Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Viola odorata Extract (VOE)
2.3. Preparation of Potato Starch/VOE Active Films
2.3.1. Determination of Thickness and Mechanical Properties
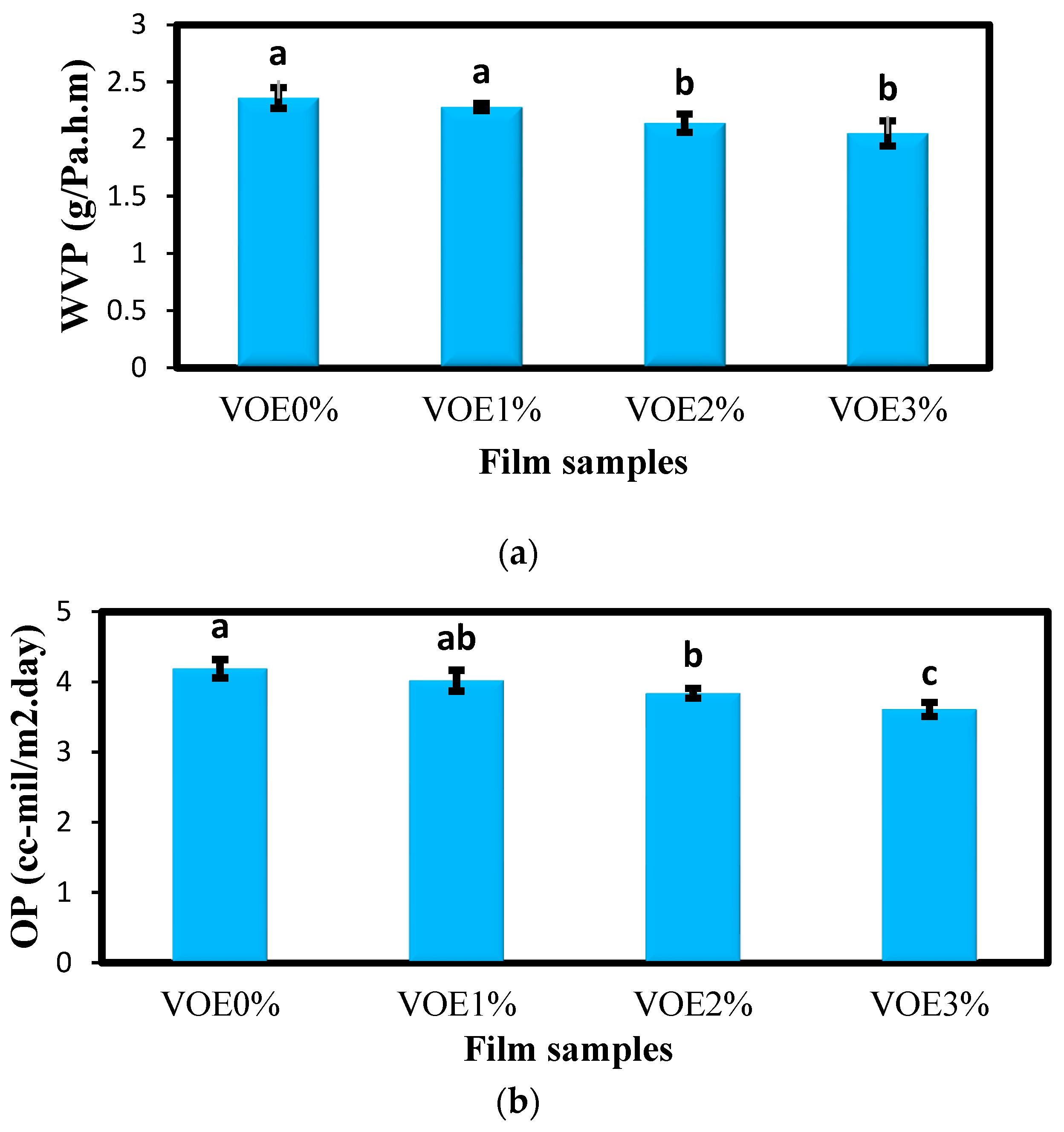
2.3.2. Determination of Barrier Properties
2.3.3. Determination of Opacity and UV–Visible Transmission
2.3.4. Determination of Total Phenol Content and Antioxidant Activity
2.3.5. Determination of Antibacterial Activity
2.4. Preparation of Chicken Fillets
2.4.1. Determination of pH and TVB-N
2.4.2. Determination of Fat Oxidation
2.4.3. Determination of Protein Oxidation
2.4.4. Determination of Metmyoglobin Content
2.4.5. Determination of Microbial Load
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of the Active Film
3.1.1. Thickness and Mechanical Properties
3.1.2. Barrier Properties
3.1.3. Opacity and Transmission of UV–Visible Light
3.1.4. Total Phenol Content and Antioxidant Activity
3.1.5. Antibacterial Activity
3.2. The Characteristic of Chicken Fillets
3.2.1. PH and Total Volatile Basic Nitrogen (TVB-N)
3.2.2. Oxidation of Fats
3.2.3. Protein Oxidation
3.2.4. Metmyoglobin
3.2.5. Microbial Load
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ajaykumar, V.; Mandal, P.K. Modern concept and detection of spoilage in meat and meat products. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–349. [Google Scholar]
- Jafarzadeh, S.; Hadidi, M.; Forough, M.; Nafchi, A.M.; Mousavi Khaneghah, A. The control of fungi and mycotoxins by food active packaging: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–19, in press. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.M.; Pereira, C.; Calhelha, R.C.; Barros, L.; Petrovic, J.; Sokovic, M.; Barreiro, M.F.; Ferreira, I.C.F.R.; Castro, M.C.R.; Rodrigues, P.V.; et al. Evaluation of plant extracts as an efficient source of additives for active food packaging. Food Front. 2022, 3, 480–488. [Google Scholar] [CrossRef]
- Abedinia, A.; Alimohammadi, F.; Teymori, F.; Razgardani, N.; Saeidi Asl, M.R.; Ariffin, F.; Mohammadi Nafchi, A.; Huda, N.; Roslan, J. Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics. Foods 2021, 10, 1761. [Google Scholar] [CrossRef]
- Ebrahimi, V.; Mohammadi Nafchi, A.; Bolandi, M.; Baghaei, H. Fabrication and characterization of a pH-sensitive indicator film by purple basil leaves extract to monitor the freshness of chicken fillets. Food Packag. Shelf Life 2022, 34, 100946. [Google Scholar] [CrossRef]
- Esfahani, A.; Mohammadi Nafchi, A.; Baghaei, H.; Nouri, L. Fabrication and characterization of a smart film based on cassava starch and pomegranate peel powder for monitoring lamb meat freshness. Food Sci. Nutr. 2022, 10, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Babapour, H.; Johari, S.; Alimohammadi, F.; Teymori, F.; Nafchi, A.M.; Shahrai, N.N.; Huda, N.; Abedinia, A. Application of Poultry Gelatin to Enhance the Physicochemical, Mechanical, and Rheological Properties of Fish Gelatin as Alternative Mammalian Gelatin Films for Food Packaging. Foods 2023, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Wijekoon, M.M.J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef]
