The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Organic Acids
2.2. Methodology
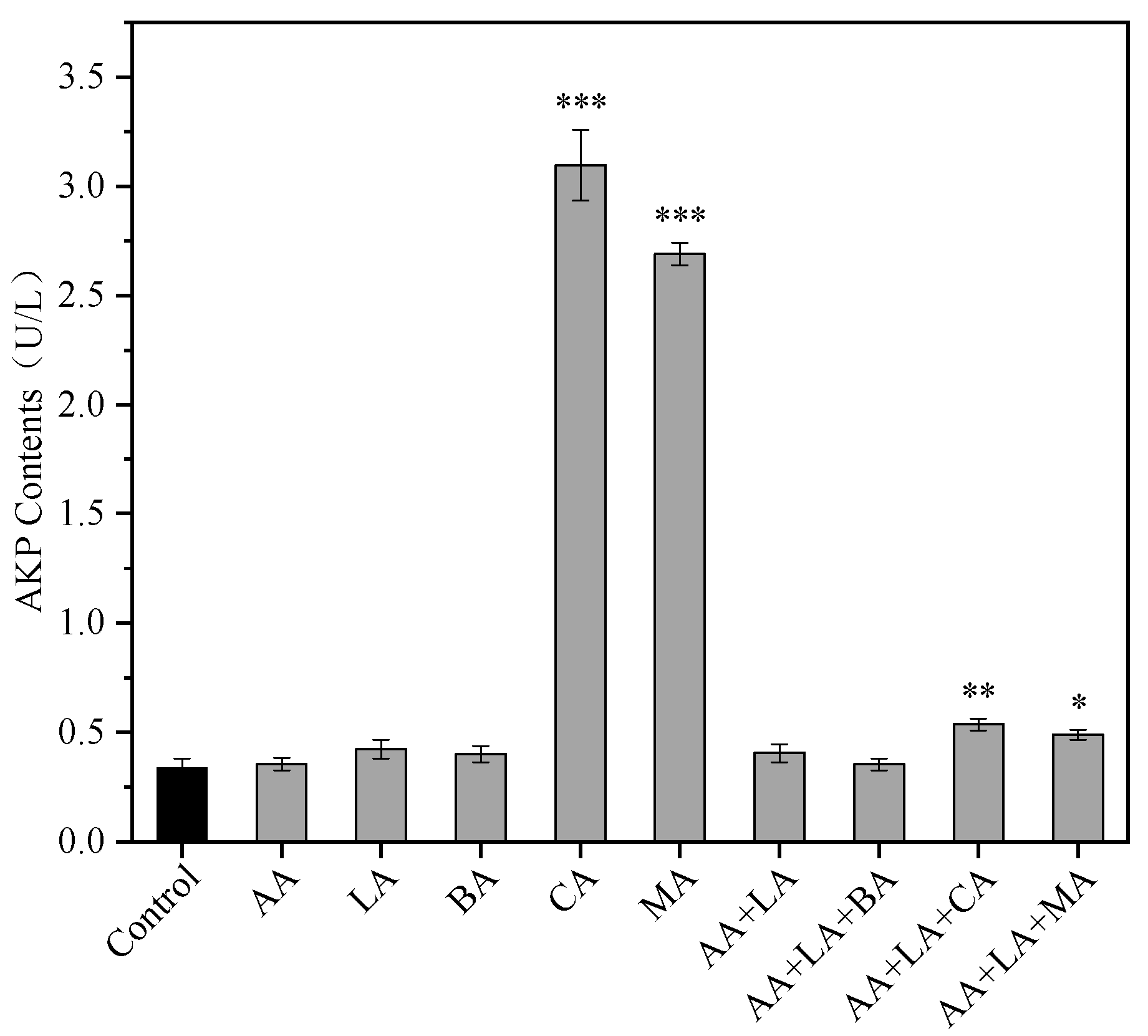
2.2.1. Alkaline Phosphatase (AKP) Activity Determination
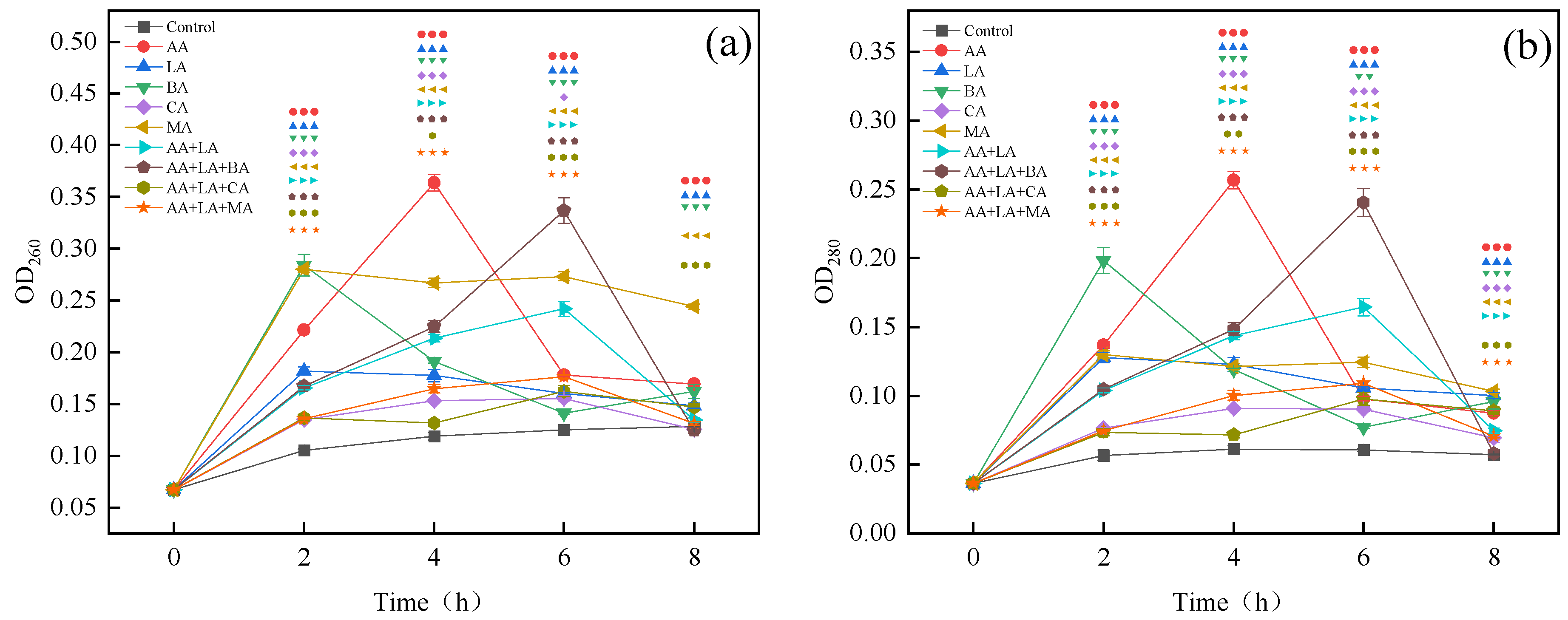
2.2.2. Extracellular Nucleic Acids and Proteins Measurement
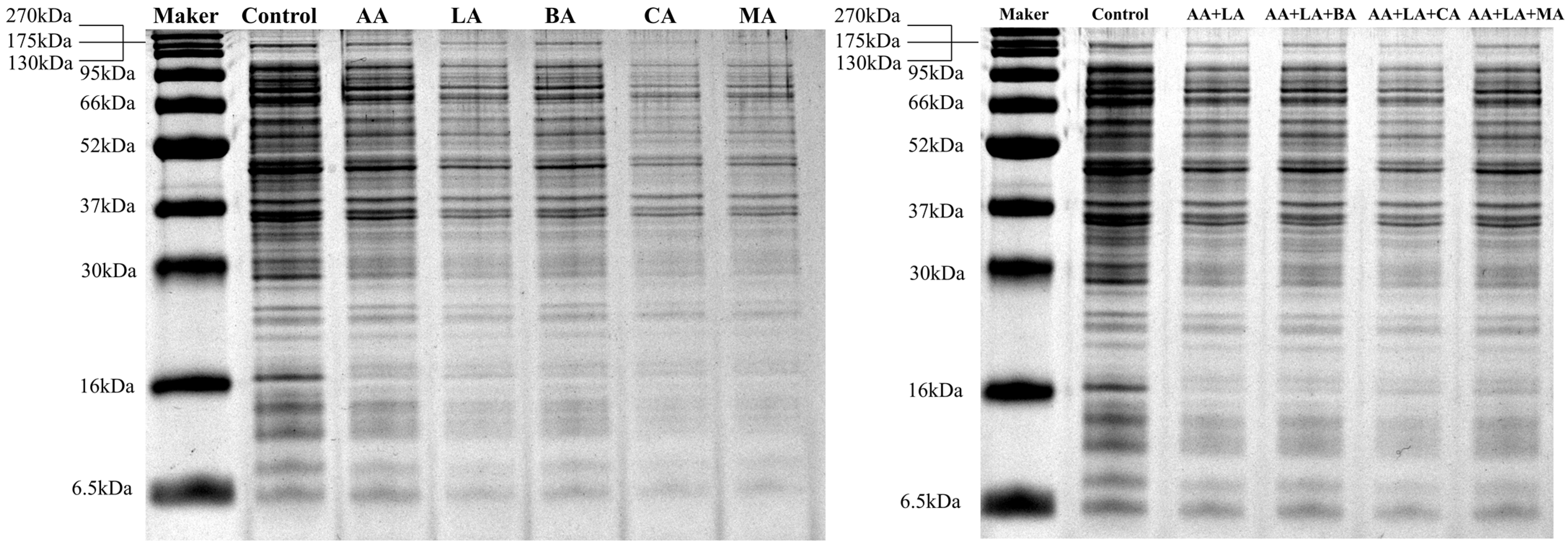
2.2.3. Intracellular Proteins Measurement
2.2.4. OmpF, OmpW, OmpX, OmpA, FadR, and PagP Gene Expression Analysis
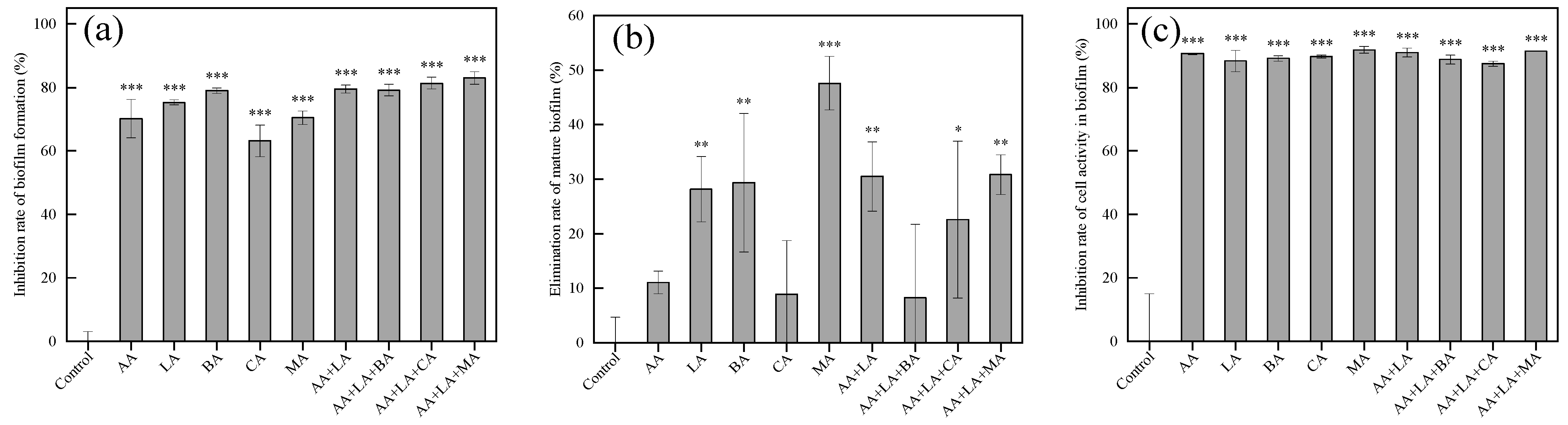
2.2.5. Biofilm Formation, Mature Biofilms, and Cell Activity within Biofilm Measurement
2.3. Statistical Analysis
3. Results and Discussion
3.1. The AKP Leakage of E. coli
3.2. Nucleic Acid and Protein Leakage of E. coli
3.3. Intracellular Protein Analysis
3.4. Gene Expression of OmpF, OmpW, OmpX, OmpA, FadR, and PagP
3.5. Effects of Organic Acids and Their Combinations on Biofilm Formation, Mature Biofilms, and Cell Activity within Biofilms in E. coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Food Safety: Fact Sheet No. 399; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Singha, S.; Thomas, R.; Viswakarma, J.N.; Gupta, V.K. Foodborne illnesses of Escherichia coli O157origin and its control measures. J. Food Sci. Technol. 2023, 60, 1274–1283. [Google Scholar] [CrossRef]
- Tan, J.Z.; Karwe, M.V. Inactivation and removal of Enterobacter aerogenes biofilm in a model piping system using plasma-activated water (PAW). Innov. Food Sci. Emerg. Technol. 2021, 69, 102664. [Google Scholar] [CrossRef]
- Min, T.; Zhu, Z.; Sun, X.; Yuan, Z.; Zha, J.; Wen, Y. Highly efficient antifogging and antibacterial food packaging film fabricated by novel quaternary ammonium chitosan composite. Food Chem. 2020, 308, 125682. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Bastos, R.G.; Oliver, J.C.; Germano, J.d.L.; Fernandes, G.R.; VEIGA, S.M.O.M. Effectiveness evaluation of alternative sanitizers in microbiological quality of strawberry (Fragaria ananassa Duch Var. Oso Grande) after artificial contamination by Escherichia coli. Food Sci. Technol. 2019, 39, 470–474. [Google Scholar]
