Nano Freezing–Thawing of Atlantic Salmon Fillets: Impact on Thermodynamic and Quality Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Freezing–Thawing Treatment
2.2.1. FCST (Cryogenic Freezing Integrated with 4 °C Cold Storage Thawing)
2.2.2. FMT (Cryogenic Freezing Integrated with MNPs Combined Microwave Thawing)
2.2.3. NCST (MNPs Assisted Cryogenic Freezing Integrated with Cold Storage Thawing)
2.2.4. NNMT (MNPs Assisted Cryogenic Freezing Integrated with MNPs Combined Microwave Thawing)
2.3. Thermal Characteristic Parameters Determination
2.3.1. Freezing Point and Unfreezable Water Content
2.3.2. Apparent Specific Heat
2.3.3. Glass Transition Temperature (Tg)
2.3.4. Protein Thermal Stability
2.4. WHC
2.4.1. Thawing Loss, Cooking Loss, and Centrifugation Loss
2.4.2. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.4.3. Magnetic Relaxation Image (MRI)
2.5. Color
2.6. Texture Profile Analysis (TPA) and Stress Relaxation Determination
2.7. Determination of Protein and Lipid Oxidation
2.7.1. Myofibrillar Protein (MP) Extraction
2.7.2. Protein Oxidation
2.7.3. Lipid Oxidation
2.8. Protein Denaturation
2.8.1. Particle Size
2.8.2. Solubility of Protein
2.8.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Thermal Characteristic Analysis
3.1.1. Freezing Point and Unfreezable Water Content
3.1.2. Apparent Specific Heat (Capp) and Tg
3.1.3. Protein Thermal Stability
3.2. Analysis of WHC
3.2.1. Thawing Loss, Cooking Loss, and Centrifugation Loss
3.2.2. Water Distribution
3.3. Color
3.4. TPA and Stress Relaxation Parameters Analysis
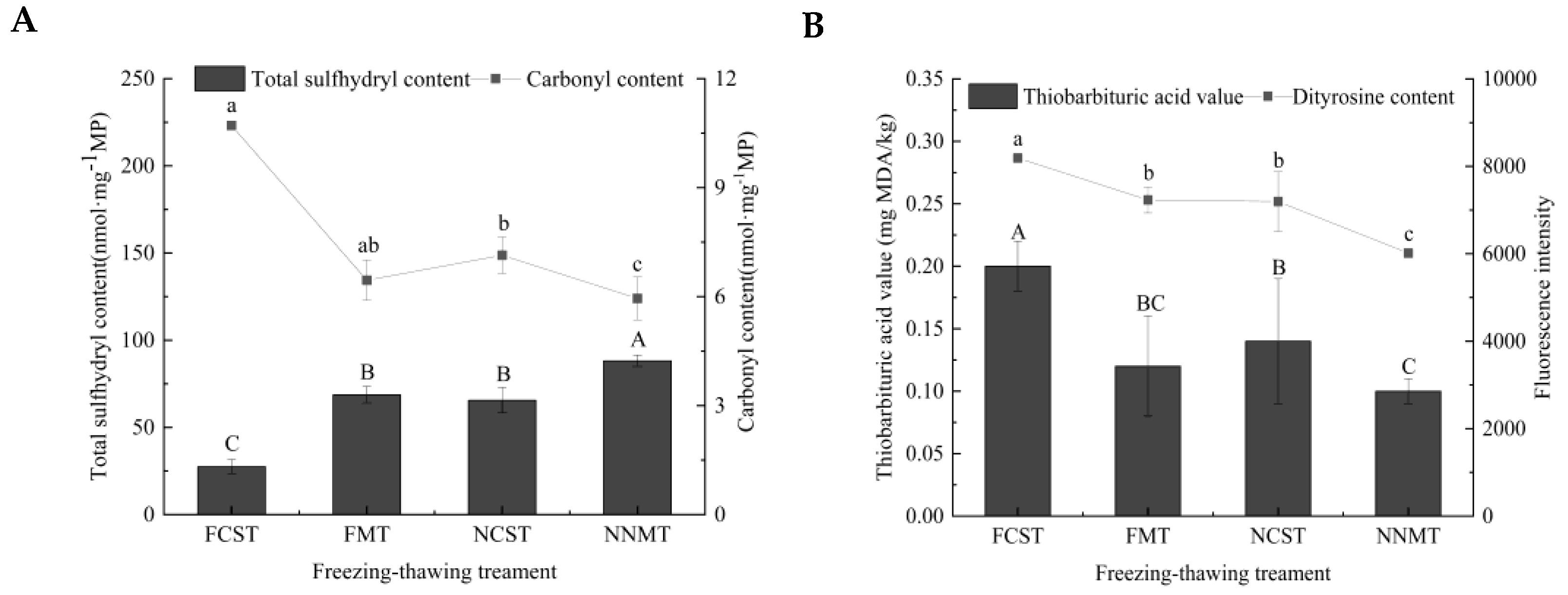
3.5. Analysis of Protein and Lipid Oxidation
3.5.1. Protein Oxidation
3.5.2. Lipid Oxidation
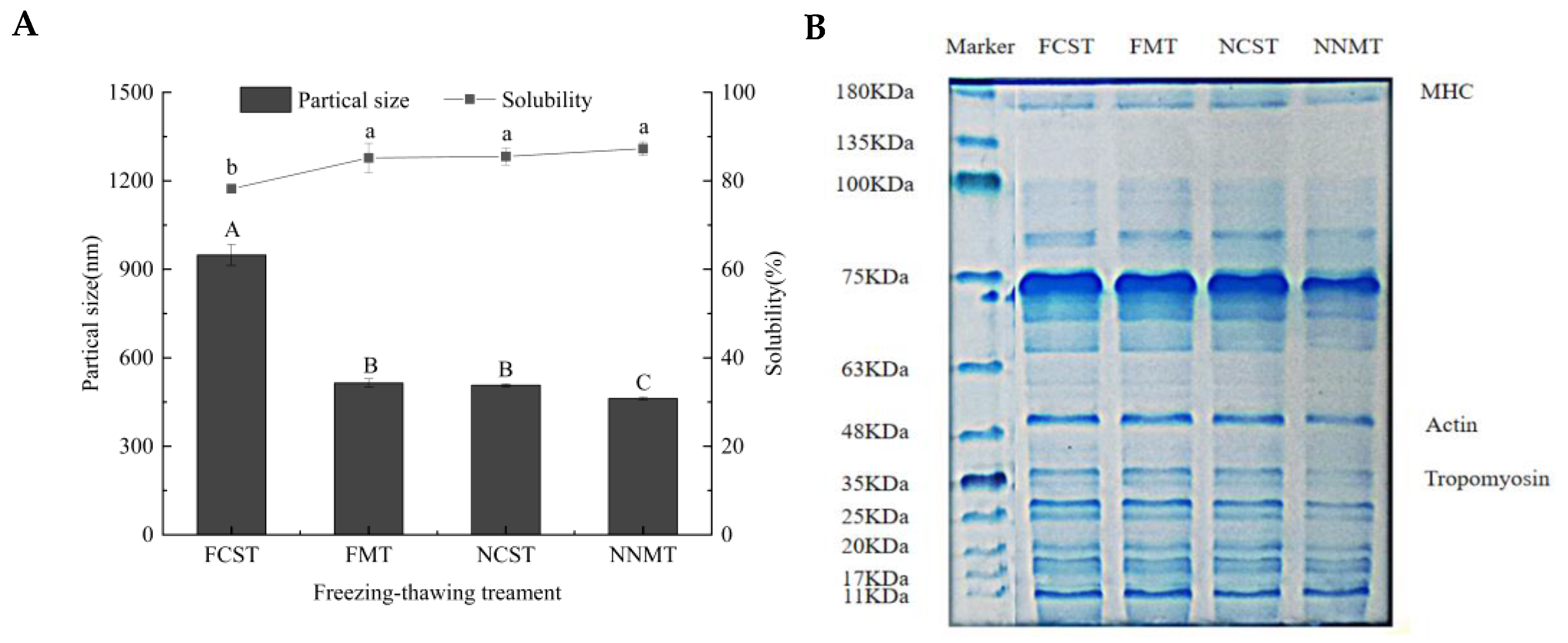
3.6. Analysis of Protein Aggregation and Degradation
3.6.1. Particle Size
3.6.2. Solubility of Protein