- Bagher Abiri, A.; Baghaei, H.; Mohammadi Nafchi, A. Preparation and Application of Active Bionanocomposite Films Based on Sago Starch Reinforced with a Combination of TiO2 Nanoparticles and Penganum harmala Extract for Preserving Chicken Fillets. Polymers 2023, 15, 2889. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural anthocyanins: Sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf Life 2022, 33, 100872. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Azlim, N.A.; Mohammadi Nafchi, A.; Oladzadabbasabadi, N.; Ariffin, F.; Ghalambor, P.; Jafarzadeh, S.; Al-Hassan, A.A. Fabrication and characterization of a pH-sensitive intelligent film incorporating dragon fruit skin extract. Food Sci. Nutr. 2022, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Khanniri, E.; Shadnoush, M.; Khorshidian, N.; Mortazavian, A.M. Development, characterization and in vitro antioxidant activity of chitosan-coated alginate microcapsules entrapping Viola odorata Linn. extract. Int. J. Biol. Macromol. 2020, 163, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Zaigham, M.; Naquibuddin, M.; Rather, G.J. Phyto-chemical profile and pharmacological activities of Banafsha (Viola odorata Linn): An important herb of Unani Medicine. J. Res. Tradit. Med. 2020, 5, 58. [Google Scholar] [CrossRef]
- Fazeenah, A.; Quamri, M.A. Banafsha (Viola odorata Linn.)—A review. World J. Pharm. Res. 2020, 9, 514–537. [Google Scholar]
- Zawiślak, A.; Francik, R.; Francik, S.; Knapczyk, A. Impact of drying conditions on antioxidant activity of red clover (Trifolium pratense), sweet violet (Viola odorata) and elderberry flowers (Sambucus nigra). Materials 2022, 15, 3317. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.; Hubinger, M.D.; Menegalli, F.C. Development of active films from pectin and fruit extracts: Light protection, antioxidant capacity, and compounds stability. J. Food Sci. 2015, 80, C2389–C2396. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Zhong, J.; Ayatollahi, S.A.; Kobarfard, F.; Faizi, M.; Khosravi-Dehaghi, N.; Suleria, H.A. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Prosopis farcta (Banks & Sol.) JF Macbr. and their potential antioxidant activities. Cell. Mol. Biol. 2021, 67, 189–200. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of total anthocyanin content, stability and antioxidant evaluation of the anthocyanin extract from Vietnamese Carissa carandas L. fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Lohvina, H.; Sándor, M.; Wink, M. Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic Composition by HPLC-ESI-MS. Diversity 2022, 14, 7. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of anthocyanin associated purple sweet potato starch and peel-based pH indicator films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Ahmadi, R.; Tanomand, A.; Kazeminava, F.; Kamounah, F.S.; Ayaseh, A.; Ganbarov, K.; Yousefi, M.; Katourani, A.; Yousefi, B.; Kafil, H.S. Fabrication and characterization of a titanium dioxide (TiO2) nanoparticles reinforced bio-nanocomposite containing Miswak (Salvadora persica L.) extract–the antimicrobial, thermo-physical and barrier properties. Int. J. Nanomed. 2019, 14, 3439–3454. [Google Scholar] [CrossRef] [Green Version]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control. 