probiotic strains and short chain fructooligosaccharides in patients with diarrhea predominant irritable bowel syndrome—A randomized double-blind, placebo-controlled study. Nutrients 2020, 12, 1999. [Google Scholar]
- de Castro Soares, G.G.; Marinho, C.H.; Pitol, R.; Andretta, C.; Oliveira, E.; Martins, C.; Riella, M.C. Sporulated Bacillus as alternative treatment for diarrhea of hospitalized adult patients under enteral nutrition: A pilot randomized controlled study. Clin. Nutr. ESPEN 2017, 22, 13–18. [Google Scholar] [CrossRef]
- Lee, Z.Y.; Lew, C.C.H.; Ortiz-Reyes, A.; Patel, J.J.; Wong, Y.J.; Loh, C.T.I.; MArtindale, R.G.; Heyland, D.K. Benefits and harm of probiotics and synbiotics in adult critically ill patients. A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Clin. Nutr. 2023, 42, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Kalam, A.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Zhou, L.; Wang, Q.; Zheng, G.; Su, S. Effect of Compound Lactic Acid Bacteria Capsules on the Small Intestinal Bacterial Overgrowth in Patients with Depression and Diabetes: A Blinded Randomized Controlled Clinical Trial. Dis. Markers 2022, 2022, 6721695. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef] [Green Version]
- Cazorla, S.I.; Maldonado-Galdeano, C.; Weill, R.; De Paula, J.; Perdigón, G.D. Oral administration of probiotics increases paneth cells and intestinal antimicrobial activity. Front. Microbiol. 2018, 9, 736. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [Green Version]
- Batra, P.; Soni, K.D.; Mathur, P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: An updated systematic review and meta-analysis of randomized control trials. J. Intensive Care 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Asghari, R.; Abri, R.; Shadvar, K.; Sanaie, S. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: A prospective double-blind randomized controlled trial. Nutr. Clin. Pract. 2019, 34, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xie, Z.; Zhang, Y.; Liu, Y.; Niu, A.; Liu, Y.; Zhang, L.; Guan, L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- BAPEN. BAPEN Publishes Results of Biggest Malnutrition Survey Ever Undertaken (Scotland). Available online: https://www.bapen.org.uk/media-centre/press-releases/377-bapen-publishes-results-of-biggest-malnutrition-survey-ever-undertaken-scotland (accessed on 14 December 2020).
- Zaher, S. Nutrition and the gut microbiome during critical illness: A new insight of nutritional therapy. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2020, 26, 290. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.A.; Soliman, M.M.; Abo-Zeid, M.A.; Shaaban, M.I. Effect of AntiInflammatory and Antimicrobial Cosupplementations on Sepsis Prevention in Critically Ill Trauma Patients at High Risk for Sepsis. Front. Pharmacol. 2021, 12, 792741. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Kabata, D.; Shintani, A.; Tasaki, O.; Ojima, M.; Ikeda, M.; Shimazu, T. Association of prophylactic synbiotics with reduction in diarrhea and pneumonia in mechanically ventilated critically ill patients: A propensity score analysis. J. Infect. Chemother. 2018, 24, 795–801. [Google Scholar] [CrossRef]
- Sun, X.; Shi, J.; Kong, L.; Shen, Q.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Recent insights into the hepatoprotective effects of lactic acid bacteria in alcoholic liver disease. Trends Food Sci. Technol. 2022, 125, 91–99. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef] [Green Version]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The effect of probiotics (MCP® BCMC® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic bacteria: A promising tool in cancer prevention and therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Wang, Y.; Huang, Y.; Cui, Y.; Xia, L.; Rao, Z.; Rao, Z.; ZHou, Y.; Wu, X. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: A prospective randomized and controlled trial. Medicine 2017, 96, e8418. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Hupp, T.; Duchnowska, R.; Marek-Trzonkowska, N.; Połom, K. Next-generation probiotics–do they open new therapeutic strategies for cancer patients? Gut Microbes 2022, 14, 2035659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Meta-analysis of randomized controlled trials of the effects of probiotics on type 2 diabetes in adults. Clin. Nutr. 2022, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Khalili, L.; Alipour, B.; Asghari Jafarabadi, M.; Hassanalilou, T.; Mesgari Abbasi, M.; Faraji, I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: A randomized controlled trial. Diabetol. Metab. Syndr. 2019, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mirjalili, M.; Sharif, A.S.; Sangouni, A.A.; Emtiazi, H.; Mozaffari-Khosravi, H. Effect of probiotic yogurt consumption on glycemic control and lipid profile in patients with type 2 diabetes mellitus: A randomized controlled trial. Clin. Nutr. ESPEN 2023, 54, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Nazari, B.; Amani, L.; Ghaderi, L.; Khanbabayi Gol, M. Effects of probiotics on prevalence of ventilator-associated pneumonia in multitrauma patients hospitalized in neurosurgical intensive care unit: A randomized clinical trial. Trauma Mon. 2020, 25, 262–268. [Google Scholar]
- Anandaraj, A.M.; Pichamuthu, K.K.; Hansdak, S.G.; Samuel, P.; Irodi, A.; Valsa, S.; Peter, J.V. A Randomised Controlled Trial of Lactobacillus in the Prevention of Ventilator Associated Pneumonia. J. Clin. Diagn. Res. 2019, 13, 1–4. [Google Scholar] [CrossRef]
- Johnstone, J.; Meade, M.; Lauzier, F.; Marshall, J.; Duan, E.; Dionne, J.; Arabi, Y.; Heels-Ansdell, D.; Thabane, L.; Lamarche, D.; et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: A randomized clinical trial. JAMA 2021, 326, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Katkowska, M.; Garbacz, K.; Kusiak, A. Probiotics: Should all patients take them? Microorganisms 2021, 9, 2620. [Google Scholar] [CrossRef] [PubMed]
