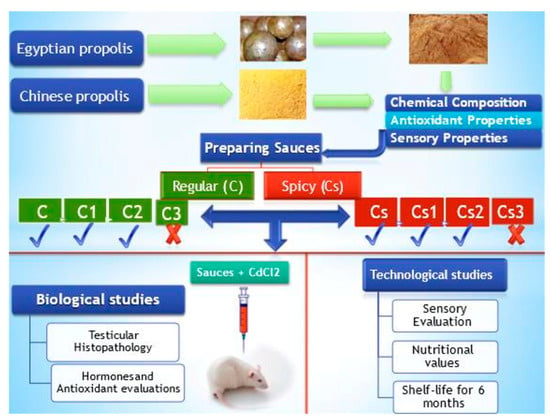
An Innovative Use of Propolis in the Production of Dipping Sauce Powder as a Functional Food to Mitigate Testicular Toxicity Induced by Cadmium Chloride: Technological and Biological Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Propolis
2.1.2. Plant Materials
2.1.3. Shrimp Shell and Head Powder
2.1.4. Chemicals and Solvents
2.2. Sauce Preparation and Properties
2.2.1. Preparation of Dipping Sauce Powder Samples and Evaluation of Palatability
2.2.2. Chemical and Sensory Properties of Propolis and Nutritional Value of Dipping Sauce Samples
2.2.3. Shelf-Life Study of Dipping Sauce Powder Samples
2.3. Biological Study Design
- Group I (negative control): Rats were fed the basal diet for 30 days;
- Group II (positive control): Rats were fed the basal diet and received CdCl2 solution orally via stomach tube every other day for 30 days at a dose of 5 mg/kg body weight [20];
- Group III: Rats were fed the basal diet containing 5% C formula and received CdCl2 solution orally via stomach tube every other day for 30 days at a dose of 5 mg/kg body weight;
- Group IV: Rats were fed the basal diet containing 5% C1 formula and received CdCl2 solution orally via stomach tube every other day for 30 days at a dose of 5 mg/kg body weight;
- Group V: Rats were fed the basal diet containing 5% C2 formula and received CdCl2 solution orally via stomach tube every other day for 30 days at a dose of 5 mg/kg body weight;
- Group VI: Rats were fed the basal diet containing 5% Cs formula and received CdCl2 solution orally via stomach every other day for 30 days at a dose of 5 mg/kg body weight;
- Group VII: Rats were fed the basal diet containing 5% Cs1 formula and received CdCl2 solution orally via stomach tube every other day for 30 days at a dose of 5 mg/kg body weight;
- Group VIII: Rats were fed the basal diet containing 5% (Cs2) formula and received CdCl2 solution orally via stomach tube every other day for 30 days at a dose of 5 mg/kg body weight.
2.3.1. Biochemical Analysis
2.3.2. Histopathological and Histomorphometric Examinations
2.4. Statistical Analysis
3. Results and Discussion
3.1. Technological Results
3.1.1. Functional and Technological Properties of Egyptian Propolis
3.1.2. Palatability Tests, Nutritive Values, and Shelf-Life Assay of Investigated Products
3.2. Biological Results
3.2.1. Histological and Morphometric Results
3.2.2. Biochemical Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Salama, A.; Mohamed, S.A.A. Propolis applications in food industries and packaging. Biomass Convers. Biorefinery 2023, 1–17. [Google Scholar] [CrossRef]
- Smith, A.F. (Ed.) The Oxford Companion to American Food and Drink; Oxford University Press: Oxford, UK, 2007; pp. 1–145. [Google Scholar]
- Rombauer, I.S.; Becker, R.M.; Becker, E. The Joy of Cooking: 2019 Edition Fully Revised and Updated; Simon & Schuster: New York, NY, USA, 2019; pp. 1–1200. [Google Scholar]
- Manouchehri, A.; Shokri, S.; Pirhadi, M.; Karimi, M.; Abbaszadeh, S.; Mirzaei, G.; Bahmani, M. The Effects of Toxic Heavy Metals Lead, Cadmium and Copper on the Epidemiology of Male and Female Infertility. JBRA Assist. Reprod. 2022, 26, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-L.; Agarwal, A. Role of sperm DNA fragmentation in male factor infertility: A systematic review. Arab J. Urol. 2018, 16, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bhattacharyya, S.; Ray, S.; Saha, R.; Ghosh, P.; Rauth, S.; Murmu, N. Resveratrol alleviates cadmium-induced damage and overexpression of epidermal growth factor receptor and its downstream signaling proteins in the reproductive system of male Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Rengga, W.D.P.; Salsabiil, K.A.; Harianingsih; Oktavia, S.E.; Ansori, M. Flavored powder from shrimp shells with bromelain enzymatic process and adding of flour and spices. J. Phys. Conf. Ser. 2019, 1367, 012080. [Google Scholar] [CrossRef]
