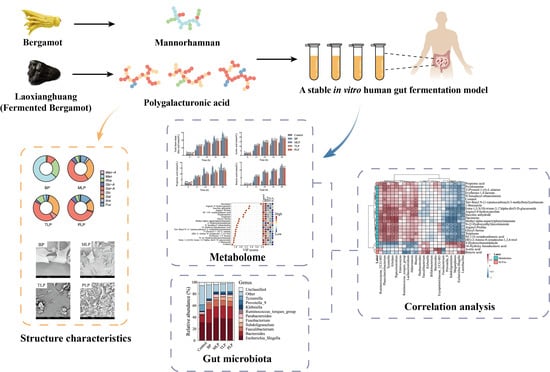
Comparative Study on In Vitro Fermentation Characteristics of the Polysaccharides Extracted from Bergamot and Fermented Bergamot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Polysaccharides
2.3. Structural Characterization
2.4. In Vitro Fermentation of Polysaccharides
2.5. DNA Extraction and 16S rRNA Gene Sequencing
2.6. Determination of SCFAs
2.7. Metabolomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Structural Characterization
3.2. Effect of Polysaccharides on Gut Microbiota
3.3. SCFAs Production
3.4. Effects of Polysaccharides on Metabolic Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, T.; Sun, R.J.; Zhang, Y.C.; Zhang, C.; Wang, Y.J.; Wang, Z.A.; Du, Y.G. Recent Research and Application Prospect of Functional Oligosaccharides on Intestinal Disease Treatment. Molecules 2022, 27, 7622. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Ramirez-Moreno, E.; Quintero-Lira, A.; Contreras-Lopez, E.; Jaimez-Ordaz, J.; Castaneda-Ovando, A.; Anorve-Morga, J.; Calderon-Ramos, Z.G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health-Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 28. [Google Scholar]
- Yu, Y.; Shen, M.Y.; Song, Q.Q.; Xie, J.H. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Tao, W.Y.; Wang, M.Z.; Liu, W.; Xing, J.R.; Yang, Y. Dendrobium officinale Xianhu 2 polysaccharide helps forming a healthy gut microbiota and improving host immune system: An in vitro and in vivo study. Food Chem. 2023, 401, 134211. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Liang, F.; Zhang, Y.; Pan, Y. Water-soluble polysaccharides from finger citron fruits (Citrus medica L. var. sarcodactylis). Carbohydr. Res. 2014, 388, 100–104. [Google Scholar] [CrossRef]
- Peng, B.; Yang, J.; Huang, W.; Peng, D.; Bi, S.; Song, L.; Wen, Y.; Zhu, J.; Chen, Y.; Yu, R. Structural characterization and immunoregulatory activity of a novel heteropolysaccharide from bergamot (Citrus medica L. var. sarcodactylis) by alkali extraction. Ind. Crop Prod. 2019, 140, 111617. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hua, J.X.; Chen, S.X.; Zhao, L.C.; Wang, Q.; Zhou, A.M. The potential mechanisms of bergamot-derived dietary fiber alleviating high-fat diet-induced hyperlipidemia and obesity in rats. Food Funct. 2022, 13, 8228–8242. [Google Scholar] [CrossRef]
- Yaqun, L.; Hanxu, L.; Wanling, L.; Yingzhu, X.; Mouquan, L.; Yuzhong, Z.; Lei, H.; Yingkai, Y.; Yidong, C. SPME-GC-MS combined with chemometrics to assess the impact of fermentation time on the components, flavor, and function of Laoxianghuang. Front. Nutr. 2022, 9, 915776. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Luo, D.H.; Lin, L.Z.; Lu, X.Y.; Gao, J.; Xiao, C.Q.; Zhao, M.M. Effect of Bergamot and Laoxianghuang Polysaccharides on Gut Microbiota Derived from Patients with Hyperlipidemia: An Integrative Analysis of Microbiome and Metabolome during In Vitro Fermentation. Foods 2022, 11, 2039. [Google Scholar] [CrossRef]
- Wan, C.; Wu, K.Z.; Lu, X.Y.; Fang, F.; Li, Y.Q.; Zhao, Y.M.; Li, S.B.; Gao, J. Integrative Analysis of the Gut Microbiota and Metabolome for In Vitro Human Gut Fermentation Modeling. J. Agric. Food Chem. 2021, 69, 15414–15424. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, L.Z.; Sun, B.G.; Zhao, M.M. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Lin, L.Z.; Chen, Z.J.; Cai, Y.J.; Xiao, C.Q.; Zhou, F.B.; Sun, B.G.; Zhao, M.M. In Vitro Digestion and Fermentation of Three Polysaccharide Fractions from Laminaria japonica and Their Impact on Lipid Metabolism-Associated Human Gut Microbiota. J. Agric. Food Chem. 2019, 67, 7496–7505. [Google Scholar] [CrossRef]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of untargeted metabolomics approach and molecular networking analysis to identify unique natural components in wild Morchella sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Xu, T.; Zou, X.; Mao, R.; Pi, X.; Wu, W.; Huang, L.; Yang, K.; Zeng, X.; et al. Digestive Characteristics of Hericium erinaceus Polysaccharides and Their Positive Effects on Fecal Microbiota of Male and Female Volunteers during in vitro Fermentation. Front. Nutr. 2022, 9, 858585. [Google Scholar] [CrossRef]
