Optimization of Ultrasound-Assisted Extraction of Dietary Fiber from Yellow Dragon Fruit Peels and Its Application in Low-Fat Alpaca-Based Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Drying and Modeling of the Kinetics of Dragon Fruit Peels
2.2.1. Drying of Dragon Fruit Peels
2.2.2. Modeling of Drying Kinetics
2.2.3. Effective Moisture Diffusivity (Deff)
2.3. Extraction, Yield of Dietary Fiber (DF), and Determination of Its Degree of Esterification (DE)
2.3.1. Extraction of DF Fractions: SDF and IDF
2.3.2. Degree of Esterification (DE)
2.3.3. Extraction Yield of DF Fractions (%)
2.4. Ultrasound-Assisted Extraction and Characterization of Optimized Dietary Fiber (SDF and IDF)
2.4.1. Optimization of Ultrasound-Assisted Extraction of IDF and SDF
2.4.2. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
2.4.3. Swelling Capacity (SC)
2.4.4. FTIR Analysis
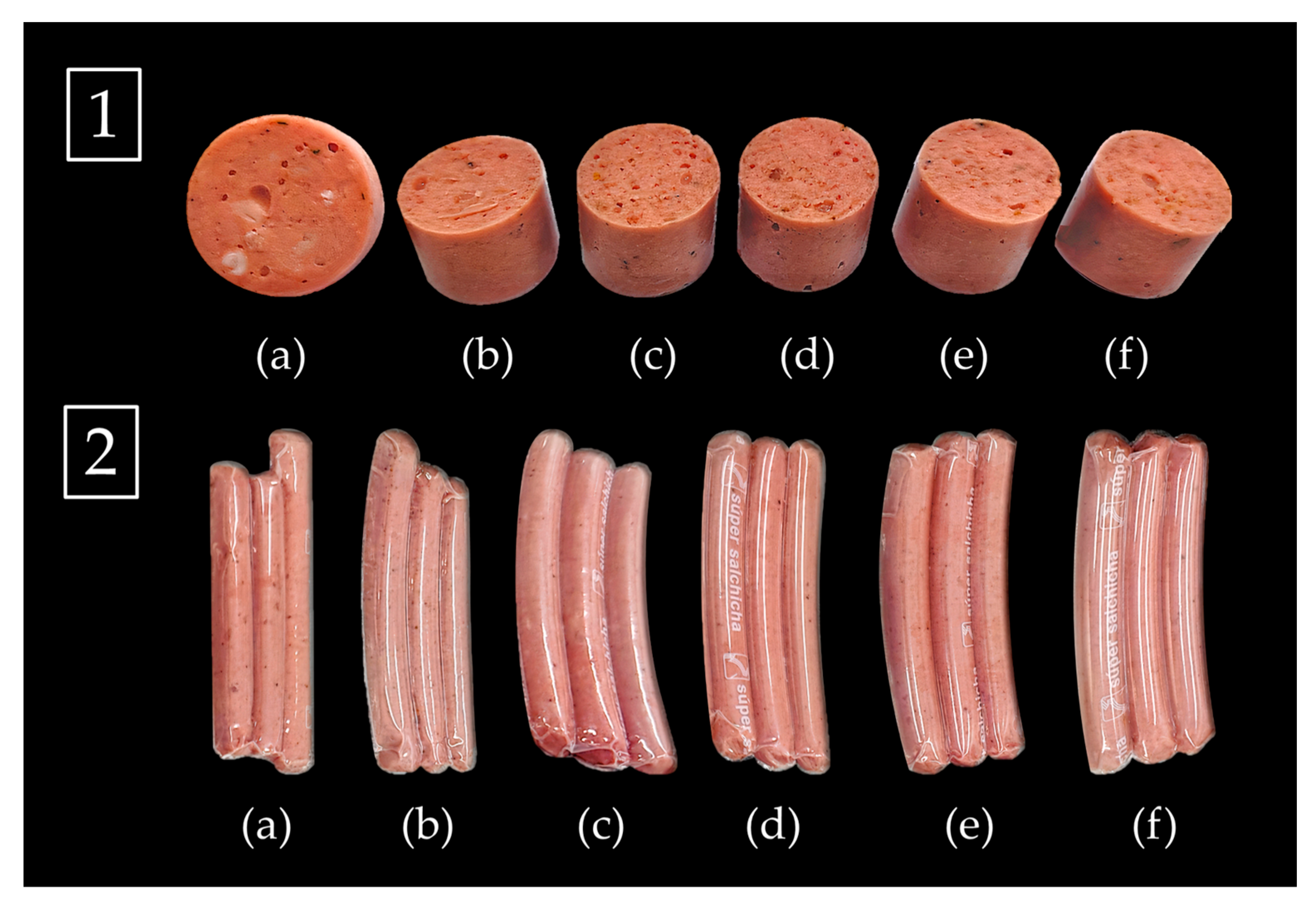
2.5. Application of Dietary Fibers Fractions in Alpaca-Based Sausage
2.5.1. Elaboration of Low-Fat Frankfurter-Type Sausages
2.5.2. Texture Profile Analysis (TPA)
2.5.3. Cooking Losses (CL), Water Activity (aw), and Processing Yield (PY)
2.5.4. Instrumental Color Analysis
2.5.5. Chemical Composition of the Alpaca-Based Sausage including Dietary Fiber from DFPP
2.6. Statistical Analysis
3. Results and Discussion
3.1. Study of the Drying Kinetics and Effect of Drying Temperature on Dragon Fruit Peels
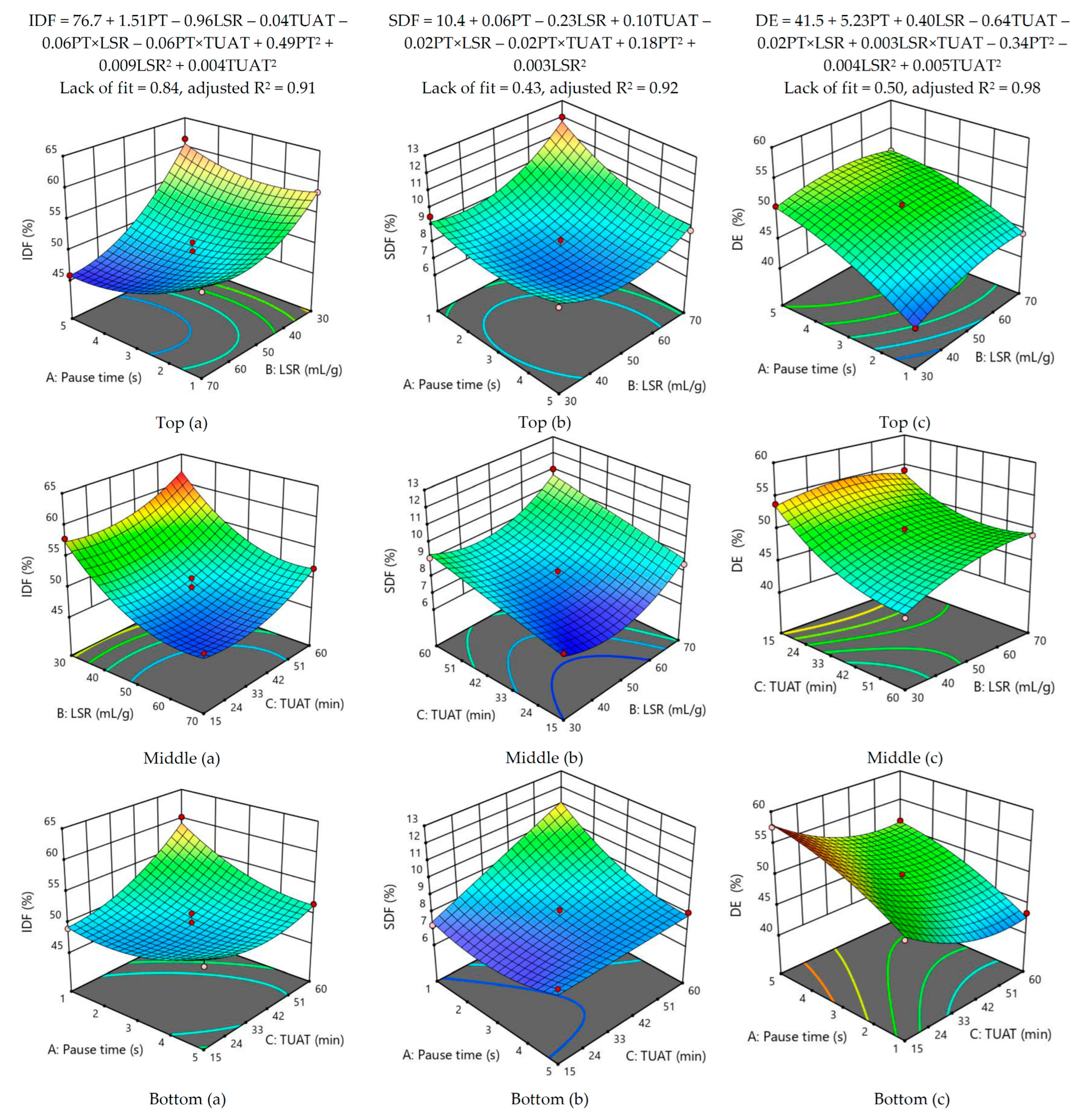
3.2. Optimization of Dietary Fiber Extraction Parameters from DFPP
3.3. Characterization of Optimized Dietary Fiber
3.3.1. Techno–Functional Properties (WHC, OHC, SC) of Dietary Fibers
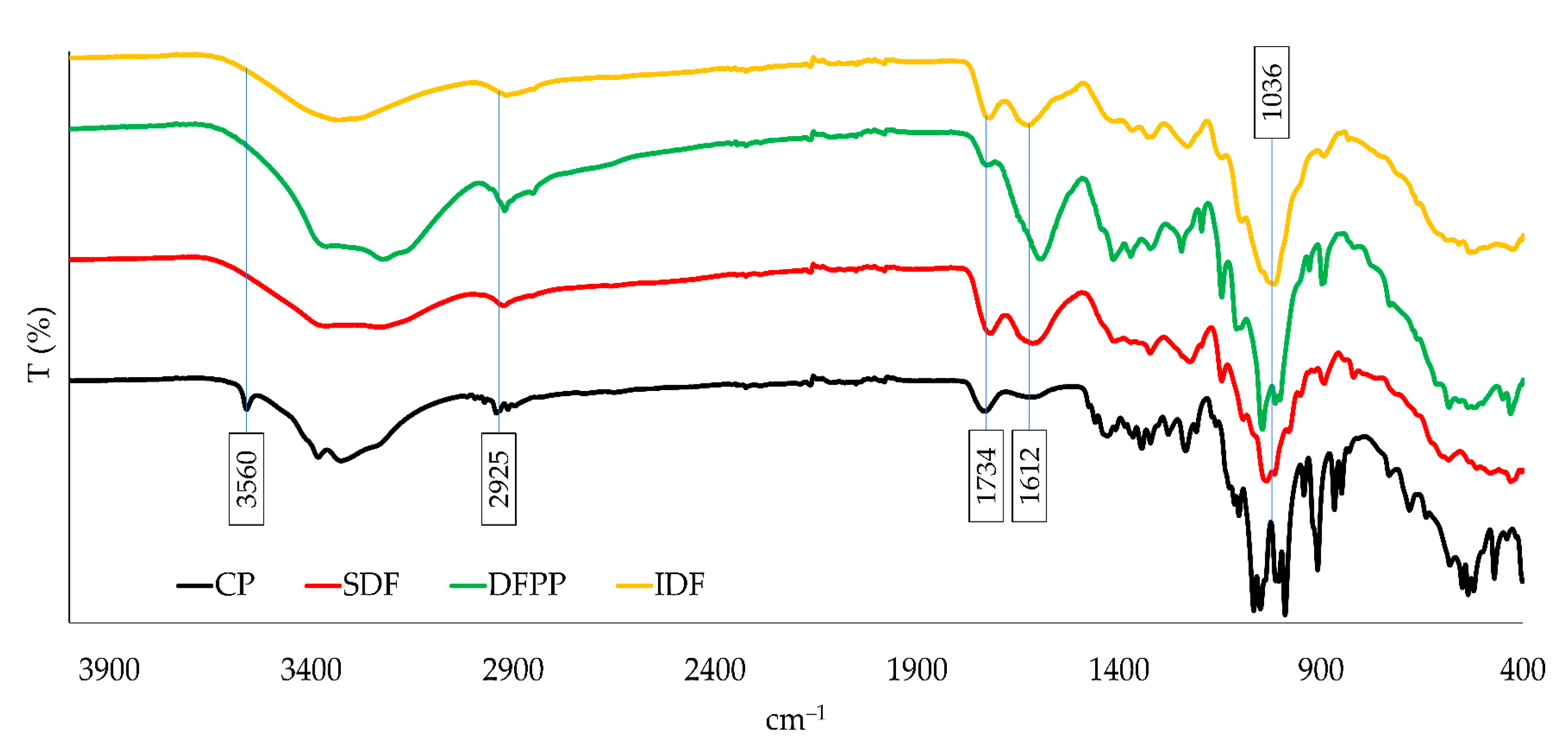
3.3.2. FTIR Analysis
3.4. Effect of the Application of Dietary Fiber in the Production of Alpaca-Based Sausages
3.4.1. Texture Profile Analysis (TPA)
3.4.2. Evaluation of Color Parameters of Frankfurter-Type Sausages and Low-Fat Alpaca-based Sausages
3.4.3. Evaluation of Cooking Losses (CL), Water Activity (aw), and Processing Yield (PY)
3.4.4. Chemical Analysis in an Alpaca-Based Sausage including SDF Obtained from DFPP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chua, B.L.; Tang, S.F.; Ali, A.; Chow, Y.H. Optimisation of Pectin Production from Dragon Fruit Peels Waste: Drying, Extraction and Characterisation Studies. SN Appl. Sci. 2020, 2, 621. [Google Scholar] [CrossRef]
- Le Bellec, F.; Vaillant, F. Pitahaya (Pitaya) (Hylocereus spp.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing Limited: Sawston, UK, 2011; Volume 4, pp. 247–273e. ISBN 9780857090904. [Google Scholar]