3.6.3. SDS-PAGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wang, H.; Lu, W.; Zhang, M.; Xue, J.; Yu, X.; Wang, H. Low-salted salmon: Effects of salt reduction on physicochemical, lipidomic, and sensory characteristics. LWT 2021, 152, 112311. [Google Scholar] [CrossRef]
- Dawson, P.; Jeddawi, W.A.; Remington, N. Effect of freezing on the shelf life of Salmon. Int. J. Food Sci. 2018, 2018, 1686121. [Google Scholar] [CrossRef] [Green Version]
- Tinacci, L.; Armani, A.; Guidi, A.; Nucera, D.; Shvartzman, D.; Miragliotta, V.; Abramo, F. Histological discrimination of fresh and frozen/thawed fish meat: European hake (Merluccius merluccius) as a possible model for white meat fish species. Food Control 2018, 92, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Chen, G.; Liu, G.; Huang, X.; Xu, X.; Li, L.; Abd El-Aty, A.M. A review on recent advances in the applications of composite Fe3O4 magnetic nanoparticles in the food industry. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Hornowski, T.; Kubovcikova, M.; Timko, M.; Koralewski, M.; Jozefczak, A. Heating induced by therapeutic ultrasound in the presence of magnetic nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 11554–11564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guan, W.; Cai, L.; Wang, X.; Zhang, Z.; Ni, Z. Effects of magnetic nanometer combined with radio frequency or microwave thawing on physicochemical properties of myofibrillary protein in sea bass. LWT-Food Sci. Technol. 2022, 154, 112585. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, H.; Han, M.; Shan, C.; Bu, Y.; Li, J.; Li, X. Evaluating the effects of nanoparticles combined ultrasonic-microwave thawing on water holding capacity, oxidation, and protein conformation in jumbo squid (Dosidicus gigas) mantles. Food Chem 2022, 402, 134250. [Google Scholar] [CrossRef]