- Ai, J.; Yang, Z.; Liu, J.; Schols, H.A.; Battino, M.; Bao, B.; Tian, L.; Bai, W. Structural Characterization and In Vitro Fermentation Characteristics of Enzymatically Extracted Black Mulberry Polysaccharides. J. Agric. Food Chem. 2022, 70, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; He, Y.; Yuan, Q.; Wang, S.P.; Gan, R.Y.; Hu, Y.C.; Zou, L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocolloids 2022, 132, 107897. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Huang, L.; Wu, D.T.; Li, J.; Lei, J.; Fu, M.X.; Zhang, Q.; Qin, W. Catabolism of Dictyophora indusiata Polysaccharide and Its Impacts on Gut Microbial Composition during In Vitro Digestion and Microbial Fermentation. Foods 2023, 12, 1909. [Google Scholar] [CrossRef]
- Tian, C.C.; Xu, H.; Li, J.; Han, Z. Characteristics and intestinal immunomodulating activities of water-soluble pectic polysaccharides from Chenpi with different storage periods. J. Sci. Food Agric. 2018, 98, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.Y.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Wang, J.; Li, J.; Hu, J.L.; Yan, H.L.; Zhao, J.L.; Zou, L.; Hu, Y.C. Physicochemical properties and biological functions of soluble dietary fibers isolated from common and Tartary buckwheat sprouts. LWT Food Sci. Technol. 2023, 183, 11494. [Google Scholar] [CrossRef]
- Zielinska, S.; Staniszewska, I.; Cybulska, J.; Zdunek, A.; Szymanska-Chargot, M.; Zielinska, D.; Liu, Z.L.; Pan, Z.L.; Xiao, H.W.; Zielinska, M. Modification of the cell wall polysaccharides and phytochemicals of okra pods by cold plasma treatment. Food Hydrocolloids 2022, 131, 10. [Google Scholar] [CrossRef]
- Mahdi, A.S.; Abd, A.M.; Awad, K.M. The Role of Nano-selenium in Alleviating the Effects of Salt Stress in Date Palm Trees (Phoenix dactylifera L.): A Fourier Transform Infrared (FTIR) Spectroscopy Study. BioNanoScience 2023, 13, 74–80. [Google Scholar]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jimenez-Escrig, A.; Ruperez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Leng, X.P.; Li, J.Z.; Miao, W.J.; Liu, Y.X.; Haider, M.S.; Song, M.; Fang, J.G.; Li, Q. Comparison of physicochemical characteristics, antioxidant and immunomodulatory activities of polysaccharides from wine grapes. Int. J. Biol. Macromol. 2023, 239, 11. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Li, T.; Zhang, W.; Fan, M.; Zhang, K.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. Comparison of Different Soluble Dietary Fibers during the In Vitro Fermentation Process. J. Agric. Food Chem. 2021, 69, 7446–7457. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Pers. Med. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Biophys. Acta Rev. Cancer. 2021, 1875, 188494. [Google Scholar]