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.; Mojaddar Langroodi, A.; Amiri, S.; Radi, M. Effect of nanocomposite alginate-based film incorporated with cumin essential oil and TiO2 nanoparticles on chemical, microbial, and sensory properties of fresh meat/beef. Food Sci. Nutr. 2022, 10, 1401–1413. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent pH-sensitive indicator based on starch-cellulose and alizarin dye to track freshness of rainbow trout fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jebelli Javan, A.; Ghazvinian, K.; Mahdavi, A.; Javaheri Vayeghan, A.; Staji, H.; Ghaffari Khaligh, S. The effect of dietary Zataria multiflora Boiss. essential oil supplementation on microbial growth and lipid peroxidation of broiler breast fillets during refrigerated storage. J. Food Process. Preserv. 2013, 37, 881–888. [Google Scholar] [CrossRef]
- Özer, C.O.; Secen, S.M. Effects of quinoa flour on lipid and protein oxidation in raw and cooked beef burger during long term frozen storage. Food Sci. Technol. 2018, 38, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Naveena, B.M.; Muthukumar, M.; Sen, A.R.; Babji, Y.; Murthy, T.R.K. Improvement of shelf-life of buffalo meat using lactic acid, clove oil and vitamin C during retail display. Meat Sci. 2006, 74, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Shange, N.; Makasi, T.; Gouws, P.; Hoffman, L.C. Preservation of previously frozen black wildebeest meat (Connochaetes gnou) using oregano (Oreganum vulgare) essential oil. Meat Sci. 2019, 148, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Babapour, H.; Jalali, H.; Mohammadi Nafchi, A. The synergistic effects of zinc oxide nanoparticles and fennel essential oil on physicochemical, mechanical, and antibacterial properties of potato starch films. Food Sci. Nutr. 2021, 9, 3893–3905. [Google Scholar] [CrossRef] [PubMed]
- García, A.V.; Álvarez-Pérez, O.B.; Rojas, R.; Aguilar, C.N.; Garrigós, M.C. Impact of olive extract addition on corn starch-based active edible films properties for food packaging applications. Foods 2020, 9, 1339. [Google Scholar] [CrossRef]
- de Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef]
- Nouri, L.; Nafchi, A.M. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fan, Y.; Cui, J.; Yang, L.; Su, H.; Yang, P.; Pan, J. Colorimetric films based on pectin/sodium alginate/xanthan gum incorporated with raspberry pomace extract for monitoring protein-rich food freshness. Int. J. Biol. Macromol. 2021, 185, 959–965. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Cheng, M.; Yan, X.; Cui, Y.; Han, M.; Wang, Y.; Wang, J.; Zhang, R.; Wang, X. Characterization and release kinetics study of active packaging films based on modified starch and red cabbage anthocyanin extract. Polymers 2022, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef]
- Karioti, A.; Furlan, C.; Vincieri, F.F.; Bilia, A.R. Analysis of the constituents and quality control of Viola odorata aqueous preparations by HPLC-DAD and HPLC-ESI-MS. Anal. Bioanal. Chem. 2011, 399, 1715–1723. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Go, E.J.; Song, K.B. Antioxidant properties of rye starch films containing rosehip extract and their application in packaging of chicken breast. Starch-Stärke 2019, 71, 1900116. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Shi, Q.; Zhang, Y.; Liu, J.; Wu, X.; Fang, Z. Development and characterization of active and pH-sensitive films based on psyllium seed gum incorporated with free and microencapsulated mulberry pomace extracts. Food Chem. 2021, 352, 129333. [Google Scholar] [CrossRef]
