Effect of Calcium Salts on the Firmness and Physicochemical and Sensorial Properties of Iranian Black Olive Cultivars
Abstract
1. Introduction
2. Materials and Methods
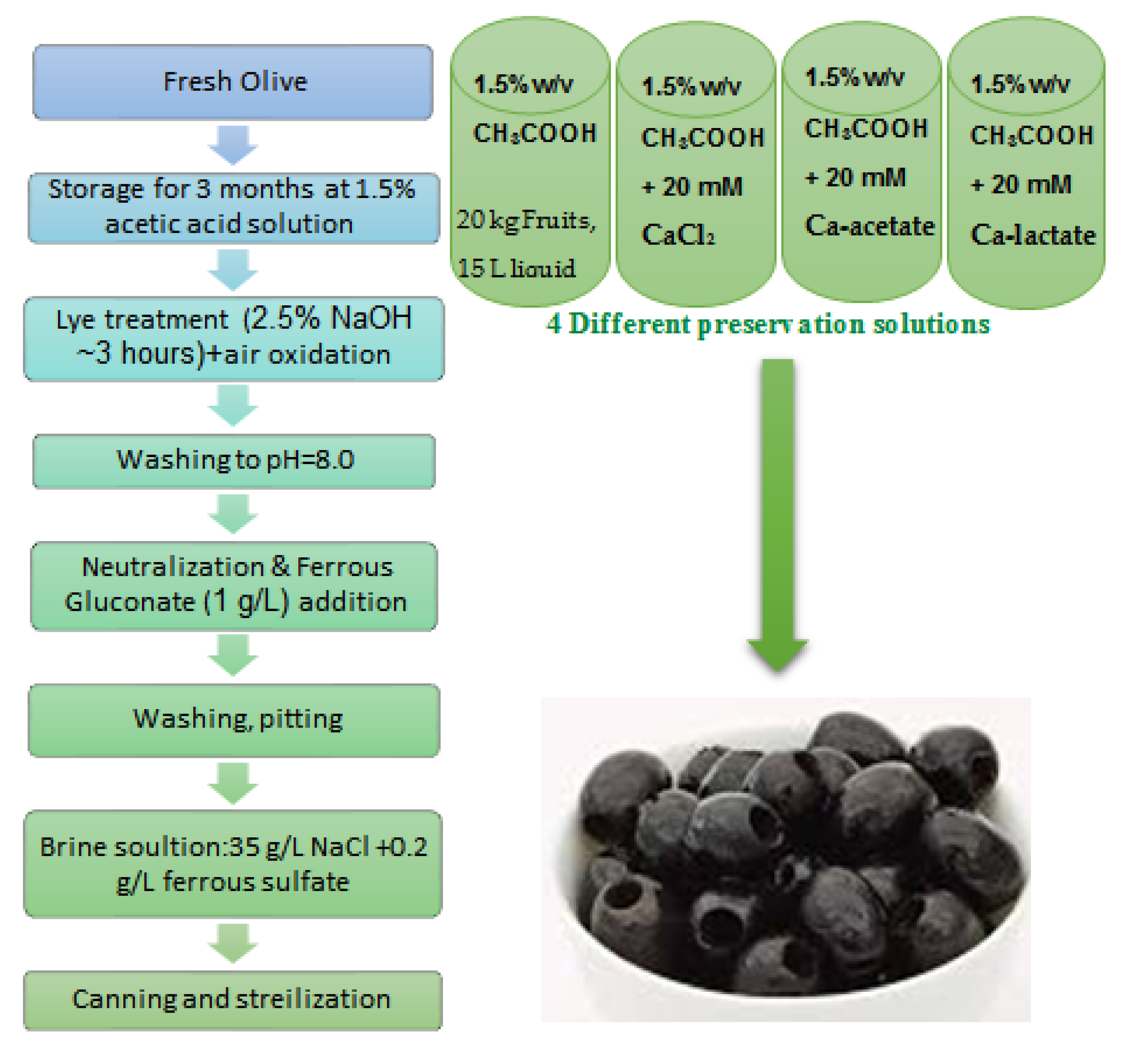
2.1. Olive Processing
2.2. Physicochemical Analysis
2.3. Calcium Measurements
2.4. Firmness
2.5. Superficial Color
2.6. Sensorial Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characteristics
3.2. Phenolic Contents
3.3. Textural Attributes
3.4. Superficial Color and Sensory Attributes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IOC (International Olive Council). Online reference included in World table olives figures: Production. 2018. Available online: http://www.internationaloliveoil.org/estaticos/view/132-world-table-olive-figures (accessed on 20 February 2022).
