The Effects of Tea Polyphenol on Chicken Protein Digestion and the Mechanism under Thermal Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Simulated Digestion of Sample
2.4. Determination of Sample Digestibility
2.5. MALDI-TOF-MS Analysis of Sample Digest
2.6. Intrinsic Fluorescence Measurement of Sample
2.7. Surface Plasmon Resonance (SPR)
2.8. Reactive Sulfhydryl Content Determination
2.9. Statistical Analysis
3. Results and Discussion
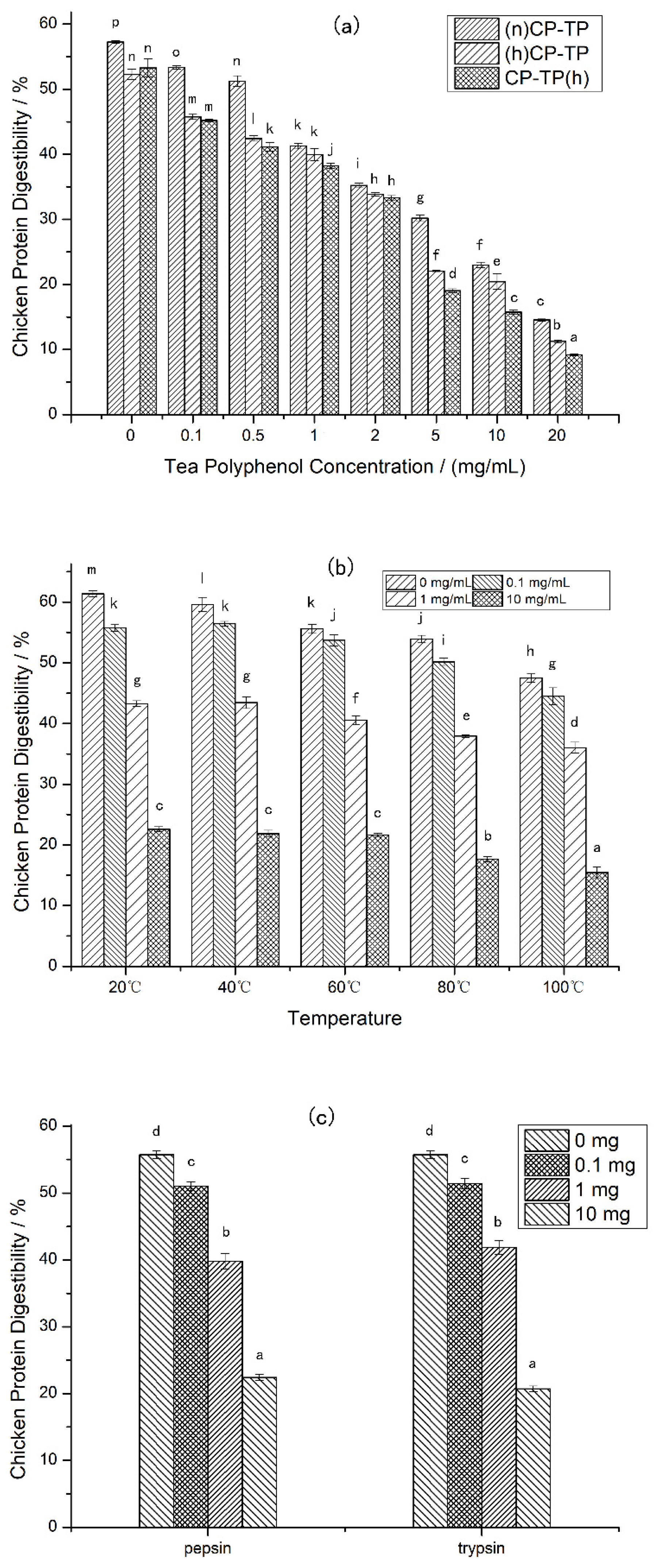
3.1. The Changes of Chicken Protein Digestibility under Different Concentrations of Tea Polyphenol
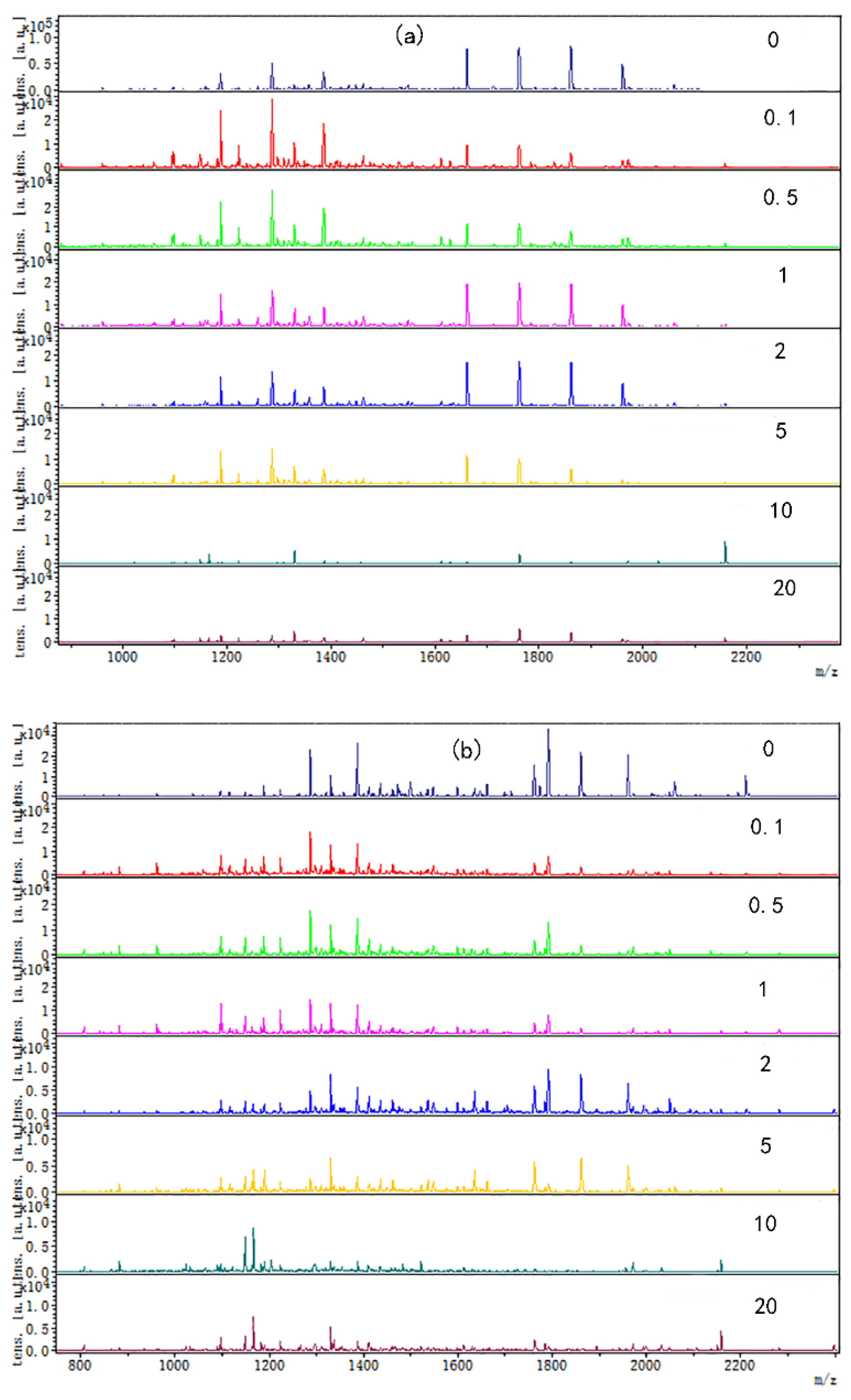
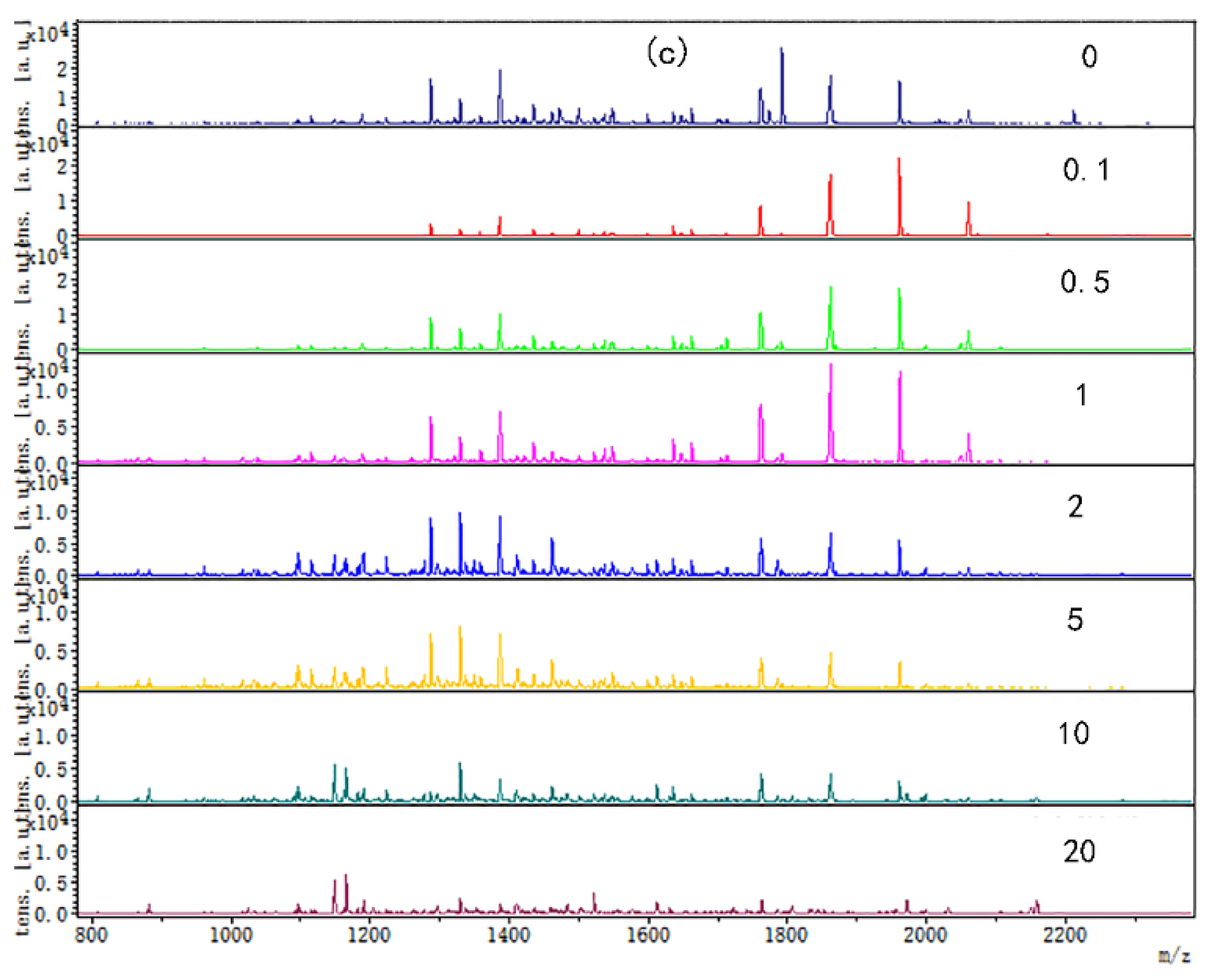
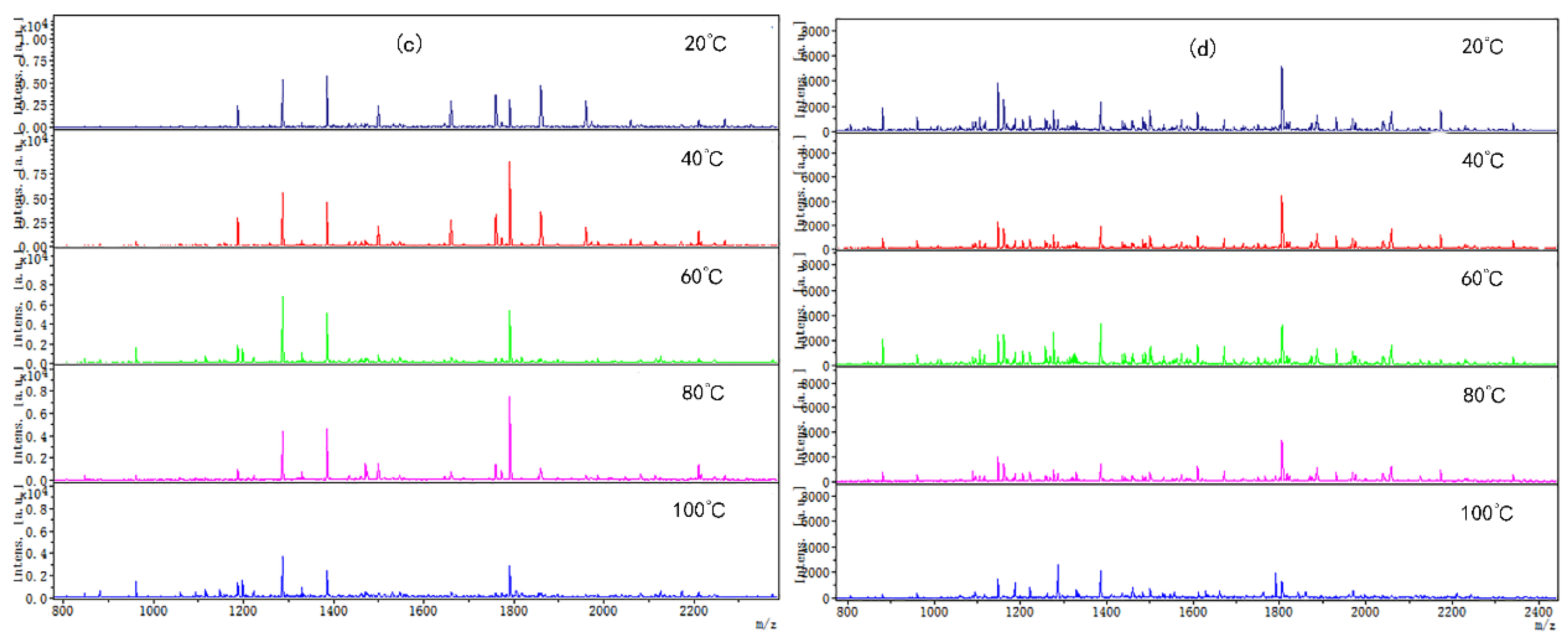
3.2. The Changes of Chicken Protein Digested Peptide Mapping at Different Tea Polyphenol Concentrations at Different Temperatures
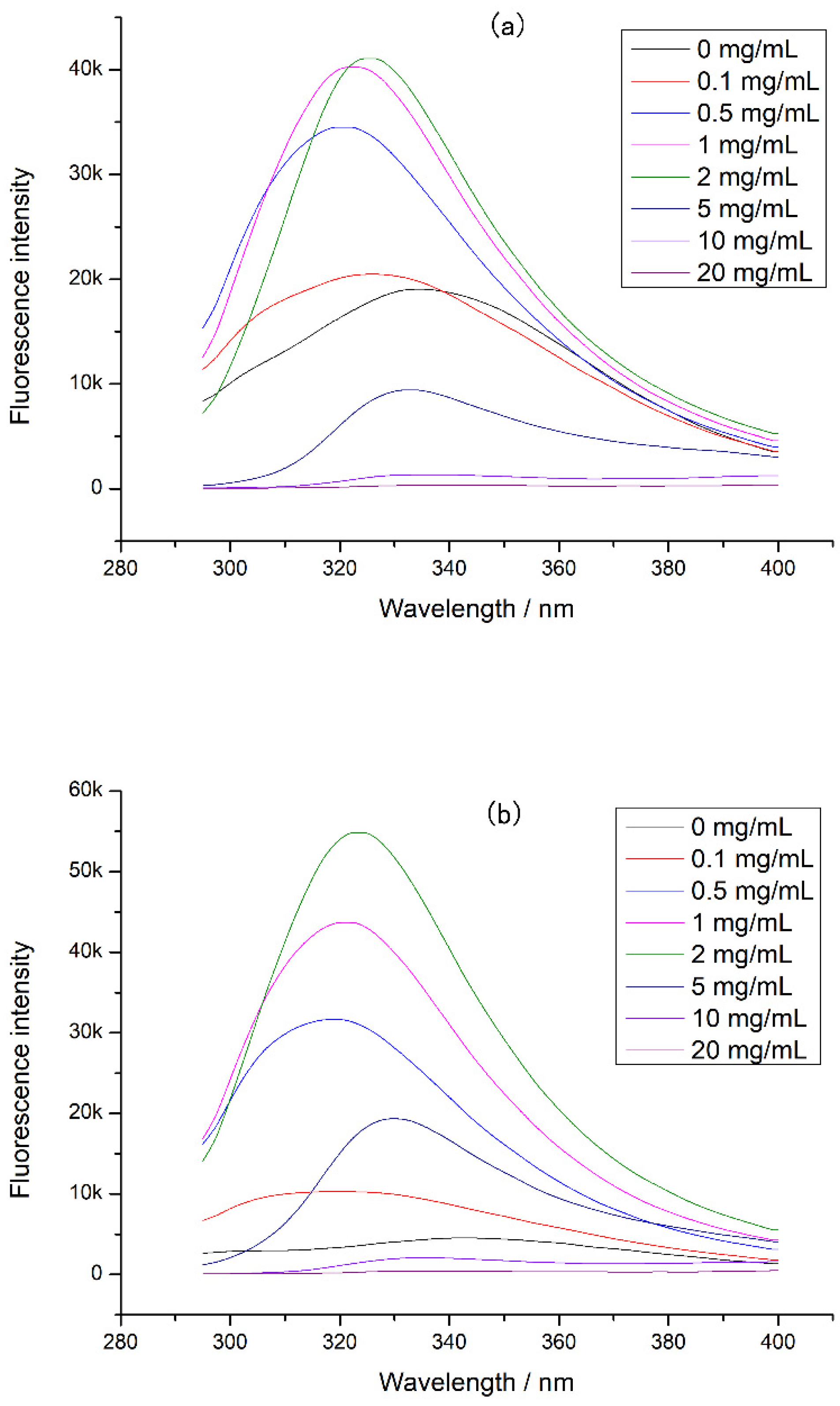
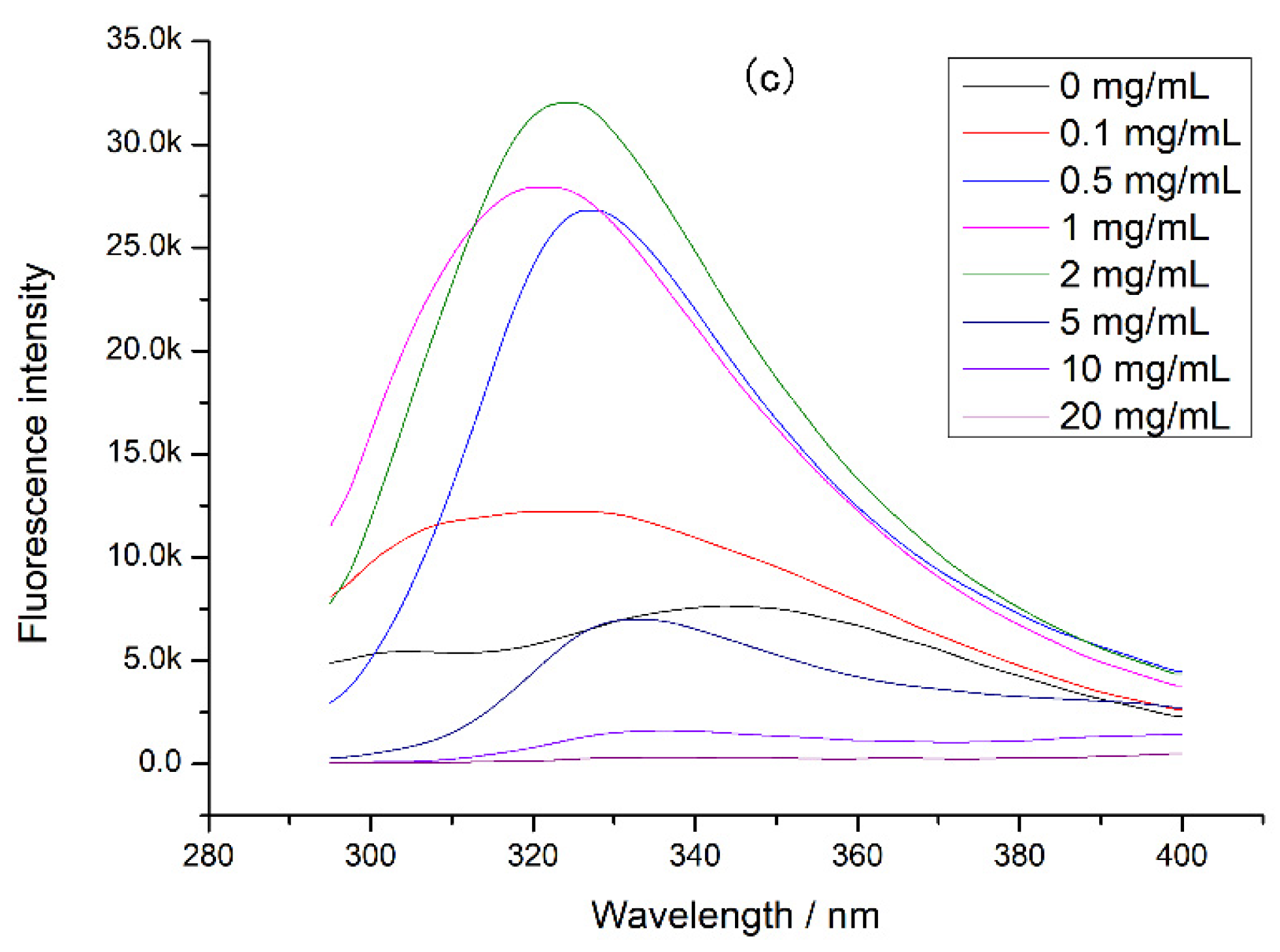
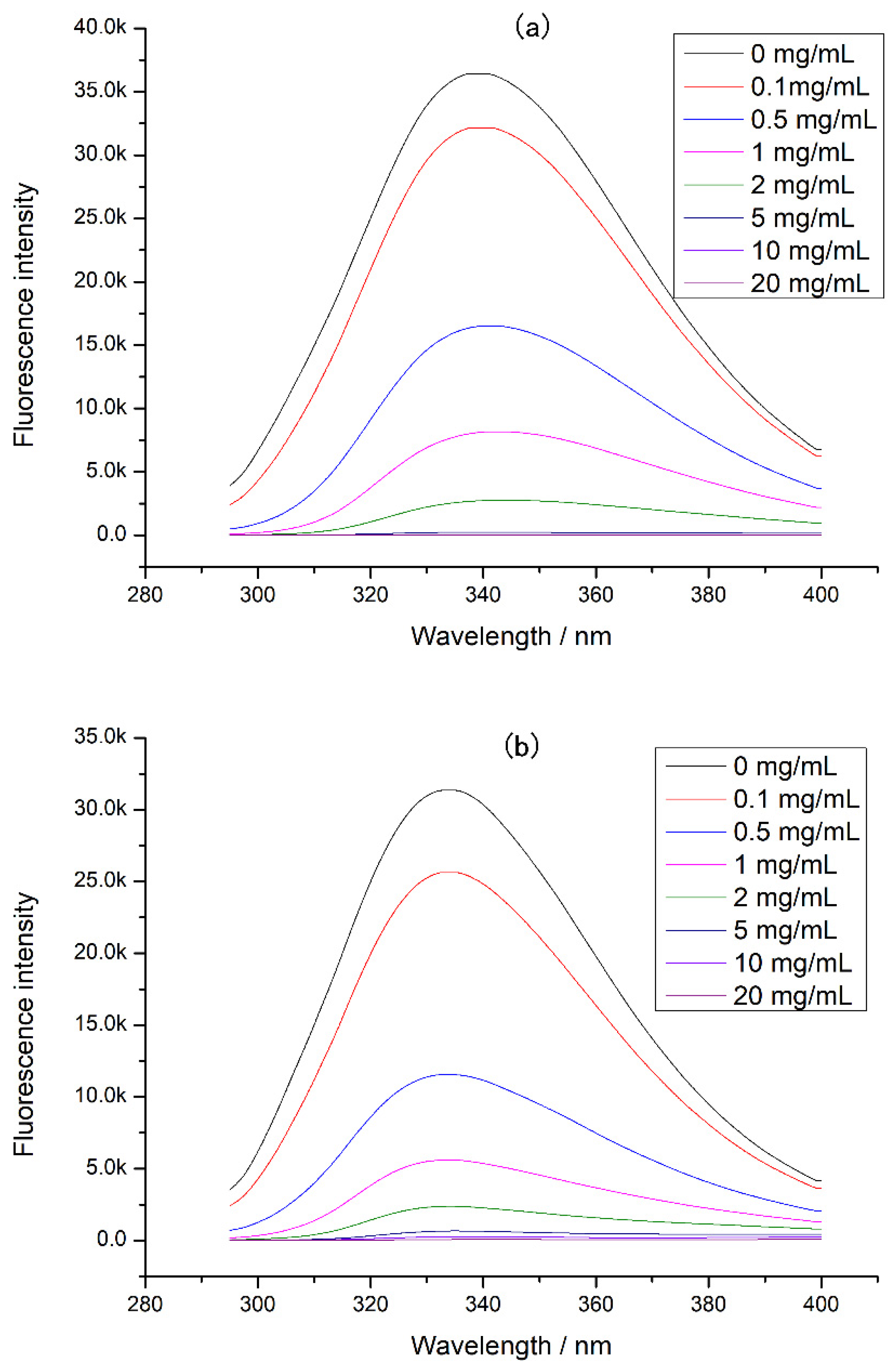