- Mercado-Silva, E.M. Pitaya—Hylocereus Undatus (Haw). In Exotic Fruits; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 339–349. ISBN 9780128031384. [Google Scholar]
- Le, N.L. Functional Compounds in Dragon Fruit Peels and Their Potential Health Benefits: A Review. Int. J. Food Sci. Technol. 2022, 57, 2571–2580. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Mucilage from Yellow Pitahaya (Selenicereus Megalanthus) Fruit Peel: Extraction, Proximal Analysis, and Molecular Characterization. Molecules 2023, 28, 786. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, Phytochemical Profile, Bioactivities and Applications in Food Industry of Pitaya (Hylocereus spp.) Peels: A Comprehensive Review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of Fruits and Vegetable By-Products for Isolation of Dietary Fibres and Its Potential Application as Functional Ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Xu, S.Y.; Liu, J.P.; Huang, X.; Du, L.P.; Shi, F.L.; Dong, R.; Huang, X.T.; Zheng, K.; Liu, Y.; Cheong, K.L. Ultrasonic-Microwave Assisted Extraction, Characterization and Biological Activity of Pectin from Jackfruit Peel. LWT—Food Sci. Technol. 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Huang, X.; Jing, H.; Ye, X.; Gao, W.; Bai, X.; Wang, H. Effects of Different Extraction Methods on Structure and Properties of Soluble Dietary Fiber from Defatted Coconut Flour. LWT—Food Sci. Technol. 2021, 143, 111031. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of Extraction Methods on the Structural Characteristics and Functional Properties of Dietary Fiber Extracted from Kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Ameer, K. The Influence of Different Extraction Methods on the Structure, Rheological, Thermal and Functional Properties of Soluble Dietary Fiber from Sanchi (Panax Notoginseng) Flower. Foods 2022, 11, 1995. [Google Scholar] [CrossRef]
- Gerschenson, L.N.; Fissore, E.N.; Rojas, A.M.; Idrovo Encalada, A.M.; Zukowski, E.F.; Higuera Coelho, R.A. Pectins Obtained by Ultrasound from Agroindustrial By-Products. Food Hydrocoll. 2021, 118, 106799. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. The Principles of Ultrasound and Its Application in Freezing Related Processes of Food Materials: A Review. Ultrason. Sonochem. 2015, 27, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Kan, J. Polysaccharides from Ginger Stems and Leaves: Effects of Dual and Triple Frequency Ultrasound Assisted Extraction on Structural Characteristics and Biological Activities. Food Biosci. 2021, 42, 101166. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Sarabia, L.A.; Ortiz, M.C. Response Surface Methodology. Compr. Chemom. 2009, 1, 345–390. [Google Scholar] [CrossRef]