- Sánchez, A.H.; López-López, A.; Cortés-Delgado, A.; de Castro, A.; Montaño, A. Aroma profile and volatile composition of black ripe olives (Manzanilla and Hojiblanca cultivars). Food Res. Int. 2020, 127, 108733. [Google Scholar] [CrossRef]
- López-López, A.; Cortés-Delgado, A.; de Castro, A.; Sánchez, A.H.; Montaño, A. Changes in volatile composition during the processing and storage of black ripe olives. Food Res. Int. 2019, 125, 108568. [Google Scholar] [CrossRef] [PubMed]
- Chatzisymeon, E.; Stypas, E.; Bousios, S.; Xekoukoulotakis, N.P.; Mantzavinos, D. Photocatalytic treatment of black table olive processing wastewater. J. Hazard. Mater. 2008, 154, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- García-Serrano, P.; Romero, C.; García-García, P.; Brenes, M. Influence of the type of alkali on the processing of black ripe olives. LWT 2020, 126, 109318. [Google Scholar] [CrossRef]
- Fernández, A.G.; Adams, M.R.; Fernandez-Diez, M.J. Table Olives: Production and Processing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- de Castro, A.; García, P.; Romero, C.; Brenes, M.; Garrido, A. Industrial implementation of black ripe olive storage under acid conditions. J. Food Eng. 2007, 80, 1206–1212. [Google Scholar] [CrossRef]
- López-López, A.; Sánchez-Gómez, A.H.; Montaño, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Sensory characterisation of black ripe table olives from Spanish Manzanilla and Hojiblanca cultivars. Food Res. Int. 2019, 116, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Brenes, M.; García-Serrano, P.; Medina, E.; García-García, P. Packing black ripe olives in retortable pouches with different oxygen permeability. Food Packag. Shelf Life 2019, 20, 100323. [Google Scholar] [CrossRef]
- Mousavi, S.M.R.; Rafe, A.; Yeganehzad, S. Textural, mechanical, and microstructural properties of restructured pimiento alginate-guar gels. J. Texture Stud. 2018, 50, 155–164. [Google Scholar] [CrossRef]
- Mojarrad, L.S.; Rafe, A. Rheological characteristics of binary composite gels of wheat flour and high amylose corn starch. J. Texture Stud. 2018, 49, 320–327. [Google Scholar] [CrossRef]
- Panagou, E.; Hondrodimou, O.; Mallouchos, A.; Nychas, G.-J. A study on the implications of NaCl reduction in the fermentation profile of Conservolea natural black olives. Food Microbiol. 2011, 28, 1301–1307. [Google Scholar] [CrossRef]
- Fleming, H.; Mcdonald, L.; Mcfeeters, R.; Thompson, R.; Humphries, E. Fermentation of Cucumbers Without Sodium Chloride. J. Food Sci. 1995, 60, 312–315. [Google Scholar] [CrossRef]
- Wolkers-Rooijackers, J.; Thomas, S.; Nout, M. Effects of sodium reduction scenarios on fermentation and quality of sauerkraut. LWT 2013, 54, 383–388. [Google Scholar] [CrossRef]