- Cai, L.; Nian, L.; Zhao, G.; Zhang, Y.; Sha, L.; Li, J. Effect of herring antifreeze protein combined with chitosan magnetic nanoparticles on quality attributes in red sea bream (Pagrosomus major). Food Bioprocess Technol. 2019, 12, 409–421. [Google Scholar] [CrossRef]
- Ohkuma, C.; Kawai, K.; Viriyarattanasak, C.; Mahawanich, T.; Tantratian, S.; Takai, R.; Suzuki, T. Glass transition properties of frozen and freeze-dried surimi products: Effects of sugar and moisture on the glass transition temperature. Food Hydrocoll. 2008, 22, 255–262. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Zhang, Z.; Xu, X.; He, X. Magnetic induction heating of superparamagnetic nanoparticles during rewarming augments the recovery of hUCM-MSCs cryopreserved by vitrification. Acta Biomater. 2016, 33, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, Y.; Hajiaghalou, S.; Baniasadi, F.; Mahabadi, V.P.; Ghalamboran, M.R.; Fathi, R. Fe3O4 magnetic nanoparticles improve the vitrification of mouse immature oocytes and modulate the pluripotent genes expression in derived pronuclear-stage embryos. Cryobiology 2021, 100, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rao, J.S.; Han, Z.; Gangwar, L.; Bischof, J.C. Vitrification and nanowarming of kidneys. Cryobiology 2021, 103, 168. [Google Scholar] [CrossRef]
- Hamdami, N.; Monteau, J.Y.; Bail, A.L. Thermophysical properties evolution of French partly baked bread during freezing. Food Res. Int. 2004, 37, 703–713. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, Y.; Chen, H.; Li, Z.; Xue, C. Glass transition and state diagram for freeze-dried horse mackerel muscle. Thermochim. Acta 2009, 493, 55–60. [Google Scholar] [CrossRef]