3.3. The Changes of Chicken Protein Intrinsic Fluorescence
3.4. The Binding Constants of EGCG with Different Proteins
3.5. The Changes of Chicken Protein Reactive Sulfhydryl Contents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Li, L.; Zhong, Q.; Cai, R.; Yin, T. Myofibrillar protein-curcumin nanocomplexes prepared at different ionic strengths to improve oxidative stability of marinated chicken meat products. LWT—Food Sci. Technol. 2018, 99, 69–76. [Google Scholar] [CrossRef]
- Cheng, J.R.; Xu, L.; Xiang, R.; Liu, X.M.; Zhu, M.J. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Harris, P.; Kaur, A.; Pastranac, L.; Jauregi, P. Characterisation of β-lactoglobulin nanoparticles and their binding to caffeine. Food Hydrocoll. 2017, 71, 85–93. [Google Scholar] [CrossRef]
- Reyer, H.; Zentek, J.; Mnner, K.; Youssef, I.M.I.; Aumiller, T.; Weghuber, J.; Wimmers, K.; Mueller, A.S. Possible molecular mechanisms by which an essential oil blend from star anise, rosemary, thyme, and oregano and saponins increase the performance and ileal protein digestibility of growing broilers. J. Agr. Food Chem. 2017, 65, 6821–6830. [Google Scholar] [CrossRef]
- Buitimea-Cantua, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic-Protein Interactions: Effects on Food Properties and Health Benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Cheng, J.R.; Lin, Y.S.; Tang, D.B.; Yang, H.G.; Liu, X.M. Structural and gelation properties of five polyphenols-modified pork myofibrillar protein exposed to hydroxyl radicals. LWT—Food Sci. Technol. 2022, 156, 113073. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, J.X.; Zhang, C.X.; Wang, W.F.; Qu, W.L.; Wang, W.H.; Li, W.X.; Wu, H. Preparation of mechanically strong and active composite films based on fish myofibrillar proteins: The dual effects of oxidized polyphenol crosslinking and layered double hydroxide reinforcement. Food Hydrocoll. 2022, 129, 107616. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Cross-linking activity of ethanolic coconut husk extract toward sardine (Sardinella albella) muscle proteins. J. Food Biochem. 2017, 41, e12283. [Google Scholar] [CrossRef]
- Zhou, C.F.; Zhang, L.J.; Zaky, A.A.; Tie, S.S.; Cui, G.X.; Liu, R.G.; Abd El-Aty, A.M.; Tan, M.Q. High internal phase Pickering emulsion by Spanish mackerel proteins-procyanidins: Application for stabilizing astaxanthin and surimi. Food Hydrocoll. 2022, 133, 107999. [Google Scholar] [CrossRef]
- Temdee, W.; Benjakul, S. Effect of oxidized kiam wood and cashew bark extracts on gel properties of gelatin from cuttlefish skins. Food Biosci. 2014, 7, 95–104. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.L.; Wu, N.; Wu, F.; Yu, D.Y.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Cheng, J.R.; Zhu, M.J.; Liu, X.M. Insight into the conformational and functional properties of myofibrillar protein modified by mulberry polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar] [CrossRef]
- Rezende, J.D.; Hudson, E.A.; De Paula, H.M. Human serum albumin-resveratrol complex formation: Effect of the phenolic chemical structure on the kinetic and thermodynamic parameters of the interactions. Food Chem. 2020, 307, 125514. [Google Scholar] [CrossRef]
- Xu, M.; Wu, Y.; Hou, G.G.; Du, X.F. Evaluation of different tea extracts on dough, textural, and functional properties of dry Chinese white salted noodle. LWT—Food Sci. Technol. 2019, 101, 456–462. [Google Scholar] [CrossRef]
- Tang, C.B.; Zhang, W.G.; Wang, Y.S.; Xing, L.J.; Xu, X.L.; Zhou, G.H. Identification of Rosmarinic Acid-Adducted Sites in Meat Proteins in a Gel Model under Oxidative Stress by Triple TOF MS/MS. J. Agr. Food Chem. 2016, 64, 6466–6476. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Sun, L.J.; Meng, Y.H.; Yang, X.B.; Guo, Y.R. Antioxidant activities of young apple polyphenols and its preservative effects on