- McFeeters, R.F.; Pérez-Díaz, I. Fermentation of Cucumbers Brined with Calcium Chloride Instead of Sodium Chloride. J. Food Sci. 2010, 75, C291–C296. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.E.; Carrerasanchez, C.; Rodriguezpatino, J.; Pilosof, A.M. Adsorption dynamics and surface activity at equilibrium of whey proteins and hydroxypropyl–methyl–cellulose mixtures at the air-water interface. Food Hydrocoll. 2007, 21, 794–803. [Google Scholar] [CrossRef]
- McMurtrie, E.K.; Johanningsmeier, S.D.; Breidt, F.; Price, R.E. Effect of Brine Acidification on Fermentation Microbiota, Chemistry, and Texture Quality of Cucumbers Fermented in Calcium or Sodium Chloride Brines. J. Food Sci. 2019, 84, 1129–1137. [Google Scholar] [CrossRef]
- García-Serrano, P.; Romero, C.; Medina, E.; García-García, P.; de Castro, A.; Brenes, M. Effect of calcium on the preservation of green olives intended for black ripe olive processing under free-sodium chloride conditions. LWT 2020, 118, 108870. [Google Scholar] [CrossRef]
- Ngamchuachit, P.; Sivertsen, H.K.; Mitcham, E.J.; Barrett, D.M. Effectiveness of Calcium Chloride and Calcium Lactate on Maintenance of Textural and Sensory Qualities of Fresh-Cut Mangos. J. Food Sci. 2014, 79, C786–C794. [Google Scholar] [CrossRef]
- Phanumong, P.; Sangsuwan, J.; Kim, S.M.; Rattanapanone, N. The Improvement of Texture and Quality of Minimally Processed Litchi Fruit Using Various Calcium Salts. J. Food Process. Preserv. 2016, 40, 1297–1308. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, X.; Ramaswamy, H.S.; Zhu, S.; Li, H. High Pressure Processing Treatment of Fresh-Cut Carrots: Effect of Presoaking in Calcium Salts on Quality Parameters. J. Food Qual. 2018, 2018, 7863670. [Google Scholar] [CrossRef]
- Luna-Guzmán, I.; Barrett, D.M. Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh-cut cantaloupes. Postharvest Biol. Technol. 2000, 19, 61–72. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. Effect of calcium additives on physicochemical aspects of cell wall pectin and sensory attributes of canned peach (Prunus persica (L) Batsch cv Andross). J. Sci. Food Agric. 2005, 85, 1773–1778. [Google Scholar] [CrossRef]
- Silveira, A.C.; Aguayo, E.; Chisari, M.; Artés, F. Calcium salts and heat treatment for quality retention of fresh-cut ‘Galia’melon. Postharvest Biol. Technol. 2011, 62, 77–84. [Google Scholar] [CrossRef]
- Tataridou, M.; Kotzekidou, P. Fermentation of table olives by oleuropeinolytic starter culture in reduced salt brines and inactivation of Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 122–130. [Google Scholar] [CrossRef]
- Ramírez, E.; Gandul-Rojas, B.; Romero, C.; Brenes, M.; Gallardo-Guerrero, L. Composition of pigments and color changes in green table olives related to processing type. Food Chem. 2015, 166, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kitsawad, K.; Sigal, A.; Flynn, D.; Guinard, J.-X. Sensory Properties and Consumer Acceptance of Imported and Domestic Sliced Black Ripe Olives. J. Food Sci. 2012, 77, S439–S448. [Google Scholar] [CrossRef]