- Amerine, M.A.; Pangborn, R.M.; Rocssler, E. Principles of Sensory Evolution of Foods; Academic Press: New York, NY, USA, 1965; pp. 1–602. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Fraser, J.R.; Holmes, D.C. Proximate analysis of wheat flour carbohydrates. IV.—Analysis of wholemeal flour and some of its fractions. J. Sci. Food Agric. 1959, 10, 506–512. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kunimasa, K.; Ohta, T.; Kumazawa, S.; Kamihira, M.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Nakayama, T. Suppression of tumor-induced angiogenesis by Brazilian propolis: Major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett. 2007, 252, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Elslimani, F.A.; Elmhdwi, M.F.; Elabbar, F.; Dakhil, O.O. Estimation of antioxidant activities of fixed and volatile oils extracted from Syzygium aromaticum (clove). Der Chem. Sin. 2013, 4, 120–125. [Google Scholar]
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Vladimir-Knezević, S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004, 54, 65–72. [Google Scholar] [PubMed]
- James, C.S. General food studies. In Analytical Chemistry of Foods; James, C.S., Ed.; Springer: Boston, MA, USA, 1995; pp. 137–171. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Andrews, W.H.; June, G.A.; Sherrod, P.S.; Hammack, T.S.; Amaguana, R.M. FDA. Bacteriological Analytical Manual; AOAC International: Gaithersburg, MD, USA, 1995; p. 614. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Hassan, W.A.; El-Kashlan, A.M.; Mohamed, N. Egyptian Date Palm Pollen Ameliorates Testicular Dysfunction Induced by Cadmium Chloride in Adult Male Rats. J. Am. Sci. 2012, 8, 659–669. [Google Scholar]
- Odell, W.D.; Parlow, A.F. Estimation of FSH test assay. J. Clin. Investig. 1981, 47, 25–51. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxidase in animal tissue by thiobaubituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Drury, R.A.; Wallington, E.A. Carleton’s Histological Techniques, 5th ed.; Oxford University Press: Oxford, UK, 1980; p. 362. [Google Scholar]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997; p. 666. [Google Scholar]
- Abd El-Hady, N.A.A.; El-Sayed, A.I.; El-Saadany, S.S. Effect Of The Egyptian Propolis on the Bioactive Compounds Content in Tomato Plants. Zagazig J. Agric. Res. 2020, 47, 579–586. [Google Scholar] [CrossRef]
- Segueni, N.; Boutaghane, N.; Asma, S.T.; Tas, N.; Acaroz, U.; Arslan-Acaroz, D.; Shah, S.R.A.; Abdellatieff, H.A.; Akkal, S.; Peñalver; et al. Review on Propolis Applications in Food Preservation and Active Packaging. Plants 2023, 12, 1654. [Google Scholar] [CrossRef]
- Abd Elhady, M.S.; Kandil, A.H.; Albalat, W.M. Trace Element’s Role in Male Infertility; Review Article. Egypt. J. Hosp. Med. 2021, 85, 3678–3681. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef]
- NSW Food Authority. Microbiological Quality guide for Ready-to-Eat Foods. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/microbiological_quality_guide_for_RTE_food.pdf (accessed on 13 July 2023).