- Gunst, R.F.; Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Technometrics 1996, 38, 285. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, W.; Huang, G.; Huang, H. Ultrasonic-Assisted Extraction, Analysis and Properties of Mung Bean Peel Polysaccharide. Ultrason. Sonochem. 2023, 98, 106487. [Google Scholar] [CrossRef]
- Rao, J.S.; Kumar, B. 3D Blade Root Shape Optimization. In Proceedings of the 10th International Conference on Vibrations in Rotating Machinery, London, UK, 11–13 September 2012; pp. 173–188. [Google Scholar] [CrossRef]
- Borges, K.B.; Pupo, M.T.; De Freitas, L.A.P.; Bonato, P.S. Box–Behnken Design for the Optimization of an Enantioselective Method for the Simultaneous Analysis of Propranolol and 4-Hydroxypropranolol by CE. Electrophoresis 2009, 30, 2874–2881. [Google Scholar] [CrossRef]
- Usman, A.; Sutanto, M.H.; Napiah, M.B.; Yaro, N.S.A. Response Surface Methodology Optimization in Asphalt Mixtures: A Review. In Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- dos Santos, M.; Ozaki, M.M.; Ribeiro, W.O.; de Souza Paglarini, C.; Vidal, V.A.S.; Campagnol, P.C.B.; Pollonio, M.A.R. Emulsion Gels Based on Pork Skin and Dietary Fibers as Animal Fat Replacers in Meat Emulsions: An Adding Value Strategy to Byproducts. LWT—Food Sci. Technol. 2020, 120, 108895. [Google Scholar] [CrossRef]
- Hernawati; Setiawan, N.A.; Shintawati, R.; Priyandoko, D. The Role of Red Dragon Fruit Peel (Hylocereus Polyrhizus) to Improvement Blood Lipid Levels of Hyperlipidaemia Male Mice. J. Phys. Conf. Ser. 2018, 1013, 12167. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y. Pectin and Health. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015; pp. 289–293. ISBN 9780123849533. [Google Scholar]
- Candogan, K.; Kolsarici, N. The Effects of Carrageenan and Pectin on Some Quality Characteristics of Low-Fat Beef Frankfurters. Meat Sci. 2003, 64, 199–206. [Google Scholar] [CrossRef]
- Popova, T.; Tejeda, L.; Peñarrieta, J.M.; Smith, M.A.; Bush, R.D.; Hopkins, D.L. Meat of South American Camelids—Sensory Quality and Nutritional Composition. Meat Sci. 2021, 171, 108285. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Kashaninejad, M.; Jafarianlari, A. Drying Kinetics and Characteristics of Combined Infrared-Vacuum Drying of Button Mushroom Slices. Heat Mass Transf. Stoffuebertragung 2017, 53, 1751–1759. [Google Scholar] [CrossRef]