- García-Serrano, P.; Romero, C.; Brenes, M.; García-García, P. Enrichment in phenolic compounds of black ripe olives through nano-filtration and vacuum evaporation techniques. Innov. Food Sci. Emerg. Technol. 2019, 51, 73–79. [Google Scholar] [CrossRef]
- Moghaddam, M.R.A.; Rafe, A.; Taghizadeh, M. Kinetics of Color and Physical Attributes of Cookie during Deep-Fat Frying by Image Processing Techniques. J. Food Process. Preserv. 2015, 39, 91–99. [Google Scholar] [CrossRef]
- IOC (International Olive Council). Method for the Sensory Analysis of Table Olives; COI/OT/MO No 1/Rev.2 November 2011; International Olive Council: Madrid, Spain, 2011; Available online: http://www.internationaloliveoil.org/estaticos/view/70-metodos-de-evaluacion (accessed on 20 February 2022).
- Shahidi, S.-A. Effect of solvent type on ultrasound-assisted extraction of antioxidant compounds from Ficaria kochii: Optimization by response surface methodology. Food Chem. Toxicol. 2022, 163, 112981. [Google Scholar] [CrossRef]
- Moreno, M.D.M.C.; Barreto-Palacios, V.; Gonzalez-Carrascosa, R.; Iborra-Bernad, C.; Andres-Bello, A.; Martínez-Monzó, J.; García-Segovia, P. Evaluation of Textural and Sensory Properties on Typical Spanish Small Cakes Designed Using Alternative Flours. J. Culin. Sci. Technol. 2015, 13, 19–28. [Google Scholar] [CrossRef]
- Pérez-Díaz, I.M.; McFeeters, R.F.; Moeller, L.; Johanningsmeier, S.D.; Hayes, J.; Fornea, D.S.; Rosenberg, L.; Gilbert, C.; Custis, N.; Beene, K.; et al. Commercial Scale Cucumber Fermentations Brined with Calcium Chloride Instead of Sodium Chloride. J. Food Sci. 2015, 80, M2827–M2836. [Google Scholar] [CrossRef]
| Salts | Parameters | Mari | Zard | Rowghani | Shengeh | Dakal | Dezful | Fishomi |
|---|---|---|---|---|---|---|---|---|
| Control | pH | 3.97 ± 0.04 aA | 3.96 ± 0.05 aA | 3.61 ± 0.02 cB | 3.87 ± 0.06 bA | 3.86 ± 0.05 bA | 4.04 ± 0.03 aB | 3.85 ± 0.02 bA |
| Acidity (%) | 1.52 ± 0.11 aA | 1.56 ± 0.23 aA | 1.57 ± 0.22 aA | 1.24 ± 0.10 dB | 1.38 ± 0.27 cB | 1.45 ± 0.05 bB | 1.27 ± 0.27 dC | |
| Lactic acid (%) | 0.36 ± 0.08 cB | 0.44 ± 0.11 bB | 0.54 ± 0.15 aB | 0.47 ± 0.19 bB | 0.38 ± 0.11 cB | 0.44 ± 0.08 bB | 0.34 ± 0.11 cC | |
| Acetic acid (%) | 1.30 ± 0.04 cB | 1.41 ± 0.12 bB | 1.53 ± 0.10 aB | 1.41 ± 0.15 bB | 1.36 ± 0.17 cB | 1.18 ± 0.09 dB | 1.09 ± 0.03 dB | |
| Ethanol (%) | 0.19 ± 0.06 bB | 0.24 ± 0.03 bA | 0.23 ± 0.05 bB | 0.28 ± 0.03 aA | 0.27 ± 0.07 aB | 0.16 ± 0.02 cA | 0.17 ± 0.03 dA | |
| Fructose/mannitol ratio | 0.51 | 1 | 0.6 | 0.42 | 0.75 | 0.08 | 0.05 | |
| CaCl2 | pH | 3.84 ± 0.02 bB | 3.82 ± 0.02 bB | 3.68 ± 0.03 eA | 3.77 ± 0.05 cB | 3.80 ± 0.02 cB | 4.06 ± 0.03 aB | 3.73 ± 0.02 dB |
| Acidity (%) | 1.47 ± 0.32 bA | 1.45 ± 0.23 bB | 1.34 ± 0.17 dC | 1.38 ± 0.12 dA | 1.41 ± 0.17 cB | 1.57 ± 0.05 aA | 1.38 ± 0.19 dB | |
