Effect of Ball-Milling on Starch Crystalline Structure, Gelatinization Temperature, and Rheological Properties: Towards Enhanced Utilization in Thermosensitive Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corn Starch
2.2. Starch Ball Milling
2.3. Microscopy Analysis
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Amylose Content
2.6. Thermogravimetric Analysis (TGA)
2.7. Differential Scanning Calorimetry (DSC)
2.8. X-ray Diffraction (XRD)
2.9. Swelling Power (SP) and Solubility (S%)
2.10. Rheology
2.11. Film-Forming Ability
2.12. ANOVA Analysis
3. Results and Discussion
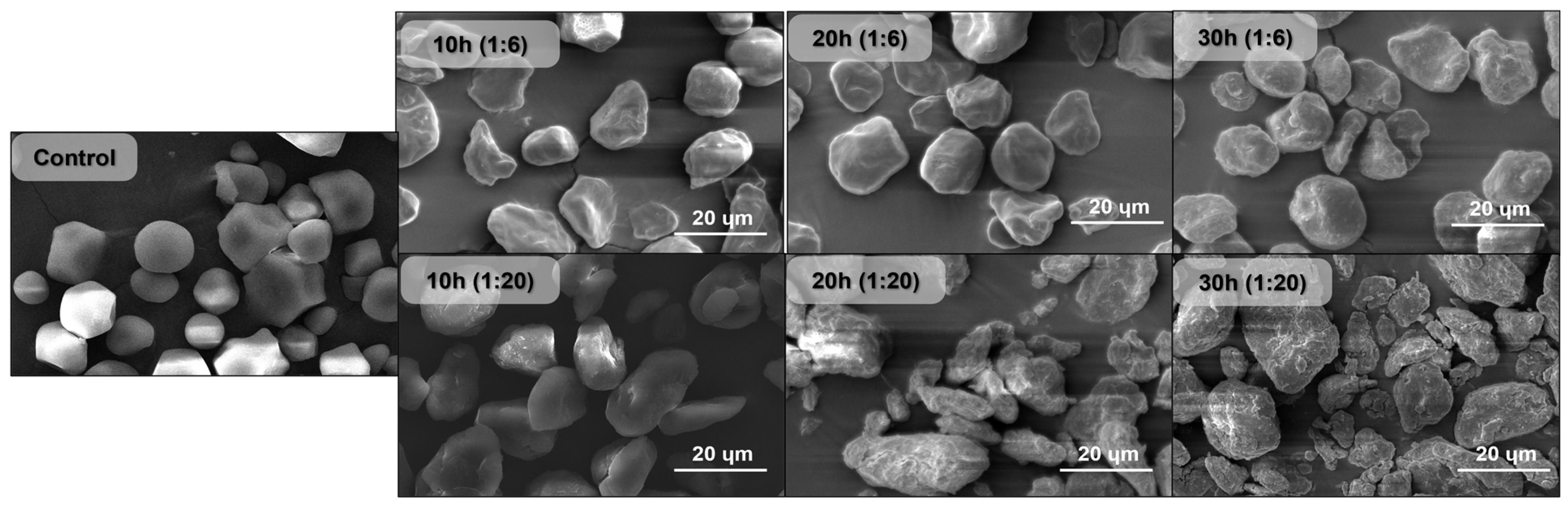
3.1. Microscopic Analysis
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Amylose Content
3.4. Thermogravimetric Analysis (TGA)
3.5. Differential Scanning Calorimetry (DSC)
3.6. X-ray Diffraction (XRD)
3.7. Swelling Power (SP) and Solubility (S%)
3.8. Rheology
3.9. Film Forming Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zia-ud-Din; Xiong, H.; Fei, P. Physical and Chemical Modification of Starches: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [CrossRef]
- Han, N.; Fan, J.L.; Chen, N.; Chen, H.Q. Effect of Ball Milling Treatment on the Structural, Physicochemical and Digestive Properties of Wheat Starch, A- and B-Type Starch Granules. J. Cereal Sci. 2022, 104, 103439. [Google Scholar] [CrossRef]
- Li, E.; Dhital, S.; Hasjim, J. Effects of Grain Milling on Starch Structures and Flour/Starch Properties. Starch 2014, 66, 15–27. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The Molecular Structures of Starch Components and Their Contribution to the Architecture of Starch Granules: A Comprehensive Review. Starch 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch Nanoparticles: A Review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Y. Nanostarch: Preparation, Modification, and Application in Pickering Emulsions. J. Agric. Food Chem. 2021, 69, 6929–6942. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Xie, X.L.; Chen, Y.; Lu, J.P.; Tong, Z.F. Ball-Milling Treatment Effect on Physicochemical Properties and Features for Cassava and Maize Starches. C. R. Chim. 2008, 11, 73–79. [Google Scholar] [CrossRef]
- Chakraborty, I.; N, P.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An Insight into the Gelatinization Properties Influencing the Modified Starches Used in Food Industry: A Review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Synthesis, Characteristics, and Applications of Modified Starch Nanoparticles: A Review. Int. J. Biol. Macromol. 2022, 194, 289–305. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, L.; Li, Z.; Li, C.; Hong, Y.; Gu, Z. The Degree of Substitution of OSA-Modified Starch Affects the Retention and Release of Encapsulated Mint Flavour. Carbohydr. Polym. 2022, 294, 119781. [Google Scholar] [CrossRef]
- Liu, C.; An, F.; He, H.; He, D.; Wang, Y.; Song, H. Pickering Emulsions Stabilized by Compound Modified Areca Taro (Colocasia esculenta (L.) Schott) Starch with Ball-Milling and OSA. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 185–194. [Google Scholar] [CrossRef]
- Tamaki, S.; Hisamatsu, M.; Teranishi, K.; Adachi, T.; Yamada, T. Structural Change of Maize Starch Granules by Ball-Mill Treatment. Starch 1998, 8, 342–348. [Google Scholar] [CrossRef]
- Bangar, S.P.; Singh, A.; Ashogbon, A.O.; Bobade, H. Ball-Milling: A Sustainable and Green Approach for Starch Modification. Int. J. Biol. Macromol. 2023, 237, 124069. [Google Scholar] [CrossRef]