- Mewa, E.A.; Okoth, M.W.; Kunyanga, C.N.; Rugiri, M.N. Experimental Evaluation of Beef Drying Kinetics in a Solar Tunnel Dryer. Renew. Energy 2019, 139, 235–241. [Google Scholar] [CrossRef]
- Bochek, A.M.; Zabivalova, N.M.; Petropavlovskii, G.A. Determination of the Esterification Degree of Polygalacturonic Acid. Russ. J. Appl. Chem. 2001, 74, 796–799. [Google Scholar] [CrossRef]
- Valencia, F.E.; Román, M.O. Caracterización Fisicoquímica y Funcional de Tres Concentrados Comerciales de Fibra Dietaria. Vitae 2006, 13, 54–60. [Google Scholar]
- Chau, C.F.; Huang, Y.L. Comparison of the Chemical Composition and Physicochemical Properties of Different Fibers Prepared from the Peel of Citrus sinensis L. Cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Characterization of Pectin from Grape Pomace: A Comparison of Conventional and Pulsed Ultrasound-Assisted Extraction Techniques. Foods 2022, 11, 2274. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Q.; Zhuang, X.; Wang, Y.; Zhou, G.; Zhang, W. Effect of Regenerated Cellulose Fiber on the Physicochemical Properties and Sensory Characteristics of Fat-Reduced Emulsified Sausage. LWT 2018, 97, 157–163. [Google Scholar] [CrossRef]
- Wongpattananukul, S.; Nungarlee, U.; Ruangprach, A.; Sulong, S.; Sanporkha, P.; Adisakwattana, S.; Ngamukote, S. Effect of Inca Peanut Oil on Omega-3 Polyunsaturated Fatty Acids, Physicochemical, Texture and Sensory Properties in Chicken Sausage. LWT 2022, 163, 113559. [Google Scholar] [CrossRef]
- Encina-Zelada, C.R.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Optimization of Quality Properties of Gluten-Free Bread by a Mixture Design of Xanthan, Guar, and Hydroxypropyl Methyl Cellulose Gums. Foods 2019, 8, 156. [Google Scholar] [CrossRef]
- Oshima, T.; Kato, K.; Imaizumi, T. Effects of Blanching on Drying Characteristics, Quality, and Pectin Nanostructures of Dried Cut-Persimmons. LWT—Food Sci. Technol. 2021, 143, 111094. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the Association of Official Analytical Chemists. Official Methods of Analysis of AOAC INTERNATIONAL, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Chau, J. Package “gslnls”—GSL Nonlinear Least-Squares Fitting. 2022. Available online: https://cran.r-project.org/web/packages/gslnls/gslnls.pdf (accessed on 25 June 2023).