| Lactic acid (%) | 0.35 ± 0.07 bB | 0.37 ± 0.12 bB | 0.33 ± 0.15 cC | 0.36 ± 0.18 bC | 0.37 ± 0.07 bB | 0.38 ± 0.06 bB | 0.48 ± 0.06 aB | |
| Acetic acid (%) | 1.27 ± 0.06 cB | 1.42 ± 0.17 bB | 1.50 ± 0.11 aB | 1.41 ± 0.17 bB | 1.36 ± 0.17 cB | 1.18 ± 0.09 dB | 1.09 ± 0.03 dB | |
| Ethanol (%) | 0.21 ± 0.04 bB | 0.22 ± 0.04 bA | 0.28 ± 0.08 aA | 0.26 ± 0.04 aA | 0.26 ± 0.04 aB | 0.18 ± 0.01 cA | 0.13 ± 0.05 cA | |
| Fructose/mannitol ratio | 0.60 | 1.1 | 0.61 | 0.42 | 0.72 | 0.09 | 0.07 | |
| Ca-acetate | pH | 3.78 ± 0.03 bC | 3.81 ± 0.05 bB | 3.62 ± 0.02 cB | 3.85 ± 0.03 bA | 3.78 ± 0.05 bB | 4.10 ± 0.03 aA | 3.76 ± 0.02 bB |
| Acidity (%) | 1.46 ± 0.12 bA | 1.48 ± 0.13 bB | 1.46 ± 0.12 bB | 1.40 ± 0.16 cA | 1.49 ± 0.13 bA | 1.62 ± 0.04 aA | 1.44 ± 0.18 bA | |
| Lactic acid (%) | 0.34 ± 0.04 cB | 0.38 ± 0.11 cB | 0.44 ± 0.08 bB | 0.46 ± 0.22 aB | 0.38 ± 0.15 cB | 0.47 ± 0.05 aB | 0.42 ± 0.17 bB | |
| Acetic acid (%) | 1.67 ± 0.05 bA | 1.68 ± 0.11 bA | 1.78 ± 0.17 aA | 1.66 ± 0.08 bA | 1.45 ± 0.09 cA | 1.38 ± 0.11 cA | 1.28 ± 0.03 dA | |
| Ethanol (%) | 0.22 ± 0.03 bB | 0.22 ± 0.03 bA | 0.20 ± 0.07 cB | 0.27 ± 0.05 aA | 0.29 ± 0.05 aA | 0.17 ± 0.03 dA | 0.15 ± 0.02 dA | |
| Fructose/mannitol ratio | 0.52 | 1.2 | 0.62 | 0.44 | 0.74 | 0.10 | 0.08 | |
| Ca-lactate | pH | 3.78 ± 0.02 cC | 3.79 ± 0.04 cB | 3.66 ± 0.02 dA | 3.88 ± 0.04 bA | 3.84 ± 0.03 bA | 4.13 ± 0.02 aA | 3.79 ± 0.02 cA |
| Acidity (%) | 1.49 ± 0.11 bA | 1.52 ± 0.07 bA | 1.38 ± 0.16 dC | 1.37 ± 0.15 dA | 1.44 ± 0.15 cA | 1.63 ± 0.02 aA | 1.39 ± 0.12 dB | |
| Lactic acid (%) | 0.58 ± 0.11 cA | 0.68 ± 0.23 bA | 0.76 ± 0.08 aA | 0.66 ± 0.14 bA | 0.65 ± 0.10 bA | 0.67 ± 0.06 bA | 0.72 ± 0.13 aA | |
| Acetic acid (%) | 1.32 ± 0.04 cB | 1.41 ± 0.15 bB | 1.51 ± 0.14 aB | 1.38 ± 0.08 cC | 1.42 ± 0.17 bA | 1.27 ± 0.09 dC | 1.22 ± 0.03 dA | |
| Ethanol (%) | 0.27 ± 0.02 aA | 0.20 ± 0.04 bA | 0.21 ± 0.07 bB | 0.21 ± 0.07 bB | 0.23 ± 0.06 bB | 0.16 ± 0.02 cA | 0.18 ± 0.04 cA | |
| Fructose/mannitol ratio | 0.53 | 1.1 | 0.60 | 0.43 | 0.71 | 0.11 | 0.09 |
| Salts | Phenolic Compounds | Mari | Zard | Rowghani | Shengeh | Dakal | Dezful | Fishomi |
|---|---|---|---|---|---|---|---|---|
| Control | Hydroxytyrosol | 971 ± 24 bA | 963 ± 15 bA | 987 ± 20 aA | 856 ± 33 dA | 847 ± 18 dA | 935 ± 14 cA | 967 ± 22 bA |
| Tyrosol | 122 ± 11 bA | 116 ± 23 cA | 157 ± 22 aA | 124 ± 10 bA | 138 ± 27 bA | 145 ± 15 aA | 127 ± 23 bB | |
| Verbascoside | 42 ± 8 bB | 44 ± 11 bA | 54 ± 15 aA | 47 ± 19 aA | 42 ± 16 bA | 44 ± 18 bB | 43 ± 28 bB | |
| Oleuropein | 241 ± 9 dA | 333 ± 15 cA | 448 ± 11 bA | 361 ± 19 cA | 374 ± 27 cA | 250 ± 19 dA | 568 ± 43 aA | |
| Luteolin | 69 ± 16 cB | 62 ± 11 cA | 50 ± 25 cA | 60 ± 14 cA | 63 ± 18 cA | 76 ± 22 bA | 86 ± 18 aA | |
| CaCl2 | Hydroxytyrosol | 887 ± 16 dC | 958 ± 18 bA | 977 ± 34 aA | 868 ± 18 eA | 859 ± 24 eA | 937 ± 12 cA | 966 ± 21 aA |