- Jiang, Y.-L.; Qiu, W.; Zhou, X.-D.; Li, H.; Lu, J.-Z.; Xu, H.H.; Peng, X.; Li, M.-Y.; Feng, M.-Y.; Cheng, L. Quaternary ammonium-induced multidrug tolerant Streptococcus mutans persisters elevate cariogenic virulence in vitro. Int. J. Oral Sci. 2017, 9, e7. [Google Scholar] [CrossRef] [PubMed]
- Jaskulski, I.B.; Scheik, L.K.; Kleinubing, N.; Haubert, L.; Kroning, I.; Lopes, G.V.; Silva, W. Listeria monocytogenes from food and food industry environments with reduced susceptibility to benzalkonium chloride, sodium hypochlorite, and peracetic acid. FEMS Microbiol. Lett. 2023, 370, fnad019. [Google Scholar]
- Lahiri, D.; Nag, M.; Dutta, B.; Sarkar, T.; Pati, S.; Basu, D.; Abdul Kari, Z.; Wei, L.S.; Smaoui, S.; Wen Goh, K. Bacteriocin: A natural approach for food safety and food security. Front. Bioeng. Biotechnol. 2022, 10, 1005918. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- Korber, D.; Mangalappalli-Illathu, A.; Vidović, S. Biofilm formation by food spoilage microorganisms in food processing environments. In Biofilms in the Food and Beverage Industries; Elsevier: Amsterdam, The Netherlands, 2009; pp. 169–199. [Google Scholar]
- US FDA. Generally Recognized as Safe (GRAS); FDA: Silver Spring, ML, USA, 2017. [Google Scholar]
- Mani-López, E.; García, H.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Castro, V.S.; Mutz, Y.D.S.; Rosario, D.K.A.; Cunha-Neto, A.; Figueiredo, E.E.D.S.; Conte-Junior, C.A. Inactivation of multi-drug resistant non-typhoidal Salmonella and wild-type Escherichia coli STEC using organic acids: A potential alternative to the food industry. Pathogens 2020, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.M.; Yohannes, E.; Bondurant, S.S.; Radmacher, M.; Slonczewski, J.L. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 2005, 187, 304–319. [Google Scholar]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical Preservatives and Natural Antimicrobial Compounds. In Food Microbiology; ASM Press: Washington, DC, USA, 2012; pp. 765–801. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- McLaggan, D.; Naprstek, J.; Buurman, E.T.; Epstein, W. Interdependence of K+ and glutamate accumulation during osmotic adaptation of Escherichia coli. J. Biol. Chem. 1994, 269, 1911–1917. [Google Scholar] [CrossRef]