- Li, Y.; Bu, Y.; Guo, H.; Zhu, W.; Li, J.; Li, X. The drip loss inhibitory mechanism of nanowarming in jumbo squid (Dosidicus gigas) mantles: Protein structure and molecular dynamics simulation. J. Sci. Food Agric. 2022, 102, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, Y.; Bu, Y.; Li, J.; Li, X. Effects of nanowarming on water holding capacity, oxidation and protein conformation changes in jumbo squid (Dosidicus gigas) mantles. LWT-Food Sci. Technol. 2020, 129, 109511. [Google Scholar] [CrossRef]
- Hong, H.; Zheng, Y.; Roy, W. Nanomaterials for efficiently lowering the freezing point of anti-freeze coolants. J. Nanosci. Nanotechnol. 2007, 7, 3180–3184. [Google Scholar] [CrossRef]
- Fasina, O.O. Thermophysical properties of sweet potato puree at freezing and refrigeration temperatures. Int. J. Food Prop. 2005, 8, 151–160. [Google Scholar] [CrossRef]
- Linnenkugel, S.; Paterson, A.H.J.; Huffman, L.M.; Bronlund, J.E. The effect of polysaccharide blends and salts on the glass transition temperature of the monosaccharide glucose. J. Food Eng. 2022, 322, 110961. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Zhu, L.; Fang, Z.; Shi, Q. Effect of carrier types on the physicochemical and antioxidant properties of spray-dried black mulberry juice powders. J. Food Meas. Charact. 2020, 14, 1201–1212. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Pochan, J.M.; Frechet, J.M.J. Physical properties of dendritic macromolecules: A study of glass transition temperature. Macromolecules 1993, 26, 1514–1519. [Google Scholar] [CrossRef]
- Roos, Y.H. Glass transition and re-crystallization phenomena of frozen materials and their effect on frozen food quality. Foods 2021, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz-Montoya, E.; Rinaldi, C. Synthesis and characterization of polymer nanocomposites containing magnetic nanoparticles. J. Appl. Phys. 2010, 107, 09B506. [Google Scholar] [CrossRef]