| Tyrosol | 113 ± 22 cB | 118 ± 16 cB | 149 ± 22 aA | 134 ± 10 bA | 128 ± 27 cA | 125 ± 18 cB | 133 ± 16 bA | |
| Verbascoside | 53 ± 12 aA | 35 ± 16 cB | 38 ± 11 cB | 56 ± 23 aA | 56 ± 12 aA | 49 ± 20 bA | 41 ± 16 bA | |
| Oleuropein | 239 ± 17 eA | 347 ± 18 cA | 446 ± 16 bA | 358 ± 24 cA | 363 ± 12 cA | 263 ± 32 cA | 574 ± 23 aA | |
| Luteolin | 71 ± 18 bA | 62 ± 16 cA | 51 ± 21 dA | 52 ± 18 dA | 61 ± 15 cA | 71 ± 17 bA | 89 ± 15 aA | |
| Ca-acetate | Hydroxytyrosol | 897 ± 17 cC | 973 ± 16 aA | 970 ± 24 aA | 857 ± 21 dA | 864 ± 20 dA | 947 ± 18 bA | 976 ± 27 aA |
| Tyrosol | 123 ± 17 bA | 117 ± 23 bB | 138 ± 22 aA | 128 ± 11 bA | 114 ± 19 bB | 121 ± 12 bB | 136 ± 28 aA | |
| Verbascoside | 55 ± 18 aA | 37 ± 10 bB | 42 ± 12 aA | 48 ± 18 aA | 55 ± 10 aA | 53 ± 17 aA | 54 ± 15 aA | |
| Oleuropein | 228 ± 14 eB | 340 ± 24 dA | 450 ± 14 bA | 360 ± 23 cA | 371 ± 20 cA | 248 ± 22 eB | 580 ± 16 aA | |
| Luteolin | 76 ± 11 aA | 61 ± 10 bA | 48 ± 22 cA | 56 ± 21 cA | 58 ± 12 bA | 68 ± 12 aA | 81 ± 11 aA | |
| Ca-lactate | Hydroxytyrosol | 923 ± 16 cB | 955 ± 18 bA | 987 ± 12 aA | 863 ± 13 dA | 874 ± 19 dA | 938 ± 22 cA | 960 ± 12 bA |
| Tyrosol | 106 ± 23 cB | 166 ± 30 aA | 127 ± 32 bB | 124 ± 27 bA | 108 ± 31 cC | 114 ± 16 cC | 133 ± 17 bA | |
| Verbascoside | 64 ± 19 aA | 57 ± 11 bA | 38 ± 24 cB | 53 ± 16 bA | 68 ± 12 aA | 51 ± 12 bA | 48 ± 14 cA | |
| Oleuropein | 258 ± 16 dA | 278 ± 13 dB | 452 ± 10 bA | 367 ± 17 cA | 368 ± 18 cA | 259 ± 29 dA | 568 ± 43 aA | |
| Luteolin | 82 ± 14 bA | 62 ± 13 bA | 56 ± 19 cA | 58 ± 20 cA | 65 ± 17 bA | 73 ± 21 aA | 76 ± 16 aB |
| Salts | Properties * | Mari | Zard | Rowghani | Shengeh | Dakal | Dezful | Fishomi |
|---|---|---|---|---|---|---|---|---|
| Control | Firmness † | 1455 ± 17 aD | 1394 ± 38 bC | 1364 ± 63 cB | 1422 ± 71 aD | 1379 ± 23 cD | 1265 ± 44 dD | 1391 ± 54 bC |
| Superficial color | 4.67 ± 0.14 aB | 4.56 ± 0.09 bB | 3.78 ± 0.10 eC | 4.03 ± 0.17 dC | 4.43 ± 0.23 cC | 3.36 ± 0.11 eC | 3.27 ± 0.15 eB | |
| ∆E | 20.74 ± 0.08 aB | 19.29 ± 0.21 bB | 19.42 ± 0.16 bC | 18.07 ± 0.27 cB | 17.59 ± 0.06 cB | 16.61 ± 0.16 dC | 17.43 ± 0.19 cB | |
| Ca in flesh | 612 ± 27 bD | 598 ± 36 cD | 593 ± 46 cC | 554 ± 29 cD | 481 ± 23 dC | 763 ± 42 aD | 645 ± 58 bC | |
| CaCl2 | Firmness | 1549 ± 22 aC | 1417 ± 27 bB | 1352 ± 16 cB | 1567 ± 22 aC | 1387 ± 18 cC | 1320 ± 25 dC | 1401 ± 38 bC |
| Superficial color | 4.36 ± 0.17 aC | 4.31 ± 0.15 aC | 3.55 ± 0.23 cD | 3.85 ± 0.20 bD | 4.29 ± 0.09 aD | 3.23 ± 0.08 dD | 3.08 ± 0.09 eC | |
| ∆E | 19.43 ± 0.15 aC | 18.55 ± 0.14 bC | 18.55 ± 0.27 bD | 17.85 ± 0.31 cC | 16.43 ± 0.15 dC | 15.44 ± 0.25 eD | 16.54 ± 0.08 dC | |
| Ca in flesh | 926 ± 15 aC | 876 ± 32 bC | 748 ± 27 dB | 773 ± 42 dC | 687 ± 35 eB | 825 ± 22 cC | 735 ± 18 dB | |
| Ca-acetate | Firmness | 1687 ± 13 aB | 1407 ± 27 cB | 1363 ± 23 dA | 1588 ± 25 bB | 1406 ± 15 cB | 1378 ± 26 cA | 1416 ± 22 cB |