- Goiana, M.L.; de Brito, E.S.; Alves Filho, E.G.; de Castro Miguel, E.; Fernandes, F.A.N.; de Azeredo, H.M.C.; de Freitas Rosa, M. Corn Starch Based Films Treated by Dielectric Barrier Discharge Plasma. Int. J. Biol. Macromol. 2021, 183, 2009–2016. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the Structural Order of Native Starch Granules Using Combined FTIR and XRD Analysis. J. Polym. Res. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Chrastil, J. Improved Colorimetric Determination of Amylose in Starch Flours. Carbohydr. Res. 1987, 159, 154–158. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, F.; Lin, Y.; Zhao, K.; Zhu, P. Dissolution of Starch in Urea/NaOH Aqueous Solutions. J. Appl. Polym. Sci. 2016, 133, 19. [Google Scholar] [CrossRef]
- Mandala, I.G.; Bayas, E. Xanthan Effect on Swelling, Solubility and Viscosity of Wheat Starch Dispersions. Food Hydrocoll. 2004, 18, 191–201. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wang, L.J.; Li, D.; Adhikari, B. Effects of Partial Gelatinization on Structure and Thermal Properties of Corn Starch after Spray Drying. Carbohydr. Polym. 2012, 88, 1319–1325. [Google Scholar] [CrossRef]
- Shi, J.; Sweedman, M.C.; Shi, Y.C. Structure, Birefringence and Digestibility of Spherulites Produced from Debranched Waxy Maize Starch. Int. J. Biol. Macromol. 2021, 183, 1486–1494. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Masoodi, F.A.; Rizvi, S.H. Influence of Ball Milling on the Production of Starch Nanoparticles and Its Effect on Structural, Thermal and Functional Properties. Int. J. Biol. Macromol. 2020, 151, 85–91. [Google Scholar] [CrossRef]
- Ma, H.; Liu, M.; Liang, Y.; Zheng, X.; Sun, L.; Dang, W.; Li, J.; Li, L.; Liu, C. Research Progress on Properties of Pre-Gelatinized Starch and Its Application in Wheat Flour Products. Grain Oil Sci. Technol. 2022, 5, 87–97. [Google Scholar] [CrossRef]
- Han, F.; Gao, C.; Liu, M.; Huang, F.; Zhang, B. Synthesis, Optimization and Characterization of Acetylated Corn Starch with the High Degree of Substitution. Int. J. Biol. Macromol. 2013, 59, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.V.; da Silva, A.P.M.; Barros, M.O.; Souza Filho, M.S.M.; Rosa, M.F.; Azeredo, H.M.C. Nanocomposite Films from Mango Kernel or Corn Starch with Starch Nanocrystals. Starch 2018, 70, 1800028. [Google Scholar] [CrossRef]
- Marenco-Orozco, G.A.; Rosa, M.F.; Fernandes, F.A.N. Effects of Multiple-Step Cold Plasma Processing on Banana (Musa Sapientum) Starch-Based Films. Packag. Technol. Sci. 2022, 35, 589–601. [Google Scholar] [CrossRef]
- Van Soest, J.J.; Tournois, H.; de Wit, D.; Vliegenthart, J.F. Short-Range Structure in (Partially) Crystalline Potato Starch Determined with Attenuated Total Reflectance Fourier-Transform IR Spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Cheng, F.; Lin, Y.; Shi, L.; Zhu, P.X. Preparation of Oxidized Corn Starch with High Degree of Oxidation by Fenton-like Oxidation Assisted with Ball Milling. Mater. Today Commun. 2020, 22, 100793. [Google Scholar] [CrossRef]
- Apostolidis, E.; Stergiou, A.; Kioupis, D.; Amin, S.; Paximada, P.; Kakali, G.; Mandala, I. Production of Nanoparticles from Resistant Starch via a Simple Three-Step Physical Treatment. Food Hydrocoll. 2023, 137, 108412. [Google Scholar] [CrossRef]