- Hamner, B.; Frasco, M.; LeDell, E. Package “Metrics” —Evaluation Metrics for Machine Learning. 2022. Available online: https://cran.r-project.org/web/packages/Metrics/Metrics.pdf (accessed on 25 June 2023).
- de Mendiburu, F. Package “agricolae”—Statistical Procedures for Agricultural Research. 2021. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 25 June 2023).
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the Thin-Layer Drying of Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.A.; Hameed, I.A.; Hassan, A.K. Forecasting by Using the Optimal Time Series Method. Adv. Intell. Syst. Comput. AISC 2020, 1152, 148–154. [Google Scholar]
- Liemohn, M.W.; Shane, A.D.; Azari, A.R.; Petersen, A.K.; Swiger, B.M.; Mukhopadhyay, A. RMSE Is Not Enough: Guidelines to Robust Data-Model Comparisons for Magnetospheric Physics. J. Atmos. Solar-Terr. Phys. 2021, 218, 105624. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, G.; Mu, G.; Zhao, C.; Huang, H.; Kang, C.; Li, X.; Zhang, Q. Design of a Greenhouse Solar-Assisted Heat Pump Dryer for Kelp (Laminaria Japonica): System Performance and Drying Kinetics. Foods 2022, 11, 3509. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Fudholi, A.; Muthuvalu, M.S.; Sulaiman, J.; Yasir, S.M. Implications of Drying Temperature and Humidity on the Drying Kinetics of Seaweed. In Proceedings of the AIP Conference Proceedings, Kedah, Malaysia, 4–7 December 2017; 2017; 1905. [Google Scholar] [CrossRef]
- Thewes, R.F.; Both, V.; Brackmann, A.; Thewes, R.F.; Soldateli, J.F.; Pasquetti Berghetti, R.M.; Ludwig, V.; Mallmann Wendt, L.; Ribas Schiefelbein, H. Dynamic and Static Drying Temperatures for ‘Barton’ Pecans: Impacts on the Volatile Compounds Profile and Kernel Color. LWT—Food Sci. Technol. 2022, 161, 113393. [Google Scholar] [CrossRef]
- Reis, S.; Klein, B.; Machado Ribeiro, Q.; Duarte dos Santos, I.; Gomes Genro, A.L.; de Freitas Ferreira, D.; Janner Hamann, J.; Smanioto Barin, J.; Cichoski, A.J.; Fronza, D.; et al. Chemical Composition and Oxidative Stability of Eleven Pecan Cultivars Produced in Southern Brazil. Food Res. Int. 2020, 136, 109596. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.F.; Rinaudo, M. Chain Association of Pectic Molecules during Calcium-induced Gelation. Biopolymers 1986, 25, 455–468. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of Soluble Dietary Fiber-Polysaccharide from Papaya Peel Obtained through Alkaline or Ultrasound-Assisted Alkaline Extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current Trends of Tropical Fruit Waste Utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Khalid, M.; Younis, K. Interaction Study of Dietary Fibers (Pectin and Cellulose) with Meat Proteins Using Bioinformatics Analysis: An In-Silico Study. LWT—Food Sci. Technol. 2020, 119, 108889. [Google Scholar] [CrossRef]
- Choe, J.; Kim, H.Y. Quality Characteristics of Reduced Fat Emulsion-Type Chicken Sausages Using Chicken Skin and Wheat Fiber Mixture as Fat Replacer. Poult. Sci. 2019, 98, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Yadegari, R.J.; Kaya, E.E.; Vural, H. Thermal and Microscopic Properties and Quality Characteristics of Low-Fat Frankfurters and Emulsions Produced with Carboxymethyl Cellulose, Methyl Cellulose and Pectin. Carpathian J. Food Sci. Technol. 2021, 13, 150–164. [Google Scholar] [CrossRef]
- Ozdemir, Y.; Oncel, B.; Keceli, M. Purification of Crude Fiber from Carob Molasses Pulp and Uses in Traditional Turkish Sucuk. Int. J. Gastron. Food Sci. 2021, 25, 100410. [Google Scholar] [CrossRef]