- García, I.; Tercjak, A.; Zafeiropoulos, N.E.; Stamm, M.; Mondragon, I. Self-assembling nanomaterials using magnetic nanoparticles modified with polystyrene brushes. Macromol. Rapid Commun. 2007, 28, 2361–2365. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Satarkar, N.S.; Gall, K.; Likos, R.; Hilt, J.Z. Shape-memory polymer networks with Fe3O4 nanoparticles for remote activation. J. Appl. Polym. Sci. 2009, 112, 3166–3176. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Y.; Yu, H. Research on ice crystal growth inside the vitrified Vs55 with magnetic nanoparticles during devitrification by cryomicroscopy. Chem Res. Chin. Univ. 2019, 35, 542–548. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Y.; Yang, N.; Jin, Y.; Sun, H.; Xu, X. Influence of oscillating uniform magnetic field and iron supplementation on quality of freeze-thawed surimi. RSC Adv. 2019, 9, 33163–33169. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, L.; Huang, Q.; Xiong, S.; Yin, T.; Liu, Z. Water migration, ice crystal formation, and freeze-thaw stability of silver carp surimi as affected by inulin under different additive amounts and polymerization degrees. Food Hydrocoll. 2022, 124, 107267. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wu, J.; Chen, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on metabolite profiles of tilapia (Oreochromis niloticus) fillets during storage. Food Chem. 2019, 293, 418–428. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, Y.; Gong, T.; Mei, J.; Xie, J. Effects of different thawing methods on the physicochemical changes, water migration and protein characteristic of frozen pompano (Trachinotus ovatus). J. Food Biochem. 2021, 45, 13826. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of power ultrasound in freezing and thawing Processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020, 68, 105230. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. LWT-Food Sci. Technol. 2003, 36, 625–631. [Google Scholar] [CrossRef]