- Ibraheem, R.; Mhawesh, A.; Abood, K. Estimation of the whole flavonoid, antioxidant, anti bacterial challenge concerning viola odorata (banafsha) methanolic extract. Iraqi J. Agric. Sci. 2018, 49, 655. [Google Scholar] [CrossRef]
- Alipanah, H.; Bigdeli, M.R.; Esmaeili, M.A. Inhibitory effect of Viola odorata extract on tumor growth and metastasis in 4T1 breast cancer model. Iran. J. Pharm. Res. IJPR 2018, 17, 276. [Google Scholar]
- Granato, D.; Nunes, D.S.; Barba, F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Ramezani, M.; Zarrinkamar, F.; Bagheri, M.; Rajabnia, R. Study of environment temperature effect on the antibacterial activity of water extract of different organs of Viola odorata in the different stages of growth. J. Babol Univ. Med. Sci. 2012, 14, 16–21. [Google Scholar]
- Gautam, S.S.; Navneet; Kumar, S. The Antibacterial and Phytochemical Aspects of Viola odorata Linn. Extracts Against Respiratory Tract Pathogens. Proc. Natl. Acad. Sci. USA 2012, 82, 567–572. [Google Scholar] [CrossRef]
- Ekramian, S.; Abbaspour, H.; Roudi, B.; Amjad, L.; Mohammadi Nafchi, A. An experimental study on characteristics of sago starch film treated with methanol extract from Artemisia sieberi Besser. J. Food Meas. Charact. 2021, 15, 3298–3306. [Google Scholar] [CrossRef]
- Barbălată-Mândru, M.; Serbezeanu, D.; Butnaru, M.; Rîmbu, C.M.; Enache, A.A.; Aflori, M. Poly (vinyl alcohol)/Plant Extracts Films: Preparation, Surface Characterization and Antibacterial Studies against Gram Positive and Gram Negative Bacteria. Materials 2022, 15, 2493. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Omar-Aziz, M.; Gharaghani, M.; Khodaiyan, F.; Hosseini, S.S.; Kennedy, J.F. Effect of mung bean protein isolate/pullulan films containing marjoram (Origanum majorana L.) essential oil on chemical and microbial properties of minced beef meat. Int. J. Biol. Macromol. 2022, 201, 318–329. [Google Scholar] [CrossRef]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-Rashed, S.; Hetta, H.F.; Jeandet, P.; Yahia, R.; El-Saber Batiha, G.; Ali, E. Effects of olive leaf extracts as natural preservative on retailed poultry meat quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf life assessment of fresh poultry meat packaged in novel bionanocomposite of chitosan/montmorillonite incorporated with ginger essential oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Yue, Q.; Liu, W.; Wu, J.; Yi, Y.; Wang, H. Characterization of spoilage bacterial communities in chilled duck meat treated by kojic acid. Food Sci. Hum. Wellness 2021, 10, 72–77. [Google Scholar] [CrossRef]
- Tooryan, F.; Amiri, M.R. Evaluation of Chemical and Microbial Spoilage of Chicken Fillet Coated with Chitosan, Ginger Essential Oil (Zingiber officinale) and Medlar concentrate (Mespilus germanica L.) during refrigerated storage. Res. Innov. Food Sci. Technol. 2020, 8, 391–404. [Google Scholar] [CrossRef]