- Rasch, M. The influence of temperature, salt and pH on the inhibitory effect of reuterin on Escherichia coli. Int. J. Food Microbiol. 2002, 72, 225–231. [Google Scholar]
- Pieterse, B.; Leer, R.J.; Schuren, F.H.; van der Werf, M.J. Unravelling the multiple effects of lactic acid stress on Lactobacillus plantarum by transcription profiling. Microbiology 2005, 151, 3881–3894. [Google Scholar] [CrossRef] [Green Version]
- Roe, A.J.; O’Byrne, C.; McLaggan, D.; Booth, I.R. Inhibition of Escherichia coli growth by acetic acid: A problem with methionine biosynthesis and homocysteine toxicity. Microbiology 2002, 148, 2215–2222. [Google Scholar]
- Wang, J.; Sun, Y.; Tao, D.; Wang, S.; Li, C.; Zheng, F.; Wu, Z. Reduction of Escherichia coli O157:H7, Listeria monocytogenes, and naturally present microbe counts on lettuce using an acid mixture of acetic and lactic acid. Microorganisms 2019, 7, 373. [Google Scholar] [CrossRef] [Green Version]
- El-Saadony, M.T.; Umar, M.; Hassan, F.-U.; Alagawany, M.; Arif, M.; Taha, A.E.; Elnesr, S.S.; El-Tarabily, K.A.; Abd El-Hack, M.E. Applications of butyric acid in poultry production: The dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim. Health Res. Rev. 2022, 23, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.; Menezes, R.; Chitolina, G.Z.; Kunert-Filho, H.C.; Wilsmann, D.E.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.d.S.; do Nascimento, V.P. Antibiofilm activity of the biosurfactant and organic acids against foodborne pathogens at different temperatures, times of contact, and concentrations. Braz. J. Microbiol. 2022, 53, 1051–1064. [Google Scholar] [PubMed]
- Kundukad, B.; Udayakumar, G.; Grela, E.; Kaur, D.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Weak acids as an alternative anti-microbial therapy. Biofilm 2020, 2, 100019. [Google Scholar] [CrossRef] [PubMed]