- Zhan, X.; Sun, D.W.; Zhu, Z.; Wang, Q.J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: A review. Crit. Rev. Food Sci. 2018, 58, 2925–2938. [Google Scholar] [CrossRef]
- Supavititpatana, T.; Apichartsrangkoon, A. Combination effects of ultra-high pressure and temperature on the physical and thermal properties of ostrich meat sausage (yor). Meat. Sci. 2007, 76, 555–5860. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Y.; Song, Z.; Chen, X. A review of natural polysaccharides for food cryoprotection: Ice crystals inhibition and cryo-stabilization. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100291. [Google Scholar] [CrossRef]
- Sabikun, N.; Bakhsh, A.; Ismail, I.; Hwang, Y.H.; Rahman, M.S.; Joo, S.T. Changes in physicochemical characteristics and oxidative stability of pre-and post-rigor frozen chicken muscles during cold storage. J. Food Sci. Technol. 2019, 56, 4809–4816. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Niaz, N.; Ahmad, M.N.; Wang, P. Cryoprotective effect of egg white proteins and xylooligosaccharides mixture on oxidative and structural changes in myofibrillar proteins of culter alburnus during frozen storage. Int. J. Biol. Macromol. 2020, 158, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wan, J.; Li, X.; Li, J. Effects of different thawing methods on physicochemical properties and structure of largemouth bass (Micropterus salmoides). J. Food Sci. 2020, 85, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, H.; Muhammad, A.I.; Song, L.; Guo, M.; Liu, D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020, 320, 126614. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, Y.L. Comparative time-course of lipid and myofibrillar protein oxidation in different biphasic systems under hydroxyl radical stress. Food Chem. 2018, 243, 231–238. [Google Scholar] [CrossRef]