- El-Habibi, E.; El-Komy, M.; Saad, H. Protective Effect of Date Palm Extracts on Cadmium-Induced Infertility in Male Rats. Egypt. J. Hosp. Med. 2017, 69, 2181–2190. [Google Scholar] [CrossRef]
- El-Neweshy, M.S.; El-Maddawy, Z.K.; El-Sayed, Y.S. Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia 2012, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Eleawa, S.M.; Alkhateeb, M.A.; Alhashem, F.H.; Bin-Jaliah, I.; Sakr, H.F.; Elrefaey, H.M.; Elkarib, A.O.; Alessa, R.M.; Haidara, M.A.; Shatoor, A.S.; et al. Resveratrol Reverses Cadmium Chloride-induced Testicular Damage and Subfertility by Downregulating p53 and Bax and Upregulating Gonadotropins and Bcl-2 gene Expression. J. Reprod. Develop. 2014, 60, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Monsefi, M.; Alaee, S.; Moradshahi, A.; Rohani, L. Cadmium-induced infertility in male mice. Environ. Toxicol. 2010, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Lee, S.H.; Jin, Y.; Choi, J.H.; Han, C.H.; Lee, M.H. Genotoxicity and toxicological effects of acrylamide on reproductive system in male rats. J. Vet. Sci. 2005, 6, 103–109. [Google Scholar] [CrossRef]
- Qadori, Y.T.; Al-shaikh, M.N. Effects of high and low dose of cadmium chloride on male Reproductive system in mice. J. Fac. Med. Baghdad 2012, 54, 110–114. [Google Scholar]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef]
- Ikediobi, C.O.; Badisa, V.L.; Ayuk-Takem, L.T.; Latinwo, L.M.; West, J. Response of antioxidant enzymes and redox metabolites to cadmium-induced oxidative stress in CRL-1439 normal rat liver cells. Int. J. Mol. Med. 2004, 14, 87–92. [Google Scholar] [CrossRef]
- Ali, W.; Ma, Y.; Zhu, J.; Zou, H.; Liu, Z. Mechanisms of Cadmium-Induced Testicular Injury A Risk to Male Fertility. Cells 2022, 11, 3601. [Google Scholar] [CrossRef]
- Abu-Almaaty, A.H.; El-Aziz, A.; Yasmin, M.; Omar, N.A.; Abdeen, A.M. Estimation of the prophylactic effect of the Egyptian propolis extract against aluminum silicate toxicity on some organs of albino rats: Growth performance and histochemical studies. Egy. J. Hosp. Med. 2019, 76, 4564–4569. [Google Scholar] [CrossRef]
- El-Amawy, A.A.B.; Zaahkouk, S.A.M.; Rasheed, H.G.A.; Mohammed, B.E.E. The protective role of propolis against multi heavy metals-induced oxidative stress-hepato-renal damage in the male of albino rats. Res. Square 2021, 1, 1–22. [Google Scholar]
- Hashem, A.S. Defensive impact of propolis against CCl4 actuated rats’ testicular damage. J. Adv. Vet. Anim. Res. 2021, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Kamel, K.I.; Hassan, M.S.; El-Morsy, A.M.A. A Protective role of propolis against reproductive toxicity of triphenyltin in male rabbits. Food Chem. Toxicol. 2010, 48, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- AbdElrazek, A.; Eldash, H.A.; Said, N.I. The role of propolis against paclitaxel induced oligospermia, sperm abnormality, oxidative stress and DNA damage in testes of male rats. Andrologia 2020, 52, 3394. [Google Scholar]
- Cilenk, K.T.; Ozturk, I.; Sonmez, M.F. Ameliorative effect of propolis on the cadmium-induced reproductive toxicity in male albino rats. Exp. Mol. Pathol. 2016, 101, 207–213. [Google Scholar] [CrossRef]