- Tosun, Ş.Y. Investigating the effect of organic acids on the survival of Listeria monocytogenes and Escherichia coli O157:H7 in Atlantic salmon stored at 4 ± 1 °C. J. Food Process. Preserv. 2021, 45, e15784. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, W.; Qian, Y.; Yan, H.; Qu, H.; Gu, R. Inhibitory of different organic acid combinations against Escherichia coli. Food Ferment. Ind. 2023, 1–8. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, X.; Su, Y.; Xu, W.; Liu, H.; Liu, Z.; Chen, W.; Wang, J. Dimethyl phthalate damaged the cell membrane of Escherichia coli K12. Ecotoxicol. Environ. Saf. 2019, 180, 208–214. [Google Scholar]
- Izzo, L.; Matrella, S.; Mella, M.; Benvenuto, G.; Vigliotta, G. Escherichia coli as a model for the description of the antimicrobial mechanism of a cationic polymer surface: Cellular target and bacterial contrast response. ACS Appl. Mater. Interfaces 2019, 11, 15332–15343. [Google Scholar] [CrossRef]
- Lund, P.; Tramonti, A.; De Biase, D. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef]
- Wei-Qing, L.; Jing, X.; Wei-Feng, H.; Da-Wen, L. Antimicrobial Activity and Mechanism of Complex Biological Fresh-keeping Agents against Staphylococcus sciuri. Nat. Prod. Res. Dev. 2012, 24, 741–753. [Google Scholar]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A.; Abu Hasfa, S.H.; Smqadri, S.Q.; Haik, Y. Antimicrobial Activity of Copper Alone and in Combination with Lactic Acid against Escherichia coli O157:H7 in Laboratory Medium and on the Surface of Lettuce and Tomatoes. J. Pathog. 2011, 2011, 650968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alakomi, H.L.; Puupponen-Pimia, R.; Aura, A.M.; Helander, I.M.; Nohynek, L.; Oksman-Caldentey, K.M.; Saarela, M. Weakening of Salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J. Agric. Food Chem. 2007, 55, 3905–3912. [Google Scholar] [CrossRef]