- Cai, L.; Nian, L.; Cao, A.; Zhang, Y.; Li, X. Effect of carboxymethyl chitosan magnetic nanoparticles plus herring antifreeze protein on conformation and oxidation of myofibrillar protein from red sea bream (Pagrosomus major) after freeze-thaw treatment. Food Bioprocess Tech. 2020, 13, 355–366. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, D.; Zhu, Z.; Zhang, Z. Effects of high pressure freezing (HPF) on denaturation of natural actomyosin extracted from prawn (Metapenaeus ensis). Food Chem. 2017, 229, 252–259. [Google Scholar] [CrossRef]
- Xiong, Y.; Park, D.; Ooizumi, T. Variation in the cross-linking pattern of porcine myofibrillar protein exposed to three oxidative environments. J. Agric. Food Chem. 2009, 57, 153–159. [Google Scholar] [CrossRef]
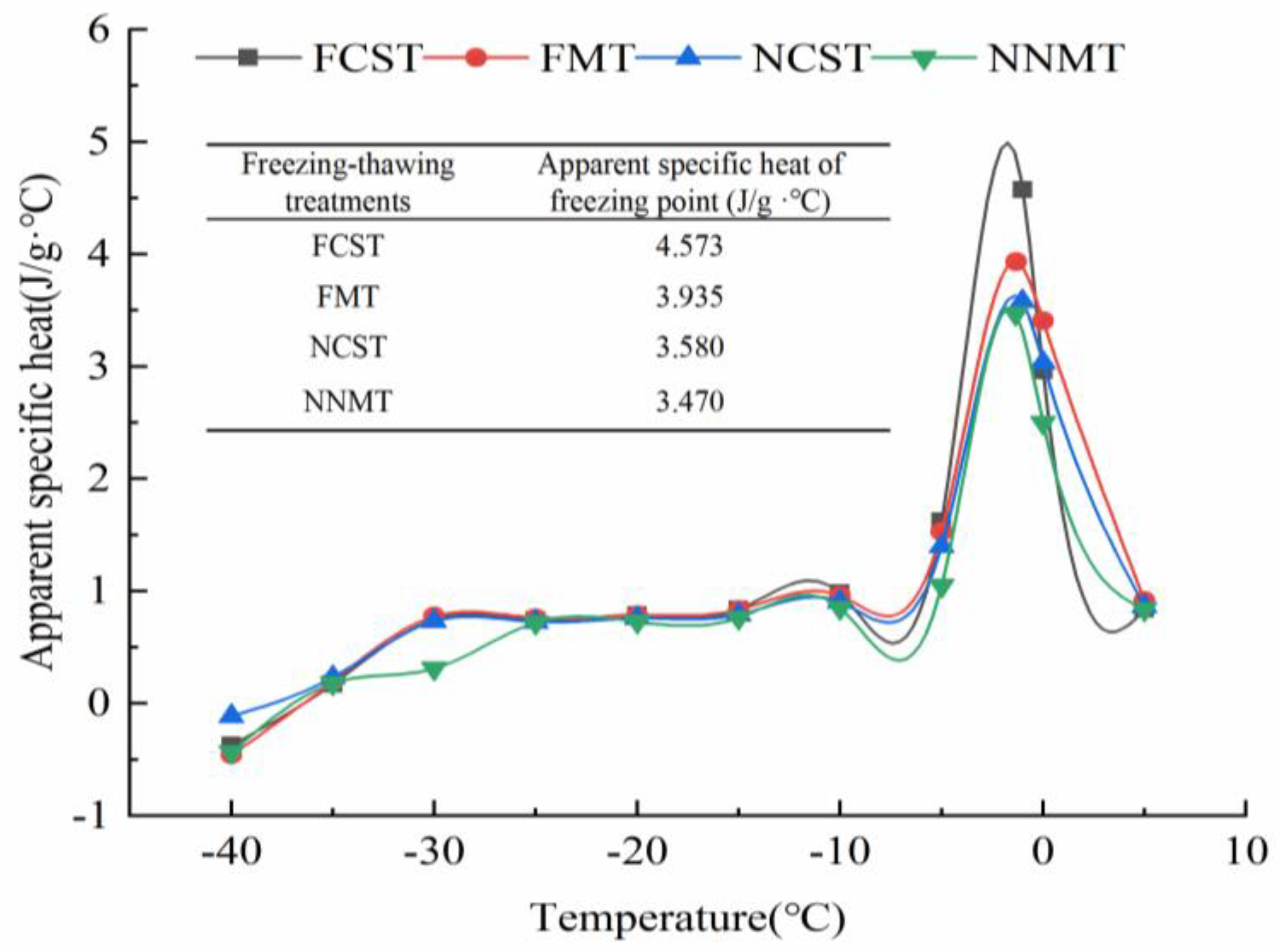
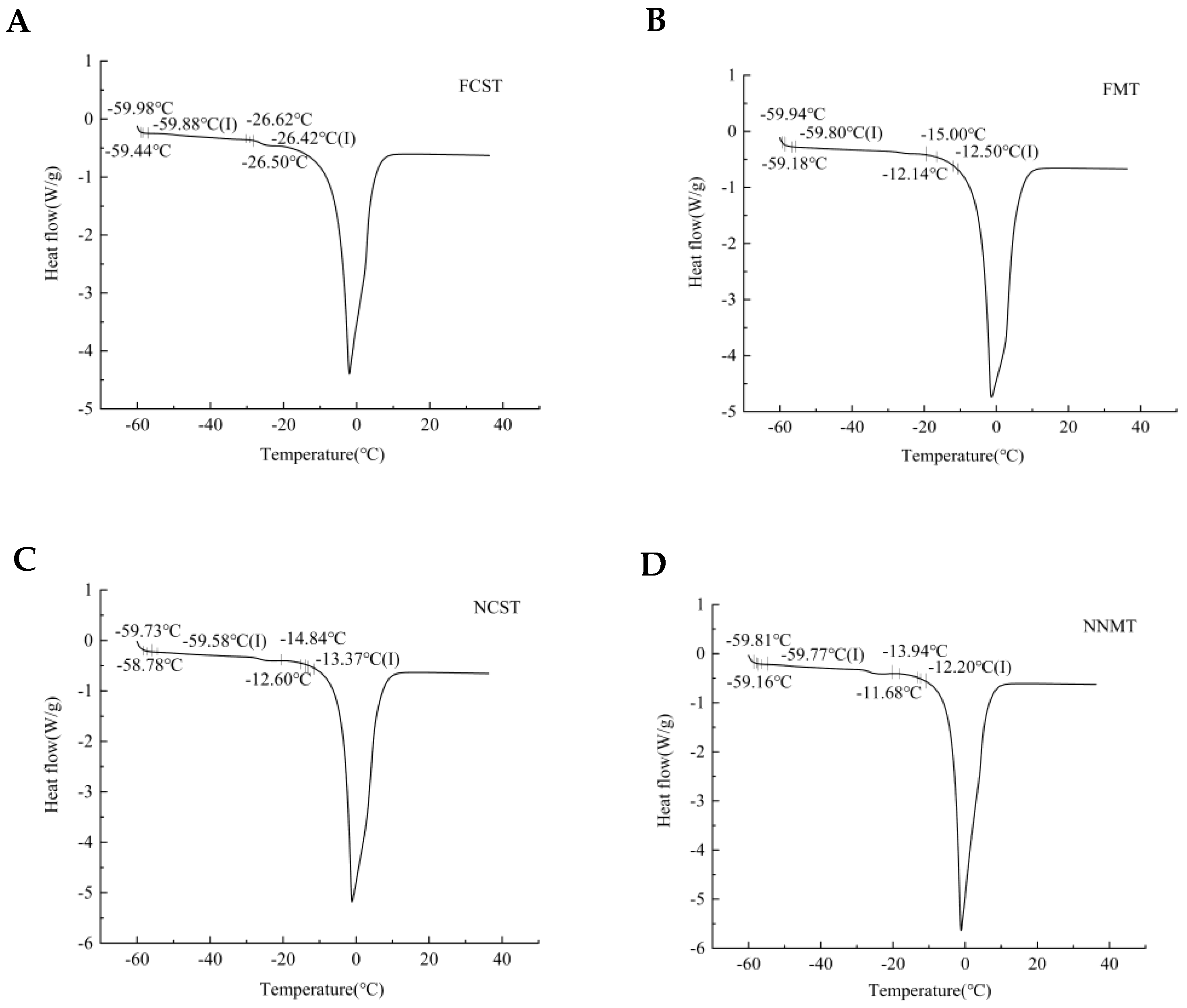
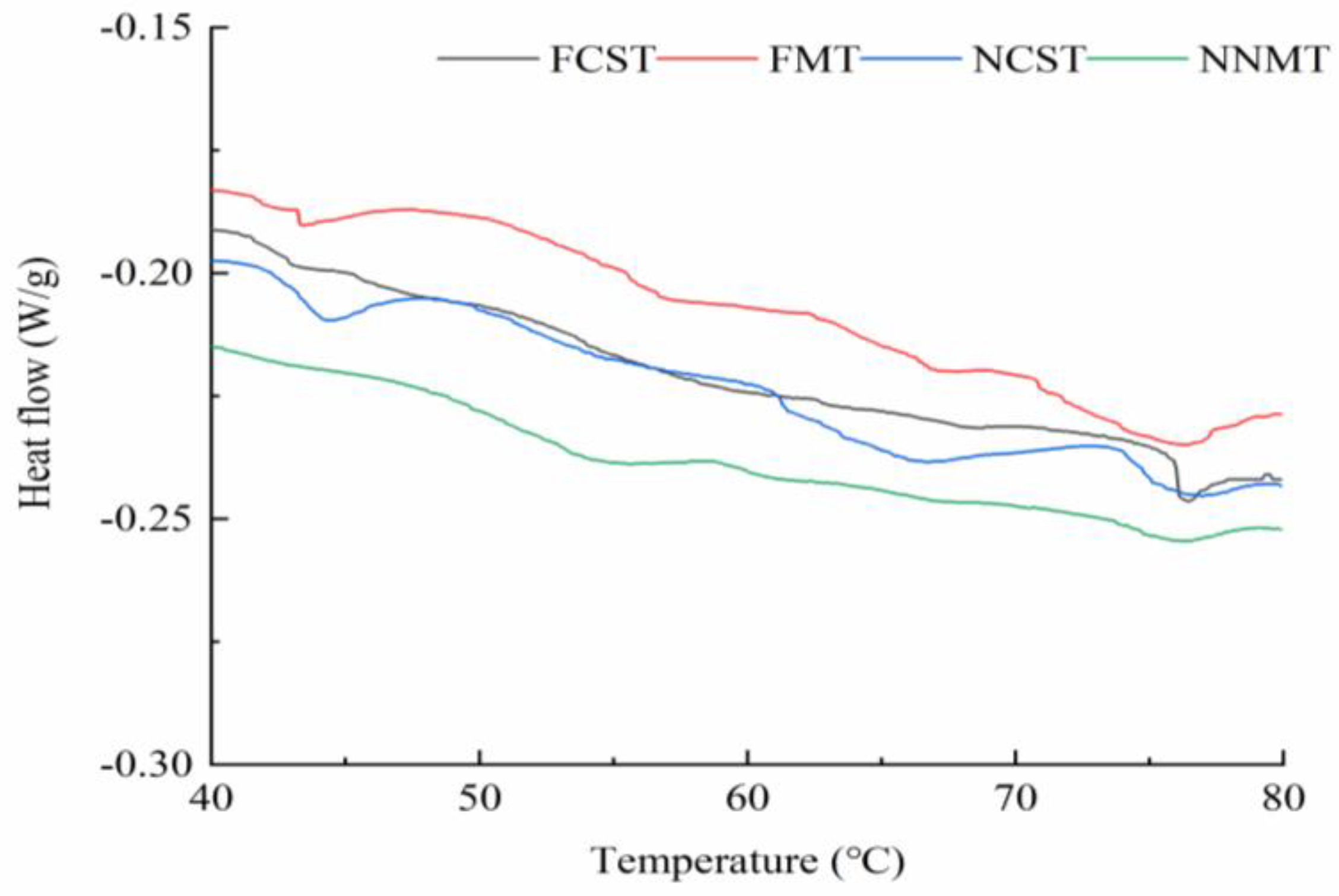
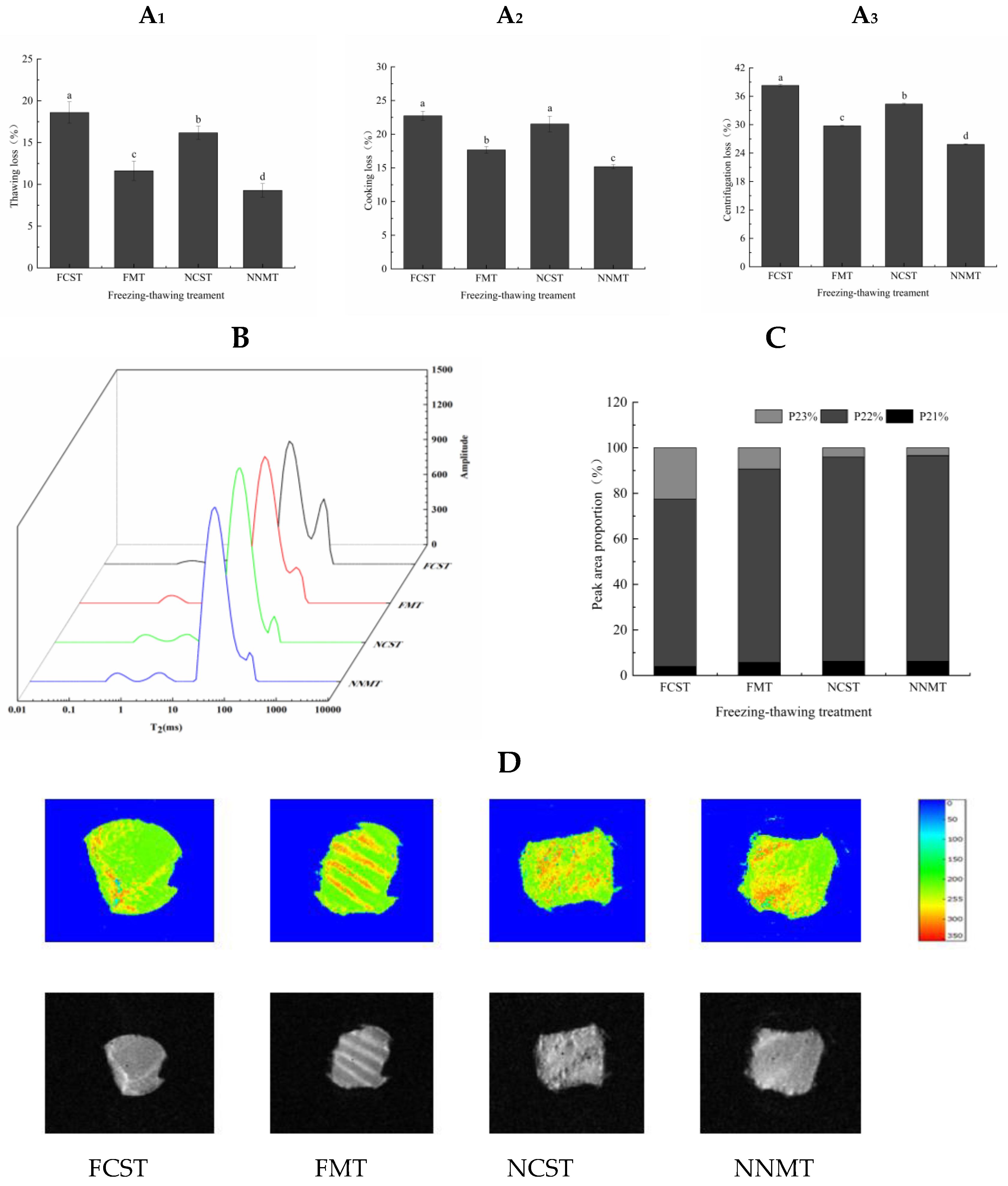
| Treatments | Freezing Point/°C | Moisture Content/% | Freezing Enthalpy/J·g−1 | Unfreezable Water Content/% |
|---|---|---|---|---|
| FCST | −1.00 ± 0.02 c | 68.11 ± 2.00 a | 194.55 ± 1.75 a | 12.68 ± 1.42 c |
| FMT | −1.31 ± 0.01 b | 66.98 ± 1.57 a | 171.60 ± 8.30 b | 18.29 ± 1.00 a |
| NCST | −1.02 ± 0.02 c | 67.81 ± 1.39 a | 175.27 ± 2.67 b | 15.30 ± 0.84 b |
| NNMT | −1.35 ± 0.03 a | 69.30 ± 1.55 a | 161.85 ± 0.95 c | 19.31 ± 1.82 a |
| Treatments | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|
| Tmax1 (°C) | ∆H1 (J/g) | Tmax2 (°C) | ∆H2 (J/g) | Tmax3 (°C) | ∆H3 (J/g) | |
| FCST | 43.08 ± 0.02 c | 0.17 ± 0.01 a | 54.06 ± 0.36 a | 0.04 ± 0.01 b | 74.63 ± 1.50 b | 0.06 ± 0.02 a |
| FMT | 42.90 ± 0.43 c | 0.18 ± 0.02 a | 55.45 ± 1.29 a | 0.08 ± 0.01 a | 76.14 ± 0.19 a | 0.34 ± 0.27 a |