- Xie, J.; Peng, G.; Hu, X.; Xie, J.; Chen, Y.; Dong, R.; Si, J.; Yang, C.; Yu, Q. Physicochemical Characteristics of Soluble Dietary Fiber Obtained from Grapefruit Peel Insoluble Dietary Fiber and Its Effects on Blueberry Jam. Foods 2022, 11, 3735. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Xie, J.; Jin, Z.; Ge, S.; Guan, F.; Liu, H.; Zheng, M.; Cai, D.; Liu, J. Removal of Bound Polyphenols and Its Effect on Structure, Physicochemical and Functional Properties of Insoluble Dietary Fiber from Adzuki Bean Seed Coat. Lwt 2022, 169, 114011. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, H.; Swallah, M.S.; Fu, H.; Shen, Y.; Guo, Z.; Tong, X.; Li, Y.; Yu, H.; Jiang, L. Structure, Properties and Potential Bioactivities of High-Purity Insoluble Fibre from Soybean Dregs (Okara). Food Chem. 2021, 364, 130402. [Google Scholar] [CrossRef]
- Zadeike, D.; Vaitkeviciene, R.; Degutyte, R.; Bendoraitiene, J.; Rukuiziene, Z.; Cernauskas, D.; Svazas, M.; Juodeikiene, G. A Comparative Study on the Structural and Functional Properties of Water-Soluble and Alkali-Soluble Dietary Fibres from Rice Bran after Hot-Water, Ultrasound, Hydrolysis by Cellulase, and Combined Pre-Treatments. Int. J. Food Sci. Technol. 2022, 57, 1137–1149. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Advances in the Functional Characterization and Extraction Processes of Dietary Fiber. Food Eng. Rev. 2016, 8, 251–271. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of Pectin Extraction from Banana Peels with Citric Acid by Using Response Surface Methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple Pomaces Derived from Mono-Varietal Asturian Ciders Production Are Potential Source of Pectins with Appealing Functional Properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef] [PubMed]
- Reichembach, L.H.; de Oliveira, L.C. Pectins from Alternative Sources and Uses beyond Sweets and Jellies: An Overview. Food Hydrocoll. 2021, 118, 106824. [Google Scholar] [CrossRef]
- Abbasi, E.; Amini Sarteshnizi, R.; Ahmadi Gavlighi, H.; Nikoo, M.; Azizi, M.H.; Sadeghinejad, N. Effect of Partial Replacement of Fat with Added Water and Tragacanth Gum (Astragalus gossypinus and Astragalus compactus) on the Physicochemical, Texture, Oxidative Stability, and Sensory Property of Reduced Fat Emulsion Type Sausage. Meat Sci. 2019, 147, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nollet, L.M.L.; Toldra, F. Handbook of Processed Meats and Poultry Analysis; Leo, M.L., Nollet, F.T., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 9781439847961. [Google Scholar]
- Martín, N.P.; Schreurs, N.M.; Morris, S.T.; López-Villalobos, N.; McDade, J.; Hickson, R.E. Meat Quality of Beef-Cross-Dairy Cattle from Angus or Hereford Sires: A Case Study in a Pasture-Based System in New Zealand. Meat Sci. 2022, 190, 108840. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Bush, R.D.; van de Ven, R.J.; Hall, E.J.S.; Greenwood, P.L.; Hopkins, D.L. The Impact of Gender and Age on the Nutritional Parameters of Alpaca (Vicugna pacos) Meat, Colour Stability and Fat Traits. Meat Sci. 2017, 123, 21–28. [Google Scholar] [CrossRef]

| Treatment | X1 | X2 | X3 |
|---|---|---|---|
| T1 | 1.00 (–1) | 30.0 (–1) | 37.5 (0) |
| T2 | 5.00 (1) | 30.0 (–1) | 37.5 (0) |
| T3 | 1.00 (–1) | 70.0 (1) | 37.5 (0) |
| T4 | 5.00 (1) | 70.0 (1) | 37.5 (0) |
| T5 | 1.00 (–1) | 50.0 (0) | 15.0 (–1) |
| T6 | 5.00 (1) | 50.0 (0) | 15.0 (–1) |
| T7 | 1.00 (–1) | 50.0 (0) | 60.0 (1) |
| T8 | 5.00 (1) | 50.0 (0) | 60.0 (1) |
| T9 | 3.00 (0) | 30.0 (–1) | 15.0 (–1) |
| T10 | 3.00 (0) | 70.0 (1) | 15.0 (–1) |
| T11 | 3.00 (0) | 30.0 (–1) | 60.0 (1) |