- Hematizad, I.; Khanjari, A.; Basti, A.A.; Karabagias, I.K.; Noori, N.; Ghadami, F.; Gholami, F.; Teimourifard, R. In vitro antibacterial activity of gelatin-nanochitosan films incorporated with Zataria multiflora Boiss essential oil and its influence on microbial, chemical, and sensorial properties of chicken breast meat during refrigerated storage. Food Packag. Shelf Life 2021, 30, 100751. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Unsalan, O.; Altunayar-Unsalan, C.; Xiong, S.; Manyande, A.; Chen, H. Development and characterization of fish myofibrillar protein/chitosan/rosemary extract composite edible films and the improvement of lipid oxidation stability during the grass carp fillets storage. Int. J. Biol. Macromol. 2021, 184, 463–475. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, J.R.A.; Almeida, K.M.; Augusto, A.S.; Vieira, É.T.; Fernando, A.L.; Souza, V.G.L. Application of Biocomposite Films of Chitosan/Natural Active Compounds for Shelf Life Extension of Fresh Poultry Meat. J. Compos. Sci. 2022, 6, 342. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Tavares, L.; Braïek, O.B.; Ennouri, K.; Abdelkafi, S.; Mellouli, L.; Khaneghah, A.M. Application of eco-friendly active films and coatings based on natural antioxidant in meat products: A review. Prog. Org. Coat. 2022, 166, 106780. [Google Scholar] [CrossRef]
- Zhu, W.; Han, M.; Bu, Y.; Li, X.; Yi, S.; Xu, Y.; Li, J. Plant polyphenols regulating myoglobin oxidation and color stability in red meat and certain fish: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–13, (in press). [Google Scholar] [CrossRef]
- Jamshed, H.; Siddiqi, H.S.; Gilani, A.u.H.; Arslan, J.; Qasim, M.; Gul, B. Studies on antioxidant, hepatoprotective, and vasculoprotective potential of Viola odorata and Wrightia tinctoria. Phytother. Res. 2019, 33, 2310–2318. [Google Scholar] [CrossRef]
- Ferreira, V.C.; Morcuende, D.; Madruga, M.S.; Silva, F.A.; Estévez, M. Role of protein oxidation in the nutritional loss and texture changes in ready-to-eat chicken patties. Int. J. Food Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef]
- da Nóbrega Santos, E.; de Albuquerque Sousa, T.C.; de Santana Neto, D.C.; Grisi, C.V.B.; da Silva Ferreira, V.C.; da Silva, F.A.P. Edible active film based on gelatin and Malpighia emarginata waste extract to inhibit lipid and protein oxidation in beef patties. LWT 2022, 154, 112837. [Google Scholar] [CrossRef]
- Munekata, P.E.; Pateiro, M.; Bellucci, E.R.B.; Domínguez, R.; da Silva Barretto, A.C.; Lorenzo, J.M. Strategies to increase the shelf life of meat and meat products with phenolic compounds. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 98, pp. 171–205. [Google Scholar]
- Wan Yahaya, W.A.; Abu Yazid, N.; Mohd Azman, N.A.; Almajano, M.P. Antioxidant activities and total phenolic content of Malaysian herbs as components of active packaging film in beef patties. Antioxidants 2019, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, M.; Dalirfardouei, R.; Sepehrizade, Z.; Kermanshahi, R. Comparison of the antimicrobial effects of semipurified cyclotides from Iranian Viola odorata against some of plant and human pathogenic bacteria. J. Appl. Microbiol. 2013, 115, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ntzimani, A.G.; Giatrakou, V.I.; Savvaidis, I.N. Combined natural antimicrobial treatments (EDTA, lysozyme, rosemary and oregano oil) on semi cooked coated chicken meat stored in vacuum packages at 4 C: Microbiological and sensory evaluation. Innov. Food Sci. Emerg. Technol. 2010, 11, 187–196. [Google Scholar] [CrossRef]