| NCST | 44.09 ± 0.71 b | 0.26 ± 0.07 a | 56.06 ± 1.31 a | 0.05 ± 0.02 b | 76.10 ± 1.18 a | 0.33 ± 0.21 a |
| NNMT | 44.89 ± 0.16 a | 0.13 ± 0.01 a | 55.57 ± 0.82 a | 0.11 ± 0.02 a | 76.58 ± 1.03 a | 0.12 ± 0.04 a |
| Treatments | L* | a* | b* |
|---|---|---|---|
| FCST | 49.06 ± 0.27 b | 9.64 ± 0.98 ab | 16.35 ± 1.46 b |
| FMT | 51.96 ± 0.56 a | 10.40 ± 0.41 ab | 16.93 ± 0.45 ab |
| NCST | 51.23 ± 0.65 a | 9.37 ± 0.50 b | 17.04 ± 0.96 ab |
| NNMT | 51.99 ± 0.65 a | 10.89 ± 0.52 a | 18.21 ± 0.15 a |
| Treatments | Hardness/g | Springiness/g | Cohesiveness/g | Chewiness/mJ | Relaxation time/s | Relaxation Stress/Pa |
|---|---|---|---|---|---|---|
| FCST | 217.40 ± 11.25 d | 0.74 ± 0.01 a | 0.67 ± 0.03 a | 107.42 ± 10.69 b | 1.83 ± 0.03 b | 1783.10 ± 110.25 d |
| FMT | 523.86 ± 24.91 b | 0.75 ± 0.07 a | 0.64 ± 0.05 ab | 252.02 ± 39.84 a | 1.91 ± 0.14 b | 2782.45 ± 27.05 b |
| NCST | 271.09 ± 26.05 c | 0.74 ± 0.01 a | 0.65 ± 0.02 ab | 130.58 ± 15.80 b | 1.98 ± 0.13 b | 2089.75 ± 145.15 c |
| NNMT | 614.19 ± 27.37 a | 0.70 ± 0.06 a | 0.59 ± 0.04 b | 254.33 ± 27.38 a | 2.26 ± 0.01 a | 3223.98 ± 193.80 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, W.; Bu, Y.; Li, X.; Zhu, W. Nano Freezing–Thawing of Atlantic Salmon Fillets: Impact on Thermodynamic and Quality Characteristics. Foods 2023, 12, 2887. https://doi.org/10.3390/foods12152887
Wang W, Li W, Bu Y, Li X, Zhu W. Nano Freezing–Thawing of Atlantic Salmon Fillets: Impact on Thermodynamic and Quality Characteristics. Foods. 2023; 12(15):2887. https://doi.org/10.3390/foods12152887
Chicago/Turabian StyleWang, Wenxuan, Wenzheng Li, Ying Bu, Xuepeng Li, and Wenhui Zhu. 2023. "Nano Freezing–Thawing of Atlantic Salmon Fillets: Impact on Thermodynamic and Quality Characteristics" Foods 12, no. 15: 2887. https://doi.org/10.3390/foods12152887