- Chen, R.; Williams, P.A.; Chong, D.; Luo, S.; Chen, J.; Liu, C. The Interaction of Pectin with Wheat Starch and Its Influence on Gelatinization and Rheology. Food Hydrocoll. 2023, 136, 108288. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, H.; Chang, P.R. Transitional Properties of Starch Colloid with Particle Size Reduction from Micro- to Nanometer. J. Colloid Interface Sci. 2009, 339, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Z.; Zhao, M.; Milosavljević, V.; Cullen, P.J.; Scally, L.; Sun, D.W.; Tiwari, B.K. Low-Pressure Plasma Modification of the Rheological Properties of Tapioca Starch. Food Hydrocoll. 2022, 125, 107380. [Google Scholar] [CrossRef]
- Liu, C.; Hong, J.; Zheng, X. Effect of Heat-Moisture Treatment on Morphological, Structural and Functional Characteristics of Ball-Milled Wheat Starches. Starch 2017, 69, 1500141. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors Influencing the Physico-Chemical, Morphological, Thermal and Rheological Properties of Some Chemically Modified Starches for Food Applications—A Review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Yang, S.; Dhital, S.; Zhang, M.N.; Wang, J.; Chen, Z.G. Structural, Gelatinization, and Rheological Properties of Heat-Moisture Treated Potato Starch with Added Salt and Its Application in Potato Starch Noodles. Food Hydrocoll. 2022, 131, 107802. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal Degradation and Stability of Starch under Different Processing Conditions. Starch 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Oliveira, A.V.; Pontes, S.M.A.; Pereira, A.L.S.; Souza Filho, M.d.s.M.; Rosa, M.F.; Azeredo, H.M.C. Mango Kernel Starch Films as Affected by Starch Nanocrystals and Cellulose Nanocrystals. Carbohydr. Polym. 2019, 211, 209–216. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline Structures of the Main Components of Starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Delcour, J.A. Impact of Swelling Power and Granule Size on Pasting of Blends of Potato, Waxy Rice and Maize Starches. Food Hydrocoll. 2016, 52, 69–77. [Google Scholar] [CrossRef]
- Abegunde, O.K.; Mu, T.H.; Chen, J.W.; Deng, F.M. Physicochemical Characterization of Sweet Potato Starches Popularly Used in Chinese Starch Industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Narpinder, S.; Jaspreet, S.; Lovedeep, K.; Navdeep, S.S.; Balmeet, S.G. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar]
- Desam, G.P.; Dehghani, N.L.; Narsimhan, G.; Narsimhan, V. Characterization of Storage Modulus of Starch Suspensions during the Initial Stages of Pasting Using Stokesian Dynamics Simulations. Food Hydrocoll. 2021, 121, 268–273. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of Konjac Glucomannan on Pasting and Rheological Properties of Corn Starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Sikora, M.; Adamczyk, G.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Berski, W.; Lukasiewicz, M.; Izak, P. Thixotropic Properties of Normal Potato Starch Depending on the Degree of the Granules Pasting. Carbohydr. Polym. 2015, 121, 254–264. [Google Scholar] [CrossRef] [PubMed]







| Sample | 995/1044 cm−1 (%) | Sample | 995/1044 cm−1 (%) |
|---|---|---|---|
| Control | 64.9 ± 0.1 d | 1 h (1:20 ratio) | 65.6 ± 0.6 c,d |
| 1 h (1:6 ratio) | 65.4 ± 0.5 c,d | 5 h (1:20 ratio) | 66.9 ± 0.3 c |
| 5 h (1:6 ratio) | 66.4 ± 0.6 c | 10 h (1:20 ratio) | 67.9 ± 0.5 c |