- He, N.; Wang, P.Q.; Wang, P.Y.; Ma, C.Y.; Kang, W.Y. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. Bmc Complement. Altern. Med. 2018, 18, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhao, M.Y.; Phey, C.P.; Yang, H. Efficacy of low concentration acidic electrolysed water and levulinic acid combination on fresh organic lettuce (Lactuca sativa Var. Crispa L.) and its antimicrobial mechanism. Food Control 2019, 101, 241–250. [Google Scholar]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Kannan, S.; Rao, V.A.; Biboy, J.; Vollmer, D.; Erickson, S.W.; Lewis, R.J.; Young, K.D.; Vollmer, W. The redundancy of peptidoglycan carboxypeptidases ensures robust cell shape maintenance in Escherichia coli. MBio 2016, 7, 10–1128. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Tarallo, R.; Elena Della Pepa, M.; D’Oriano, V.; Galdiero, M. Microbe-host interactions: Structure and role of Gram-negative bacterial porins. Curr. Protein Pept. Sci. 2012, 13, 843–854. [Google Scholar]
- Yoon, Y.; Lee, H.; Lee, S.; Kim, S.; Choi, K.-H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015, 72, 25–36. [Google Scholar]
- Bekhit, A.; Fukamachi, T.; Saito, H.; Kobayashi, H. The role of OmpC and OmpF in acidic resistance in Escherichia coli. Biol. Pharm. Bull. 2011, 34, 330–334. [Google Scholar]
- Sato, M.; Machida, K.; Arikado, E.; Saito, H.; Kakegawa, T.; Kobayashi, H. Expression of outer membrane proteins in Escherichia coli growing at acid pH. Appl. Environ. Microbiol. 2000, 66, 943–947. [Google Scholar]
- Catel-Ferreira, M.; Marti, S.; Guillon, L.; Jara, L.; Coadou, G.; Molle, V.; Bouffartigues, E.; Bou, G.; Shalk, I.; Jouenne, T. The outer membrane porin OmpW of Acinetobacter baumannii is involved in iron uptake and colistin binding. FEBS Lett. 2016, 590, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalule, J.B.; Fortuin, S.; Calder, B.; Robberts, L.; Keddy, K.H.; Nel, A.J.; Garnett, S.; Nicol, M.; Warner, D.F.; Soares, N.C. Proteomic comparison of three clinical diarrhoeagenic drug-resistant Escherichia coli isolates grown on CHROMagar™ STEC media. J. Proteom. 2018, 180, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, W.C.; Yeo, K.J.; Ryu, K.-S.; Kumarasiri, M.; Hesek, D.; Lee, M.; Mobashery, S.; Song, J.H.; Kim, S.I. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012, 26, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, R.E. The lipid A palmitoyltransferase PagP: Molecular mechanisms and role in bacterial pathogenesis. Mol. Microbiol. 2005, 57, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, Y.; Wu, Q.; Guo, W.; Chen, H.; Zhang, W.; Li, Y.; Lu, Y.; Wu, Q.; Pan, W. Antibacterial effect and mechanism against Escherichia coli of polysaccharides from Armillariella tabescens mycelia. Int. J. Biol. Macromol. 2022, 207, 750–759. [Google Scholar] [CrossRef]
- Zhang, F.; Ouellet, M.; Batth, T.S.; Adams, P.D.; Petzold, C.J.; Mukhopadhyay, A.; Keasling, J.D. Enhancing fatty acid production by the expression of the regulatory transcription factor FadR. Metab. Eng. 2012, 14, 653–660. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Abdel-Samie, M.A.; Surendhiran, D.; Lin, L. Anti-Listeria monocytogenes biofilm mechanism of cold nitrogen plasma. Innov. Food Sci. Emerg. Technol. 2021, 67, 102571. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Babu, D.; Kwon, Y.M.; Mauromoustakos, A. Inhibitory effects of lactic and malic organic acids on autoinducer type 2 (AI-2) quorum sensing of Escherichia coli O157:H7 and Salmonella Typhimurium. LWT-Food-Food Sci. Technol. 2016, 66, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Tsukatani, T.; Sakata, F. Combined effects of fumaric, lactic, and ferulic acid against food-borne pathogenic biofilms. Food Control 2022, 138, 109024. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Horax, R.; Eswaranandam, S. Electrostatic spraying of organic acids on biofilms formed by E. coli O157:H7 and Salmonella Typhimurium on fresh produce. Food Res. Int. 2015, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Sadiq, F.A.; Wang, N.; Yang, Z.; He, G. Recent advances in understanding the control of disinfectant-resistant biofilms by hurdle technology in the food industry. Crit. Rev. Food Sci. Nutr. 2021, 61, 3876–3891. [Google Scholar] [CrossRef]