| T12 | 3.00 (0) | 70.0 (1) | 60.0 (1) |
| T13 | 3.00 (0) | 50.0 (0) | 37.5 (0) |
| T14 | 3.00 (0) | 50.0 (0) | 37.5 (0) |
| T15 | 3.00 (0) | 50.0 (0) | 37.5 (0) |
| Ingredient (%) | C1 | C2 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|---|
| Alpaca meat | 48.96 | 48.96 | 48.96 | 48.96 | 48.96 | 48.96 |
| Pork back fat | 23.10 | - | - | - | - | - |
| Water | 16.65 | 33.95 | 33.87 | 33.95 | 33.76 | 33.52 |
| Oil | - | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 |
| SDF | - | - | - | - | 0.80 | 0.54 |
| IDF | - | - | - | 0.70 | - | 0.54 |
| Calcium chloride | - | - | 0.08 | - | 0.08 | 0.06 |
| DFPP | - | - | 0.70 | - | - | - |
| Carrageenan | - | 0.70 | - | - | - | - |
| Sodium chloride | 1.73 | 1.73 | 1.73 | 1.73 | 1.73 | 1.73 |
| Curing salt (20% nitrites) | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Sodium erythorbate | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Sodium polyphosphate | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 |
| Sugar | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 |
| Potato starch | 3.92 | 3.92 | 3.92 | 3.92 | 3.92 | 3.92 |
| Soy concentrate | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 |
| Milk powder | 2.15 | 2.15 | 2.15 | 2.15 | 2.15 | 2.15 |
| Black pepper | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Cumin powder | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Nutmeg | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Monosodium glutamate | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Carmine colorant | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Smoke essence | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Analysis | Temperature (°C) | |||
|---|---|---|---|---|
| 40 | 55 | 70 | ||
| Drying | Time (h) | 10.0 ± 0.3278 a | 6.24 ± 0.0610 b | 5.25 ± 0.4637 c |
| Deff (m2/s) | 1.02 × 10–10 ± 3.75 × 10–12 c | 1.49 × 10–10 ± 1.06 × 10–11 b | 2.10 × 10–10 ± 2.15 × 10–11 a | |
| Color | L* | 72.0 ± 1.172 b | 74.6 ± 0.973 a | 76.2 ± 0.057 a |
| a* | 7.04 ± 0.290 a | 3.79 ± 0.497 b | 4.22 ± 0.735 b | |
| b* | 27.1 ± 0.373 b | 27.9 ± 0.800 b | 31.4 ± 0.318 a | |
| Yield (%) | IDF | 51.2 ± 0.220 b | 51.8 ± 0.337 a | 51.7 ± 0.122 ab |
| SDF | 8.60 ± 0.211 a | 8.34 ± 0.070 a | 7.49 ± 0.078 b | |
| Treatment | IDF (%) | SDF (%) | DE (%) |
|---|---|---|---|
| T1 | 58.5 ± 0.10 a | 9.54 ± 0.27 c | 41.1 ± 1.56 f |
| T2 | 61.4 ± 1.60 a | 8.77 ± 0.05 def | 49.5 ± 1.50 de |
| T3 | 51.8 ± 2.14 b | 12.0 ± 0.21 a | 43.1 ± 1.33 f |
| T4 | 45.8 ± 1.71 c | 8.82 ± 0.63 de | 51.7 ± 0.73 cd |
| T5 | 49.0 ± 1.75 bc | 7.22 ± 0.45 j | 48.1 ± 1.24 e |
| T6 | 51.2 ± 1.51 b | 8.13 ± 0.07 fghi | 58.1 ± 0.76 a |
| T7 | 60.2 ± 0.38 a | 10.8 ± 0.08 b | 41.7 ± 2.48 f |
| T8 | 51.6 ± 0.91 b | 8.09 ± 0.31 ghi | 50.5 ± 1.26 cde |
| T9 | 57.9 ± 2.09 a | 7.60 ± 0.46 hij | 55.4 ± 2.31 ab |
| T10 | 47.8 ± 0.11 bc | 8.45 ± 0.22 efg | 52.7 ± 2.04 bc |
| T11 | 60.8 ± 1.29 a | 9.13 ± 0.09 cd | 43.8± 0.03 f |
| T12 | 51.7 ± 0.93 b | 11.0 ± 0.11 b | 50.0 ± 1.46 cde |
| T13 | 46.7 ± 2.95 c | 7.82 ± 0.30 ghij | 49.8 ± 0.37 cde |
| T14 | 50.8 ± 4.16 b | 7.51 ± 0.39 ij | 49.5 ± 0.78 de |
| T15 | 49.3 ± 2.04 bc | 8.19 ± 0.13 efgh | 49.4 ± 0.49 d |
| Dependent Variables | Lower Limit | Upper Limit | Predicted Value | Experimental Value |
|---|---|---|---|---|
| IDF (%) | 61.1 | 70.1 | 65.6 a | 61.3 ± 0.578 a |
| SDF (%) | 9.98 | 12.2 | 11.1 b | 10.8 ± 0.162 b |
| DE (%) | 36.0 | 39.7 | 37.8 c | 39.7 ± 0.374 c |
| Dietary Fiber | WHC (g/g) | OHC (g/g) | SC (mL/g) |
|---|---|---|---|
| IDF | 11.0 ± 0.240 | 5.00 ± 0.347 a | 4.86 ± 0.031 |
| SDF | n.d. | 0.33 ± 0.011 b | n.d. |