lipids and proteins in grass carp (Ctenopharyngodon idellus) fillets. CyTA—J. Food 2016, 15, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Wen, W.J.; Li, S.J.; Gu, Y.; Wang, S.; Wang, J.P. Effects of Starch on the Digestibility of Gluten under Different Thermal Processing Conditions. J. Agr. Food Chem. 2019, 67, 7120–7127. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Z.; Zhou, G.G.; Xu, X.L. Comparative study on the in vitro digestibility of chicken protein after different modifications. Food Chem. 2022, 385, 132652. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of chemical composition and baking on in vitro digestibility of proteins in breads made from selected gluten-containing and gluten-free flours. Food Chem. 2017, 233, 514–524. [Google Scholar] [CrossRef]
- Gulati, P.; Li, A.; Holding, D.; Santra, D.; Zhang, Y.; Rose, D.J. Heating Reduces Proso Millet Protein Digestibility via Formation of Hydrophobic Aggregates. J. Agr. Food Chem. 2017, 65, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Zhou, J.P.; Yu, J.L.; Wang, S.; Copeland, L.; Wang, S.J. Revealing the mechanisms of starch amylolysis affected by tea catechins using surface plasmon resonance. Int. J. Biol. Macromol. 2020, 145, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Ehmke, L.; Miller, R.; Faa, P.; Smith, G.; Li, Y.H. Effect of sodium chloride and sodium bicarbonate on the physicochemical properties of soft wheat flour doughs and gluten polymerization. J. Agr. Food Chem. 2018, 66, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its structural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.A.; Vedenkina, N.S.; Ivkova, M.N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 1973, 18, 263–279. [Google Scholar] [CrossRef] [PubMed]
- SIB Swiss Institute of Bioinformatics. Enzyme Nomenclature Database. Available online: https://enzyme.expasy.org/EC3.4.23.1,EC3.4.4.4 (accessed on 8 December 2022).