- Seven, I.; Seven, P.T.; Baykalir, B.G.; Ak, T.P.; Kaya, S.O.; Yaman, M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, e13540. [Google Scholar] [CrossRef]
- Rizk, S.M.; Zaki, H.F.; Mina, M.A. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem. Toxicol. 2014, 67, 176–186. [Google Scholar] [CrossRef]
- Talas, S.Z. Propolis reduces oxidative stress in l-NAME-induced hypertension rats. Cell Biochem. Funct. 2014, 32, 150–154. [Google Scholar] [CrossRef]
- Yousef, M.I.; Salama, A.F. Propolis protection from reproductive toxicity caused by aluminum chloride in male rats. Food Chem. Toxicol. 2009, 47, 1168–1175. [Google Scholar] [CrossRef]
- Darwish, H.A.; Arab, H.H.; Abdelsalam, R.M. Chrysin alleviates testicular dysfunction in adjuvant arthritic rats via suppression of inflammation and apoptosis: Comparison with celecoxib. Toxicol. Appl. Pharmacol. 2014, 279, 129–140. [Google Scholar] [CrossRef]
- Nna, V.U.; Bakar, A.B.; Ahmad, A.; Umar, U.Z.; Suleiman, J.B.; Zakaria, Z.; Othman, Z.S.; Mohamed, M. Malaysian propolis and metformin mitigate subfertility in streptozotocin-induced diabetic male rats by targeting steroidogenesis, testicular lactate transport, spermatogenesis and mating behaviour. Andrology 2020, 8, 731–746. [Google Scholar] [CrossRef]





| Formulations | C | C1 | C2 | C3 | Cs | Cs1 | Cs2 | Cs3 | |
|---|---|---|---|---|---|---|---|---|---|
| Ingredients | |||||||||
| Propolis powder (mg/kg) | - | 293.2 | 586.5 | 879.7 | - | 304.8 | 609 | 913.5 | |
| Yellow split chickpeas (g) | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | |
| Fenugreek powder (g) | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |
| Shrimp shell powder (g) | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | |
| Red hot pepper (g) | - | - | - | - | 45 | 45 | 45 | 45 | |
| Garlic powder (g) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Onion powder (g) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Curry powder (g) | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | |
| Salt (g) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Ginger (g) | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |
| Dried tomato powder (g) | 450 | 450 | 450 | 450 | 450 | 450 | 450 | 450 | |
| Chemical Composition | Egyptian Propolis | Chinese Propolis |
|---|---|---|
| Moisture (%) | 4.11 ± 0.28 a | 5.68 ± 0.69 a |
| Proteins (%) | 10.25 ± 0.16 a | 8.98 ± 0.29 b |
| Fats (%) | 21.54 ± 0.49 b | 41.68 ± 0.90 a |
| Fibers (%) | 49.87 ± 0.18 a | 20.48 ± 0.42 b |
| Available carbohydrates (%) | 14.23 ± 0.26 b | 23.18 ± 1.22 a |
| Antioxidant Properties | ||
| Total phenolic content (mg GAE/g sample DW) | 200.70 ± 1.54 a | 135.26 ± 5.92 b |
| Total flavonoid content (mg quercetin/g sample DW) | 91.86 ± 2.51 a | 29.03 ± 3.25 b |
| DPPH scavenging activity (%) | 78.77 ± 0.82 a | 66.70 ± 0.78 b |
| Sensory Properties | ||
| Color | Brown | Tortilla brown |
| Smell | Not aromatic | Aromatic |
| Appearance | Dry | Dry |
| Sample | Protein | Fat | Ash | Fibers | Available Carbohydrate | Energy |
|---|---|---|---|---|---|---|
| % | % | % | % | % | Kcal | |
| C | 21.20 ± 0.06 c | 7.97 ± 0.27 a | 4.12 ± 0.02 e | 2.51 ± 0.01 f | 64.21 ± 0.32 a | 413.22 ± 2.13 a |
| C1 | 21.45 ± 0.05 b | 8.02 ± 0.27 a | 4.29 ± 0.02 d | 2.63 ± 0.01 d | 63.62 ± 0.32 ab | 412.42 ± 1.26 a |