- Elhariry, H.M. Attachment strength and biofilm forming ability of Bacillus cereus on green-leafy vegetables: Cabbage and lettuce. Food Microbiol. 2011, 28, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Turhan, E.U.; Polat, S.; Erginkaya, Z.; Konuray, G. Investigation of synergistic antibacterial effect of organic acids and ultrasound against pathogen biofilms on lettuce. Food Biosci. 2022, 47, 101643. [Google Scholar] [CrossRef]
| Single Organic Acid 3 | MIC 1 (μg/mL) | Organic Acid Combination 3 | Optimal Inhibitory Concentration 2 (μg/mL) |
|---|---|---|---|
| AA | 2560 | AA + LA | 640 + 640 |
| LA | 2560 | AA + LA + BA | 320 + 320 + 320 |
| BA | 2560 | AA + LA + CA | 80 + 80 + 1280 |
| CA | 5120 | AA + LA + MA | 320 + 320 + 640 |
| MA | 5120 |
| Target Genes | Sequence (5′-3′) | Description | Reference |
|---|---|---|---|
| 16S rRNA | F: AGAGGATGACCAGCCACAC R: CGGGTAACGTCAATGAGCAAAG | Reference gene | [29] |
| OmpF | F: CGGTTATGGTCAGTGGGA R: CGAAGAAGTCATCGCTGTAT | Outer membrane porin sequencing of CDC ceftolozane/tazobactam antimicrobial resistance panel | |
| OmpW | F: TGCTGGTGGTACGTTAGGAA R: TGTTGGTGGCAGATGATGAA | Associated with the transport of small molecules | |
| OmpX | F: AACGCTACGAATACGGCTCT R: TACCCGACCTACAAACACGA | Causes the development of drug resistance | |
| OmpA | F: TGAGCCTGGGTGTTTCCTAC R: ATCCAGAGCAGCCTGACCTT | Maintains outer membrane integrity, stable cell structure, and cell morphology | |
| FadR | F: GATAATTTGCTGTCGGTGCG R: CCGGTTCCGACTGGCTGGAA | Transcriptional regulator of fatty acid metabolism | [30] |
| PagP | F: GCTAACGCAGATGAGTGGATGACAAC R: CACGAGTCCTTAAATGCCATGG | Phospholipid/lipid A palmitoyl transferase |
| Group | Similarity Coefficient 1 (%) | Total Relative Protein Content 2(%) |
|---|---|---|
| Control | 100.00 | 100.00 |
| AA | 93.75 | 75.12 |
| LA | 93.75 | 50.21 |
| BA | 87.50 | 64.57 |
| CA | 84.38 | 43.99 |
| MA | 78.13 | 45.11 |
| AA + LA | 93.75 | 60.95 |
| AA + LA + BA | 100.00 | 77.17 |
| AA + LA + CA | 100.00 | 57.64 |
| AA + LA + MA | 100.00 | 74.19 |
| Group | Relative Gene Expression 1 | |||||