- Gao, J.M.; Rao, J.H.; Wei, Z.Y.; Xia, S.Y.; Huang, L.; Tang, M.T.; Hide, G.; Zheng, T.T.; Li, J.H.; Zhao, G.A.; et al. Transplantation of Gut Microbiota from High-Fat-Diet-Tolerant Cynomolgus Monkeys Alleviates Hyperlipidemia and Hepatic Steatosis in Rats. Front. Microbiol. 2022, 13, 876043. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Chen, J.W.; Yao, H.R.; Hu, H. Fusobacterium and Colorectal Cancer. Front. Oncol. 2018, 8, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaisen, M.; Flatberg, A.; Granlund, A.v.B.; Røyset, E.S.; Martinsen, T.C.; Sandvik, A.K.; Fossmark, R. Bacterial Mucosa-associated Microbiome in Inflamed and Proximal Noninflamed Ileum of Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2021, 27, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.C.; Major, G.A.; Spiller, R.C.; Kuehne, S.A.; Minton, N.P. Coinfection and Emergence of Rifamycin Resistance during a Recurrent Clostridium difficile Infection. J. Clin. Microbiol. 2016, 54, 2689–2694. [Google Scholar] [CrossRef] [Green Version]
- Nogal, A.; Louca, P.; Zhang, X.Y.; Wells, P.M.; Steves, C.J.; Spector, T.D.; Falchi, M.; Valdes, A.M.; Menni, C. Circulating Levels of the Short-Chain Fatty Acid Acetate Mediate the Effect of the Gut Microbiome on Visceral Fat. Front. Microbiol. 2021, 12, 711359. [Google Scholar] [CrossRef]
- Tan, Y.W.; Li, M.W.; Kong, K.Y.; Xie, Y.S.; Zeng, Z.; Fang, Z.F.; Li, C.; Hu, B.; Hu, X.J.; Wang, C.X.; et al. In vitro simulated digestion of and microbial characteristics in colonic fermentation of polysaccharides from four varieties of Tibetan tea. Food Res. Int. 2023, 163, 112255. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Human Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Chen, P.L.; Lei, S.Z.; Tong, M.Y.; Chang, Q.; Zheng, B.D.; Zhang, Y.; Zeng, H.L. Effect of polysaccharide fractions from Fortunella margarita on the fecal microbiota of mice and SCFA production in vitro. Food Sci. Human Wellness 2022, 11, 97–108. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.d.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Alshanwani, A.R.; Hagar, H.; Shaheen, S.; Alhusaini, A.M.; Arafah, M.M.; Faddah, L.M.; Alharbi, F.M.B.; Sharma, A.K.; Fayed, A.; Badr, A.M. A promising antifibrotic drug, pyridoxamine attenuates thioacetamide-induced liver fibrosis by combating oxidative stress, advanced glycation end products, and balancing matrix metalloproteinases. Eur. J. Pharmacol. 2022, 923, 174910. [Google Scholar] [CrossRef]
- Oh, S.; Ahn, H.; Park, H.; Lee, J.-I.; Park, K.Y.; Hwang, D.; Lee, S.; Son, K.H.; Byun, K. The attenuating effects of pyridoxamine on adipocyte hypertrophy and inflammation differ by adipocyte location. J. Nutr. Biochem. 2019, 72, 108173. [Google Scholar] [CrossRef]
- Li, J.; Deng, Q.Y.; Zhang, Y.Q.; Wu, D.R.; Li, G.L.; Liu, J.W.; Zhang, L.Y.; Wang, H.M.D. Three Novel Dietary Phenolic Compounds from Pickled Raphanus sativus L. Inhibit Lipid Accumulation in Obese Mice by Modulating the Gut Microbiota Composition. Mol. Nutr. Food Res. 2021, 65, 9. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Lu, X.; Fang, F.; Liu, J.; Gao, J.; Zheng, Y. Comparative Study on In Vitro Fermentation Characteristics of the Polysaccharides Extracted from Bergamot and Fermented Bergamot. Foods 2023, 12, 2878. https://doi.org/10.3390/foods12152878
Wu K, Lu X, Fang F, Liu J, Gao J, Zheng Y. Comparative Study on In Vitro Fermentation Characteristics of the Polysaccharides Extracted from Bergamot and Fermented Bergamot. Foods. 2023; 12(15):2878. https://doi.org/10.3390/foods12152878
Chicago/Turabian StyleWu, Kaizhang, Xingyu Lu, Fang Fang, Juncheng Liu, Jie Gao, and Yang Zheng. 2023. "Comparative Study on In Vitro Fermentation Characteristics of the Polysaccharides Extracted from Bergamot and Fermented Bergamot" Foods 12, no. 15: 2878. https://doi.org/10.3390/foods12152878
APA StyleWu, K., Lu, X., Fang, F., Liu, J., Gao, J., & Zheng, Y. (2023). Comparative Study on In Vitro Fermentation Characteristics of the Polysaccharides Extracted from Bergamot and Fermented Bergamot. Foods, 12(15), 2878. https://doi.org/10.3390/foods12152878