| Superficial color | 4.85 ± 0.11 aA | 4.77 ± 0.13 bA | 4.16 ± 0.07 dA | 4.14 ± 0.06 dA | 4.53 ± 0.15 cB | 3.57 ± 0.15 eB | 3.50 ± 0.27 eA | |
| ∆E | 24.55 ± 0.17 aA | 22.17 ± 0.16 bA | 20.96 ± 0.18 cB | 19.52 ± 0.11 cA | 18.49 ± 0.24 dA | 17.58 ± 0.22 eB | 18.48 ± 0.26 dA | |
| Ca in flesh | 967 ± 52 aB | 913 ± 18 bB | 745 ± 41 dB | 854 ± 29 cB | 796 ± 26 dA | 957 ± 34 aB | 823 ± 26 cA | |
| Ca-lactate | Firmness | 1765 ± 23 aA | 1428 ± 18 bA | 1378 ± 42 cA | 1746 ± 37 aA | 1421 ± 11 bA | 1358 ± 14 cB | 1448 ± 28 bA |
| Superficial color | 4.77 ± 0.22 aB | 4.63 ± 0.08 aA | 3.96 ± 0.12 bB | 4.11 ± 0.10 bA | 4.66 ± 0.22 aA | 3.63 ± 0.07 cA | 3.44 ± 0.12 dA | |
| ∆E | 23.45 ± 0.16 aA | 21.08 ± 0.09 bA | 21.31 ± 0.12 bA | 19.36 ± 0.21 cA | 18.27 ± 0.16 dA | 17.87 ± 0.34 eA | 18.67 ± 0.21 dA | |
| Ca in flesh | 989 ± 43 aA | 968 ± 37 bA | 768 ± 29 dA | 827 ± 35 cA | 788 ± 19 dA | 968 ± 27 bA | 847 ± 36 cA |
| Cultivars | Brine Solutions | Negative Sensation | Gustatory Sensations | Kinesthetic Sensations | ||||
|---|---|---|---|---|---|---|---|---|
| Abnormal Flavor | Salty | Bitter | Acid | Hardness | Fibrousness | Crunchiness | ||
| Mari | Control | 1.2 ± 0.1 | 5.0 ± 0.2 a | 1.2 ± 0.1 b | 1.2 ± 0.2 b | 4.9 ± 0.3 c | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.1 ± 0.2 | 5.8 ± 0.3 a | 1.8 ± 0.2 a | 1.3 ± 0.1 b | 5.7 ± 0.2 a | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 0.9 ± 0.1 | 5.5 ± 0.2 a | 1.1 ± 0.2 b | 1.8 ± 0.2 a | 5.2 ± 0.2 b | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 0.8 ± 0.1 | 5.4 ± 0.1 a | 1.2 ± 0.1 b | 1.6 ± 0.2 a | 5.3 ± 0.1 b | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
| Zard | Control | 1.1 ± 0.2 | 5.8 ± 0.3 a | 1.2 ± 0.1 b | 1.1 ± 0.2 b | 4.9 ± 0.3 c | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.0 ± 0.1 | 5.2 ± 0.4 b | 1.8 ± 0.2 a | 1.2 ± 0.1 b | 4.7 ± 0.2 a | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 0.9 ± 0.2 | 4.8 ± 0.1 b | 1.1 ± 0.2 b | 1.5 ± 0.2 a | 4.2 ± 0.2 b | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 0.8 ± 0.2 | 4.6 ± 0.2 b | 1.2 ± 0.1 b | 1.3 ± 0.2 a | 4.3 ± 0.1 b | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
| Rowghani | Control | 1.1 ± 0.1 | 5.6 ± 0.2 a | 1.2 ± 0.1 b | 1.2 ± 0.2 b | 4.9 ± 0.3 c | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.3 ± 0.2 | 4.7 ± 0.3 b | 1.8 ± 0.2 a | 1.1 ± 0.1 b | 4.7 ± 0.2 a | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 1.4 ± 0.1 | 5.7 ± 0.1 a | 1.1 ± 0.2 b | 1.4 ± 0.2 a | 4.2 ± 0.2 b | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 1.2 ± 0.3 | 5.2 ± 0.2 a | 1.2 ± 0.1 b | 1.3 ± 0.2 a | 4.3 ± 0.1 b | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
| Shengeh | Control | 1.1 ± 0.2 | 5.2 ± 0.4 a | 1.2 ± 0.1 b | 1.2 ± 0.2 b | 4.9 ± 0.3 c | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.2 ± 0.1 | 5.1 ± 0.1 a | 1.8 ± 0.2 a | 1.1 ± 0.1 b | 4.7 ± 0.2 a | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 1.0 ± 0.1 | 5.0 ± 0.2 a | 1.1 ± 0.2 b | 1.2 ± 0.2 b | 4.2 ± 0.2 b | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 1.1 ± 0.1 | 4.8 ± 0.3 a | 1.2 ± 0.1 b | 1.4 ± 0.2 a | 4.3 ± 0.1 b | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