|---|---|---|---|---|---|---|
| OmpF | OmpW | OmpX | OmpA | FadR | PagP | |
| Control | 1.02 ± 0.19 g | 1.01 ± 0.12 f | 1.00 ± 0.03 e | 1.01 ± 0.11 e | 1.00 ± 0.05 f,g | 1.00 ± 0.03 g |
| AA | 12.72 ± 1.29 e,f | 10.14 ± 0.46 b,c,d | 1.85 ± 0.39 d,e | 9.05 ± 1.14 a | 14.76 ± 0.25 b | 21.76 ± 0.40 a |
| LA | 27.25 ± 1.20 a | 6.94 ± 0.49 d,e | 5.45 ± 0.57 c | 2.15 ± 0.12 d,e | 7.62 ± 0.87 c,d | 1.85 ± 0.11 f |
| BA | 15.86 ± 0.52 c,d,e | 18.50 ± 3.00 a | 7.52 ± 0.69 b | 2.08 ± 0.40 d,e | 3.94 ± 0.62 e,f,g | 2.26 ± 0.15 e,f |
| CA | 21.95 ± 5.37 a,b,c | 9.11 ± 0.56 b,c,d | 1.63 ± 0.06 d,e | 7.18 ± 0.21 b | 27.11 ± 3.16 a | 2.44 ± 0.07 e |
| MA | 23.80 ± 2.66 b,c | 5.19 ± 1.91 e | 1.93 ± 0.31 d,e | 3.96 ± 0.50 c | 8.45 ± 0.77 c | 1.05 ± 0.14 g |
| AA + LA | 13.58 ± 1.20 d,e,f | 11.10 ± 1.34 b,c | 10.87 ± 0.81 a | 2.23 ± 0.08 d,e | 4.91 ± 0.76 d,e,f | 2.98 ± 0.10 d |
| AA + LA + BA | 9.04 ± 1.76 f | 12.48 ± 0.88 b | 1.55 ± 0.08 d,e | 2.98 ± 0.46 c,d | 6.33 ± 0.64 c,d,e | 3.96 ± 0.04 c |
| AA + LA + CA | 18.99 ± 2.67 b,c,d,e | 12.49 ± 0.97 b | 2.02 ± 0.22 d,e | 8.14 ± 0.72 a,b | 16.61 ± 1.21 b | 10.29 ± 0.17 b |
| AA + LA + MA | 19.55 ± 2.65 b,c,d | 7.75 ± 0.19 c,d,e | 2.32 ± 0.61 d | 3.66 ± 0.27 c | 2.84 ± 0.33 f,g | 1.81 ± 0.05 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Q.-Y.; Wang, W.; Yan, H.; Qu, H.; Liu, Y.; Qian, Y.; Gu, R. The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods 2023, 12, 3011. https://doi.org/10.3390/foods12163011
Ji Q-Y, Wang W, Yan H, Qu H, Liu Y, Qian Y, Gu R. The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods. 2023; 12(16):3011. https://doi.org/10.3390/foods12163011
Chicago/Turabian StyleJi, Qing-Yang, Wenqiong Wang, Haodong Yan, Hengxian Qu, Yang Liu, Yi Qian, and Ruixia Gu. 2023. "The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli" Foods 12, no. 16: 3011. https://doi.org/10.3390/foods12163011
APA StyleJi, Q.-Y., Wang, W., Yan, H., Qu, H., Liu, Y., Qian, Y., & Gu, R. (2023). The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods, 12(16), 3011. https://doi.org/10.3390/foods12163011