| 10 h (1:6 ratio) | 66.9 ± 0.2 c | 20 h (1:20 ratio) | 70.0 ± 0.9 b |
| 20 h (1:6 ratio) | 69.5 ± 0.3 b | 30 h (1:20 ratio) | 73.9 ± 1.2 a |
| 30 h (1:6 ratio) | 68.9 ± 0.7 b | -------- | -------- |
| Sample | ΔHgel (J·g−1) | Peak Temperature (°C) | End Temperature (°C) |
|---|---|---|---|
| Control | 1.86 ± 0.21 a | 71.4 | 83.5 |
| 1 h (1:6 ratio) | 1.75 ± 0.15 a | 69.6 | 82.4 |
| 5 h (1:6 ratio) | 1.48 ± 0.11 b | 69.2 | 79.4 |
| 10 h (1:6 ratio) | 0.76 ± 0.10 d | 64.9 | 77.7 |
| 20 h (1:6 ratio) | 0.72 ± 0.09 d,e | 65.7 | 77.6 |
| 30 h (1:6 ratio) | 0.64 ± 0.07 e | 61.8 | 74.6 |
| 1 h (1:20 ratio) | 0.88 ± 0.05 c | 69.9 | 81.2 |
| 5 h (1:20 ratio) | 0.84 ± 0.09 c | 65.3 | 77.3 |
| 10 h (1:20 ratio) | 0.38 ± 0.06 f | 62.3 | 75.1 |
| 20 h (1:20 ratio) | 0.24 ± 0.08 f | 61.9 | 74.3 |
| 30 h (1:20 ratio) | 0.01 ± 0.00 g | 61.5 | 66.7 |
| Sample | CrI (%) | Sample | CrI (%) |
|---|---|---|---|
| Control | 34.8 | 1 h (1:20 ratio) | 28.6 |
| 1 h (1:6 ratio) | 31.9 | 5 h (1:20 ratio) | 12.9 |
| 5 h (1:6 ratio) | 27.0 | 10 h (1:20 ratio) | 8.3 |
| 10 h (1:6 ratio) | 15.9 | 20 h (1:20 ratio) | 2.8 |
| 20 h (1:6 ratio) | 10.7 | 30 h (1:20 ratio) | 2.7 |
| 30 h (1:6 ratio) | 8.4 | -------- | -------- |
| Samples | TGel (°C) | Viscosity (ɳ) [cP] | Storage Module (G′) [Pa] | Loss Module (G″) [Pa] | Thixotropy |
|---|---|---|---|---|---|
| Control | 69.1 | 889.7 | 2.24 | 5.12 | 27.02 |
| 1 h (1:6 ratio) | 63.4 | 1601.00 | 3.33 | 9.49 | 23.77 |
| 5 h (1:6 ratio) | 61.3 | 921.20 | 1.32 | 5.64 | 17.38 |
| 10 h (1:6 ratio) | 55.9 | 969.90 | 1.28 | 5.96 | 9.89 |
| 20 h (1:6 ratio) | 51.6 | 255.90 | 0.28 | 1.58 | 9.15 |
| 30 h (1:6 ratio) | 50.1 | 119.20 | 0.12 | 0.74 | 4.75 |
| 1 h (1:20 ratio) | 62.7 | 660.70 | 1.24 | 3.97 | 16.74 |
| 5 h (1:20 ratio) | 60.1 | 352.90 | 0.76 | 2.09 | 11.14 |
| 10 h (1:20 ratio) | 51.4 | 173.20 | 0.35 | 1.03 | 2.04 |
| 20 h (1:20 ratio) | * | 148.60 | 0.23 | 0.91 | 2.16 |
| 30 h (1:20 ratio) | * | 81.62 | 0.15 | 0.49 | −0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Barros, M.; Mattos, A.L.A.; de Almeida, J.S.; de Freitas Rosa, M.; de Brito, E.S. Effect of Ball-Milling on Starch Crystalline Structure, Gelatinization Temperature, and Rheological Properties: Towards Enhanced Utilization in Thermosensitive Systems. Foods 2023, 12, 2924. https://doi.org/10.3390/foods12152924
de Oliveira Barros M, Mattos ALA, de Almeida JS, de Freitas Rosa M, de Brito ES. Effect of Ball-Milling on Starch Crystalline Structure, Gelatinization Temperature, and Rheological Properties: Towards Enhanced Utilization in Thermosensitive Systems. Foods. 2023; 12(15):2924. https://doi.org/10.3390/foods12152924
Chicago/Turabian Stylede Oliveira Barros, Matheus, Adriano Lincoln Albuquerque Mattos, Jessica Silva de Almeida, Morsyleide de Freitas Rosa, and Edy Sousa de Brito. 2023. "Effect of Ball-Milling on Starch Crystalline Structure, Gelatinization Temperature, and Rheological Properties: Towards Enhanced Utilization in Thermosensitive Systems" Foods 12, no. 15: 2924. https://doi.org/10.3390/foods12152924
APA Stylede Oliveira Barros, M., Mattos, A. L. A., de Almeida, J. S., de Freitas Rosa, M., & de Brito, E. S. (2023). Effect of Ball-Milling on Starch Crystalline Structure, Gelatinization Temperature, and Rheological Properties: Towards Enhanced Utilization in Thermosensitive Systems. Foods, 12(15), 2924. https://doi.org/10.3390/foods12152924