- Purdel, C.; Ungurianu, A.; Adam-Dima, I.; Margină, D. Exploring the potential impact of probiotic use on drug metabolism and efficacy. Biomed. Pharmacother. 2023, 161, 114468. [Google Scholar] [CrossRef]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
| Therapeutic Effects | Lactic Acid Bacteria (LAB) Strain | Remarks | References |
|---|---|---|---|
| Lactose intolerance | Lactobacillus acidophilus, | Method: Supplementation L. acidophilus Results: Abdominal symptom (LAB < control) | [96] |
| B. animalis, Lactobacillus plantarum | Methods: Supplementation of B. animalis Results: Abdominal symptoms (no significant difference) | [84] | |
| Lactobacillus plantarum, P. acidilactici | Method: Supplementation of Lactobacillus plantarum and P. acidilactici among lactose intolerance patients Results: Total symptom score of lactose intolerance (LAB < control) | [93] | |
| Gastrointestinal problem: diarrhea | Baccilus cereus | Method: Supplementation of 20 mL/day Baccilus cereus or soluble fiber (control) among patients with diarrhea on enteral feeding Results: Ceasing the diarrhea incident (no significant difference), duration to stop diarrhea (B. Cerius group < control) | [105] |
| Lactobacillus rhamnosus, Lactobacillus acidophilus, Bifidobacterium lactis, Bifidobacterium longum, Bifidobacterium bifidum | Method: Synbiotics supplementation among diarrhea-dominant IBS for 8 weeks Results: After intervention, feeling of incomplete bowel movements, flatulence, pain, stool pressure, and diarrheal stools (synbiotics group < control) | [104] | |
| Immunomodulatory effect | Bifidobacterium breve, Lactobacillus casei | Method: 3 g supplementation of synbiotics (Bifidobacterium breve and Lactobacillus casei) within 3 days after admission Results: Enteritis and penumonia incidence lowered in synbiotics group compared to control | [118] |
| Lactobacillus and Streptococcus lactis | Method: Lactic acid bacteria capsule among depression and diabetes patient Result: Reduction of self-rating anxiety scale, IL-2 and TNF-α, fasting plasma (LAB > control), and increment of CD+4 (LAB > control) Adverse effect LAB < control) | [108] | |
| Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophiles | Methods: Supplementation of 1 capsule/12 h among VAP multi-trauma patients Results: VAP (intervention group < control) | [130] | |
| Lactobacillus rhamnosus | Method: Supplementation of 2 × 109 Colony Forming Units (CFU) of Lactobacillus rhamnosus GG on a twice daily basis among ventilated medical ICU patients Results: VAP (no significant difference between LAB and the control) | [131] | |
| L rhamnosus GG | Method: Enteral L rhamnosus GG twice daily among patients on ventilation Results: VAP incidence (no significant difference between both the intervention group and the control) | [132] | |
| Hepatoprotective effect | Bifidobacterium animalis | Method: Supplementation of 300 g synbiotics yogurt (B. animalis and inulin) or conventional (control) among NAFLD patients Results: Grades of NAFLD (synbiotics group < control), reduction in serum concentration of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and γ-glutamyltransferase (synbiotics group > control) | [121] |
| Lactobacillus, Bifidobacterium | Methods: Supplementation of probiotics sachet or placebo for 6 months among NAFLD patients Results: No significant difference in LiverFAST analysis (steatosis, fibrosis, and inflammation scores), alanine aminotransferase | [122] | |
| Treatment of cancer | Bifidobacterium, lactobacillus | Method: Gastric cancer patient receiving fiber-free nutrition formula (FF group), fiber-enriched nutrition formula (FE group), and fiber- and probiotic-enriched nutrition formula (FEp group) Results: The FEP group had the lowest number of diarrhea and intestinal disorders. No significant difference in the lymphocyte count, albumin, prealbumin, and transferrin levels | [125] |
| Bifidobacteria, Lactobacillus | Method: Supplementation of probiotics + glucose solution or glucose solution (control) among colorectal cancer patients undergoing radical resection Results: Increase in intestinal micro-ecological environment and strengthening of the intestinal mucosal barrier function (glucose solution + probiotic group > glucose group), duration of early recovery of inflammatory response (glucose solution + probiotic group > glucose group) | [120] | |
| Glycemic control | Lactobacillus casei | Method: 108 CFU of L. casei supplementation for 8 weeks among type 2 diabetes mellitus Result: Serum fetuin-A level, fasting blood sugar, insulin concentration, and insulin resistance significantly decrease among L. casei supplementation compared to the control | [128] |
| Lactobacillus acidophilus, Bifidobacterium lactis | Methods: 200 g/d yogurt containing probiotic 4.65 × 106 CFU/g or placebo group received 200 g/d conventional yogurt Results: No significant different in fasting plasma glucose (FPG), hemoglobin A1c (HbA1c) | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. https://doi.org/10.3390/foods12152850
Abdul Hakim BN, Xuan NJ, Oslan SNH. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods. 2023; 12(15):2850. https://doi.org/10.3390/foods12152850
Chicago/Turabian StyleAbdul Hakim, Bibi Nabihah, Ng Jia Xuan, and Siti Nur Hazwani Oslan. 2023. "A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry" Foods 12, no. 15: 2850. https://doi.org/10.3390/foods12152850
APA StyleAbdul Hakim, B. N., Xuan, N. J., & Oslan, S. N. H. (2023). A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods, 12(15), 2850. https://doi.org/10.3390/foods12152850