- Cao, Y.G.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Stanciuc, N.; Banu, I.; Bolea, C.; Patraşcu, L.; Aprodu, I. Structural and antigenic properties of thermally treated gluten proteins. Food Chem. 2018, 267, 43–51. [Google Scholar] [CrossRef]
- Sheng, L.; Tang, G.Y.; Wang, Q.; Zou, J.; Ma, M.H.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll. 2019, 100, 105384. [Google Scholar] [CrossRef]
- Mitra, B.; Rinnan, A.; Ruiz-Carrascal, J. Tracking hydrophobicity state, aggregation behaviour and structural modifications of pork proteins under the influence of assorted heat treatments. Food Res. Int. 2017, 101, 266–273. [Google Scholar] [CrossRef]
- Liu, F.G.; Ma, C.C.; McClements, D.J.; Gao, Y.X. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Tang, C.B.; Zhang, W.G.; Dai, C.; Li, H.X.; Xu, X.L.; Zhou, G.H. Identification and Quantification of Adducts between Oxidized Rosnnarinic Acid and Thiol Compounds by UHPLC-LTQ-Orbitrap and MALDI-TOF/TOF Tandem Mass Spectrometry. J. Agr. Food Chem. 2015, 63, 902–911. [Google Scholar] [CrossRef]
- Jongberg, S.; Terkelsen, L.D.; Miklos, R.; Lund, M.N. Green tea extract impairs meat emulsion properties by disturbing protein disulfide cross-linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, G.Y.; Liu, G.; Zeng, Q.H.; Shen, X.; Hou, Y.; Li, L.; Hu, S.Q. The soluble recombinant N-terminal domain of HMW 1Dx5 and its aggregation behavior. Food Res. Int. 2015, 78, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, G.; Huang, Y.B.; Zeng, Q.H.; Song, G.S.; Hou, Y.; Li, L.; Hu, S.Q. Role of N-terminal domain of HMW 1Dx5 in the functional and structural properties of wheat dough. Food Chem. 2016, 213, 682–690. [Google Scholar] [CrossRef] [PubMed]


| Sample | Ka (M−1 s−1) | Kd × 10−4 (s−1) | KD × 10−7 (M) |
|---|---|---|---|
| Myoglobin | 1514.0 ± 79.8 b | 1.93 ± 0.07 a | 1.28 ± 0.14 a |
| Myosin | 3051.0 ± 46.0 c | 4.79 ± 0.43 c | 1.57 ± 0.02 a |
| Trypsin | 376.6 ± 6.2 a | 2.73 ± 0.20 b | 7.24 ± 0.24 b |
| TP Concentration | (n)CP-TP | (h)CP-TP | CP-TP(h) |
|---|---|---|---|
| 0 mg/mL | 93.76 ± 3.83 l | 78.6 ± 2.55 j | 78.98 ± 3.49 j |
| 0.1 mg/mL | 87.8 ± 1.54 k | 62.15 ± 2.22 h | 73.22 ± 3.22 i |
| 0.5 mg/mL | 34.71 ± 0.08 fg | 24.66 ± 0.24 d | 28.51 ± 0.32 e |
| 1 mg/mL | 20.34 ± 0.45 c | 17.31 ± 0.15 a | 17.74 ± 0.33 ab |
| 2 mg/mL | 17.34 ± 0.17 a | 15.59 ± 0.21 a | 16.29 ± 0.47 a |
| 5 mg/mL | 20.74 ± 0.40 c | 19.87 ± 0.60 bc | 20.2 ± 0.56 c |
| 10 mg/mL | 26.96 ± 0.92 de | 27.19 ± 0.59 e | 24.56 ± 0.48 d |
| 20 mg/mL | 37.06 ± 0.61 g | 34.75 ± 0.65 fg | 33.38 ± 1.53 f |
| TP Concentration | 20 °C | 40 °C | 60 °C | 80 °C | 100 °C |
|---|---|---|---|---|---|
| 0 mg/mL | 100.38 ± 0.92 k | 91.55 ± 1.92 j | 90.24 ± 1.43 ij | 85.53 ± 2.99 g | 79.81 ± 2.05 f |
| 0.1 mg/mL | 89.21 ± 1.36 hi | 90.15 ± 1.91 ij | 86.59 ± 2.55 g | 87.5 ± 2.19 gh | 72.85 ± 2.37 e |
| 1 mg/mL | 20.34 ± 0.29 b | 20.44 ± 0.43 b | 19.52 ± 0.34 ab | 18.72 ± 0.13 ab | 17.74 ± 0.25 a |
| 10 mg/mL | 26.96 ± 0.60 d | 26.06 ± 1.55 cd | 25.91 ± 0.83 cd | 24.99 ± 0.40 cd | 24.56 ± 0.37 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.; Li, S.; Wang, J. The Effects of Tea Polyphenol on Chicken Protein Digestion and the Mechanism under Thermal Processing. Foods 2023, 12, 2905. https://doi.org/10.3390/foods12152905
Wen W, Li S, Wang J. The Effects of Tea Polyphenol on Chicken Protein Digestion and the Mechanism under Thermal Processing. Foods. 2023; 12(15):2905. https://doi.org/10.3390/foods12152905
Chicago/Turabian StyleWen, Wenjun, Shijie Li, and Junping Wang. 2023. "The Effects of Tea Polyphenol on Chicken Protein Digestion and the Mechanism under Thermal Processing" Foods 12, no. 15: 2905. https://doi.org/10.3390/foods12152905
APA StyleWen, W., Li, S., & Wang, J. (2023). The Effects of Tea Polyphenol on Chicken Protein Digestion and the Mechanism under Thermal Processing. Foods, 12(15), 2905. https://doi.org/10.3390/foods12152905