| Analysis | C1 | C2 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|---|
| Hardness (N) | 43.6 ± 4.21 a | 22.6 ± 1.71 b | 16.2 ± 1.40 c | 12.8 ± 1.09 d | 24.2 ± 1.48 b | 17.3 ± 0.926 c |
| Chewiness (N) | 19.8 ± 1.36 a | 12.1 ± 1.28 b | 6.02 ± 0.768 c | 4.38 ± 0.554 d | 11.8 ± 1.37 b | 6.77 ± 0.683 c |
| Springiness | 0.800 ± 0.0001 d | 0.886 ± 0.0378 ab | 0.871 ± 0.0488 ab | 0.829 ± 0.0488 cd | 0.900 ± 0.0001 a | 0.857 ± 0.0535 bc |
| Cohesiveness | 0.571 ± 0.0488 ab | 0.600 ± 0.0001 a | 0.429 ± 0.0488 c | 0.414 ± 0.0378 c | 0.543 ± 0.0535 b | 0.457 ± 0.0535 c |
| Adhesiveness (mJ) | 0.663 ± 0.124 a | 0.256 ± 0.149 c | 0.476 ± 0.0441 b | 0.281 ± 0.176 c | 0.339 ± 0.195 bc | 0.394 ± 0.186 bc |
| Rupture force (N) | 43.8 + 3.20 a | 25.5 + 0.704 b | 14.7 + 1.43 e | 10.7 + 1.41 f | 22.1 + 2.35 c | 18.1 + 1.03 d |
| Shear stress (N) | 38.0 ± 2.89 a | 27.8 ± 7.58 b | 29.5 ± 5.09 b | 20.4 ± 3.51 c | 26.9 ± 0.786 bc | 25.2 ± 1.45 bc |
| aw | 0.978 ± 0.569 c | 0.984 ± 0.267 b | 0.988 ± 0.101 ab | 0.987 ± 0.132 ab | 0.99 ± 0.110 a | 0.99 ± 0.070 a |
| Processing yield (%) | 94.5 ± 0.467 bc | 92.4 ± 0.0564 d | 95.0 ± 0.0505 a | 91.9 ± 0.0541 e | 94.9 ± 0.104 ab | 94.3 ± 0.0594 c |
| CL | 23.2 ± 0.324 d | 25.5 ± 0.559 c | 28.0 ± 0.4170 b | 30.2 ± 0.164 a | 28.6 ± 0.660 b | 30.6 ± 0.241 a |
| L* | 43.1 ± 0.551 c | 58.9 ± 0.203 b | 59.6 ± 0.0666 a | 58.7 ± 0.356 b | 59.6 ± 0.214 a | 59.7 ± 0.459 a |
| a* | 16.1 ± 0.733 c | 17.5 ± 0.250 ab | 17.1 ± 0.247 ab | 17.7 ± 0.156 a | 17.4 ± 0.108 ab | 16.9 ± 0.197 b |
| b* | 11.4 ± 0.269 a | 9.07 ± 0.174 d | 10.8 ± 0.0458 b | 10.5 ± 0.146 b | 9.92 ± 0.246 c | 10.3 ± 0.549 bc |
| Chroma | 19.8 ± 0.650 b | 19.7 ± 0.274 b | 20.2 ± 0.231 ab | 20.6 ± 0.134 a | 20.0 ± 0.211 ab | 19.8 ± 0.356 b |
| Hue° | 35.3 ± 1.260 a | 27.3 ± 0.405 d | 32.1 ± 0.275 b | 30.7 ± 0.465 bc | 29.7 ± 0.477 c | 31.2 ± 1.330 b |
| Chemical Composition | Amount * |
|---|---|
| Moisture (%) | 70.6 ± 0.021 |
| Protein (%) | 14.0 ± 0.120 |
| Fat (%) | 4.01 ± 0.002 |
| Total carbohydrates (%) | 8.53 ± 0.136 |
| Ash (%) | 2.91 ± 0.035 |
| Crude fiber (%) | 0.30 ± 0.028 |
| Iron (ppm) | 19.0 ± 0.007 |
| Zinc (ppm) | 11.9 ± 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilcapoma, W.; de Bruijn, J.; Elías-Peñafiel, C.; Espinoza, C.; Farfán-Rodríguez, L.; López, J.; Encina-Zelada, C.R. Optimization of Ultrasound-Assisted Extraction of Dietary Fiber from Yellow Dragon Fruit Peels and Its Application in Low-Fat Alpaca-Based Sausages. Foods 2023, 12, 2945. https://doi.org/10.3390/foods12152945
Vilcapoma W, de Bruijn J, Elías-Peñafiel C, Espinoza C, Farfán-Rodríguez L, López J, Encina-Zelada CR. Optimization of Ultrasound-Assisted Extraction of Dietary Fiber from Yellow Dragon Fruit Peels and Its Application in Low-Fat Alpaca-Based Sausages. Foods. 2023; 12(15):2945. https://doi.org/10.3390/foods12152945
Chicago/Turabian StyleVilcapoma, Wilber, Johannes de Bruijn, Carlos Elías-Peñafiel, Clara Espinoza, Lucero Farfán-Rodríguez, Jorge López, and Christian R. Encina-Zelada. 2023. "Optimization of Ultrasound-Assisted Extraction of Dietary Fiber from Yellow Dragon Fruit Peels and Its Application in Low-Fat Alpaca-Based Sausages" Foods 12, no. 15: 2945. https://doi.org/10.3390/foods12152945
APA StyleVilcapoma, W., de Bruijn, J., Elías-Peñafiel, C., Espinoza, C., Farfán-Rodríguez, L., López, J., & Encina-Zelada, C. R. (2023). Optimization of Ultrasound-Assisted Extraction of Dietary Fiber from Yellow Dragon Fruit Peels and Its Application in Low-Fat Alpaca-Based Sausages. Foods, 12(15), 2945. https://doi.org/10.3390/foods12152945