| Films | Thickness (mm) | TS (MPa) | YM (MPa) | EAB (%) |
|---|---|---|---|---|
| VOE0% | 0.107 ± 0.003 a | 39.84 ± 1.22 a | 296.72 ± 1.54 a | 11.09 ± 0.27 c |
| VOE1% | 0.108 ± 0.006 a | 38.52 ± 0.93 ab | 293.19 ± 2.16 ab | 11.44 ± 0.19 bc |
| VOE2% | 0.109 ± 0.002 a | 37.37 ± 1.04 bc | 289.97 ± 2.10 bc | 11.78 ± 0.22 b |
| VOE3% | 0.111 ± 0.006 a | 36.67 ± 0.88 c | 286.44 ± 2.87 c | 12.39 ± 0.08 a |
| Films | Opacity (mm−1) | T280 (%) | T600 (%) |
|---|---|---|---|
| VOE0% | 0.347 ± 0.006 d | 44.61 ± 0.47 a | 75.24 ± 0.72 a |
| VOE1% | 0.491 ± 0.013 c | 7.39 ± 0.31 b | 34.19 ± 0.65 b |
| VOE2% | 0.758 ± 0.019 b | 0.00 ± 0.00 c | 29.93 ± 0.89 c |
| VOE3% | 1.014 ± 0.010 a | 0.00 ± 0.00 c | 17.87 ± 0.82 d |
| Films | E. coli (mm) | S. aureus (mm) | S. typhimorium (mm) |
|---|---|---|---|
| VOE0% | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| VOE1% | 11.56 ± 0.48 c | 15.61 ± 0.30 c | 14.00 ± 0.00 c |
| VOE2% | 19.46 ± 0.83 b | 24.67 ± 0.21 b | 23.33 ± 0.48 b |
| VOE3% | 29.96 ± 0.21 a | 34.10 ± 0.44 a | 31.50 ± 0.67 a |
| Samples | Storage Time (Day) | TAMB (Log CFU/g) | Coliforms (Log CFU/g) | Coagulase-Positive Staphylococci (Log CFU/g) |
|---|---|---|---|---|
| Control | 1 | 2.75 ± 0.19 D,a | 2.16 ± 0.08 D,a | 3.02 ± 0.03 D,a |
| 4 | 4.91 ± 0.05 C,a | 3.98 ± 0.07 C,a | 4.61 ± 0.09 C,a | |
| 8 | 7.49 ± 0.14 B,a | 5.13 ± 0.04 B,a | 5.99 ± 0.07 B,a | |
| 12 | 8.45 ± 0.11 A,a | 5.71 ± 0.11 A,a | 7.32 ± 0.04 A,a | |
| VOE0% | 1 | 2.77 ± 0.08 D,a | 2.19 ± 0.05 D,a | 3.04 ± 0.08 D,a |
| 4 | 4.32 ± 0.11 C,b | 3.33 ± 0.06 C,b | 4.00 ± 0.02 C,b | |
| 8 | 7.07 ± 0.07 B,b | 4.69 ± 0.04 B,b | 5.18 ± 0.03 B,b | |
| 12 | 7.42 ± 0.05 A,b | 5.27 ± 0.06 A,b | 6.25 ± 0.09 A,b | |
| VOE1% | 1 | 2.70 ± 0.12 D,a | 2.17 ± 0.03 D,a | 3.00 ± 0.05 D,a |
| 4 | 3.59 ± 0.07 C,c | 2.70 ± 0.09 C,c | 3.41 ± 0.03 C,c | |
| 8 | 4.56 ± 0.11 B,c | 3.35 ± 0.07 B,c | 3.90 ± 0.05 B,c | |
| 12 | 5.00 ± 0.17 A,c | 3.96 ± 0.08 A,c | 4.47 ± 0.02 A,c | |
| VOE2% | 1 | 2.65 ± 0.09 D,a | 2.14 ± 0.08 D,a | 2.98 ± 0.04 D,a |
| 4 | 3.28 ± 0.12 C,d | 2.48 ± 0.04 C,d | 3.24 ± 0.08 C,d | |
| 8 | 4.10 ± 0.03 B,d | 2.92 ± 0.03 B,d | 3.58 ± 0.04 B,d | |
| 12 | 4.67 ± 0.14 A,d | 3.41 ± 0.05 A,d | 3.88 ± 0.04 A,d | |
| VOE3% | 1 | 2.66 ± 0.15 D,a | 2.14 ± 0.08 D,a | 2.98 ± 0.04 D,a |
| 4 | 3.10 ± 0.04 C,e | 2.39 ± 0.02 C,e | 3.17 ± 0.02 C,d | |
| 8 | 3.81 ± 0.07 B,e | 2.80 ± 0.07 B,e | 3.44 ± 0.05 B,e | |
| 12 | 4.23 ± 0.09 A,e | 3.19 ± 0.04 A,e | 3.62 ± 0.08 A,e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikmanesh, A.; Baghaei, H.; Mohammadi Nafchi, A. Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage. Foods 2023, 12, 2955. https://doi.org/10.3390/foods12152955
Nikmanesh A, Baghaei H, Mohammadi Nafchi A. Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage. Foods. 2023; 12(15):2955. https://doi.org/10.3390/foods12152955
Chicago/Turabian StyleNikmanesh, Ali, Homa Baghaei, and Abdorreza Mohammadi Nafchi. 2023. "Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage" Foods 12, no. 15: 2955. https://doi.org/10.3390/foods12152955
APA StyleNikmanesh, A., Baghaei, H., & Mohammadi Nafchi, A. (2023). Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage. Foods, 12(15), 2955. https://doi.org/10.3390/foods12152955