| Dakal | Control | 1.1 ± 0.1 | 5.1 ± 0.2 a | 1.2 ± 0.1 b | 1.2 ± 0.2 a | 4.9 ± 0.3 a | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.1 ± 0.1 | 5.5 ± 0.2 a | 1.8 ± 0.2 a | 1.2 ± 0.1 a | 4.7 ± 0.2 b | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 1.0 ± 0.2 | 5.4 ± 0.2 a | 1.1 ± 0.2 b | 1.1 ± 0.2 a | 4.2 ± 0.2 c | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 0.9 ± 0.1 | 5.3 ± 0.4 a | 1.2 ± 0.1 b | 1.3 ± 0.2 a | 4.3 ± 0.1 c | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
| Dezful | Control | 1.0 ± 0.1 | 5.3 ± 0.2 a | 1.2 ± 0.1 b | 1.2 ± 0.2 b | 4.9 ± 0.3 a | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.1 ± 0.1 | 5.2 ± 0.2 a | 1.8 ± 0.2 s | 1.3 ± 0.1 b | 4.7 ± 0.2 b | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 0.9 ± 0.1 | 5.1 ± 0.2 a | 1.1 ± 0.2 b | 1.4 ± 0.2 a | 4.2 ± 0.2 c | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 0.8 ± 0.1 | 5.1 ± 0.1 a | 1.2 ± 0.1 b | 1.2 ± 0.2 b | 4.3 ± 0.1 c | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
| Fishomi | Control | 1.1 ± 0.2 | 5.8 ± 0.3 a | 1.2 ± 0.1 b | 1.2 ± 0.2 a | 4.9 ± 0.3 a | 4.8 ± 0.2 b | 4.2 ± 0.1 |
| CaCl2 | 1.2 ± 0.1 | 5.6 ± 0.2 a | 1.8 ± 0.2 a | 1.1 ± 0.1 b | 4.7 ± 0.2 b | 5.2 ± 0.4 a | 4.7 ± 0.3 | |
| Ca-acetate | 1.2 ± 0.2 | 5.1 ± 0.2 b | 1.1 ± 0.2 b | 1.3 ± 0.2 a | 4.2 ± 0.2 c | 4.8 ± 0.1 b | 4.6 ± 0.1 | |
| Ca-lactate | 1.0 ± 0.1 | 5.2 ± 0.2 b | 1.2 ± 0.1 b | 1.2 ± 0.2 a | 4.3 ± 0.1 c | 4.6 ± 0.1 b | 4.5 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataollahi Eshkour, M.; Ghorbani-HasanSaraei, A.; Rafe, A.; Shahidi, S.-A.; Naghizadeh Raeisi, S. Effect of Calcium Salts on the Firmness and Physicochemical and Sensorial Properties of Iranian Black Olive Cultivars. Foods 2023, 12, 2970. https://doi.org/10.3390/foods12152970
Ataollahi Eshkour M, Ghorbani-HasanSaraei A, Rafe A, Shahidi S-A, Naghizadeh Raeisi S. Effect of Calcium Salts on the Firmness and Physicochemical and Sensorial Properties of Iranian Black Olive Cultivars. Foods. 2023; 12(15):2970. https://doi.org/10.3390/foods12152970
Chicago/Turabian StyleAtaollahi Eshkour, Mahnaz, Azade Ghorbani-HasanSaraei, Ali Rafe, Seyed-Ahmad Shahidi, and Shahram Naghizadeh Raeisi. 2023. "Effect of Calcium Salts on the Firmness and Physicochemical and Sensorial Properties of Iranian Black Olive Cultivars" Foods 12, no. 15: 2970. https://doi.org/10.3390/foods12152970
APA StyleAtaollahi Eshkour, M., Ghorbani-HasanSaraei, A., Rafe, A., Shahidi, S.-A., & Naghizadeh Raeisi, S. (2023). Effect of Calcium Salts on the Firmness and Physicochemical and Sensorial Properties of Iranian Black Olive Cultivars. Foods, 12(15), 2970. https://doi.org/10.3390/foods12152970