| C2 | 21.48 ± 0.05 b | 8.07 ± 0.27 a | 4.47 ± 0.04 c | 2.76 ± 0.01 b | 63.25 ± 0.25 b | 411.43 ± 1.35 a |
| Cs | 22.34 ± 0.02 a | 8.47 ± 0.27 a | 5.05 ± 0.01 b | 2.58 ± 0.01 e | 61.54 ± 0.32 c | 411.81 ± 1.43 a |
| Cs1 | 22.37 ± 0.09 a | 8.59 ± 0.23 a | 5.11 ± 0.01 b | 2.71 ± 0.01 c | 61.26 ± 0.27 c | 411.65 ± 1.10 a |
| Cs2 | 22.47 ± 0.02 a | 8.67 ± 0.24 a | 5.23 ± 0.02 a | 2.83 ± 0.01 a | 60.80 ± 0.26 c | 411.12 ± 1.17 a |
| LSD at 0.05 | 0.17 | 0.80 | 0.07 | 0.03 | 0.90 | 4.46 |
| Sample | Micronutrient Contents | |||
|---|---|---|---|---|
| P | Fe | Zn | Se | |
| C | 57.83 ± 0.17 e | 111.67 ± 0.88 b | 146.05 ± 0.13 e | 1.33 ± 0.01 d |
| C1 | 59.50 ± 0.29 cd | 111.83 ± 0.86 b | 147.05 ± 0.64 e | 1.36 ± 0.00 c |
| C2 | 60.50 ± 0.29 bc | 113.00 ± 0.15 ab | 148.71 ± 0.38 d | 1.39 ± 0.00 b |
| Cs | 58.50 ± 0.76 de | 112.65 ± 0.31 ab | 149.71 ± 0.57 cd | 1.39 ± 0.01 b |
| Cs1 | 61.27 ± 0.03 ab | 113.23 ± 0.09 ab | 151.05 ± 0.13 bc | 1.41 ± 0.01 ab |
| Cs2 | 61.80 ± 0.06 a | 113.87 ± 0.03 a | 153.05 ± 0.87 a | 1.43 ± 0.01 a |
| LSD at 0.05 | 1.11 | 1.61 | 1.63 | 0.02 |
| Group | Seminiferous Tubule Diameter | Epithelial Height |
|---|---|---|
| GI (Control) | 394.00 ± 2.08 a | 124.00 ± 0.58 a |
| GII (CdCl2) | 254.48 ± 1.16 g | 55.70 ± 0.93 g |
| GIII | 322.85 ± 1.25 f | 86.31 ± 0.66 f |
| GIV | 366.74 ± 1.85 d | 113.67 ± 0.88 d |
| GV | 370.08 ± 0.56 d | 116.00 ± 0.58 c |
| GVI | 333.77 ± 2.04 e | 102.00 ± 1.15 e |
| GVII | 376.21 ± 1.17 c | 118.00 ± 0.58 bc |
| GVIII | 388.67 ± 1.20 b | 119.67 ± 0.88 b |
| LSD at 0.05 | 5.50 | 2.96 |
| Group | Testosterone | LH | Progesterone | MDA | TAC |
|---|---|---|---|---|---|
| (ng/mL) | (mIU/mL) | (ng/mL) | (nmol/g tissue) | (nmol/g tissue) | |
| GI (Control) | 2.89 ± 0.01 a | 2.92 ± 0.02 a | 1.86 ± 0.01 a | 39.44 ± 0.70 g | 36.18 ± 0.57 a |
| GII (CdCl2) | 1.38 ± 0.02 g | 2.02 ± 0.03 g | 1.03 ± 0.03 e | 99.45 ± 0.53 a | 12.83 ± 0.20 e |
| GIII | 1.87 ± 0.03 f | 2.19 ± 0.01 f | 1.28 ± 0.01 d | 72.62 ± 0.26 b | 13.62 ± 0.26 d |
| GIV | 2.26 ± 0.02 d | 2.34 ± 0.04 e | 1.66 ± 0.04 b | 56.76 ± 1.49 d | 18.57 ± 0.35 d |
| GV | 2.31 ± 0.02 c | 2.51 ± 0.01 d | 1.63 ± 0.01 b | 53.21 ± 0.48 e | 18.63 ± 0.20 d |
| GVI | 2.00 ± 0.03 e | 2.34 ± 0.04 e | 1.47 ± 0.02 c | 59.54 ± 0.64 c | 18.65 ± 0.32 d |
| GVII | 2.36 ± 0.02 c | 2.62 ± 0.01 c | 1.65 ± 0.03 b | 54.18 ± 1.52 de | 22.77 ± 0.38 c |
| GVIII | 2.54 ± 0.03 b | 2.77 ± 0.02 b | 1.79 ± 0.01 a | 43.54 ± 0.89 f | 25.25 ± 0.64 b |
| LSD at 0.05 | 0.07 | 0.08 | 0.07 | 2.77 | 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheir, M.A.; Serrapica, F.; Ahmed, R.A. An Innovative Use of Propolis in the Production of Dipping Sauce Powder as a Functional Food to Mitigate Testicular Toxicity Induced by Cadmium Chloride: Technological and Biological Evidence. Foods 2023, 12, 3069. https://doi.org/10.3390/foods12163069
Sheir MA, Serrapica F, Ahmed RA. An Innovative Use of Propolis in the Production of Dipping Sauce Powder as a Functional Food to Mitigate Testicular Toxicity Induced by Cadmium Chloride: Technological and Biological Evidence. Foods. 2023; 12(16):3069. https://doi.org/10.3390/foods12163069
Chicago/Turabian StyleSheir, Marwa A., Francesco Serrapica, and Rania A. Ahmed. 2023. "An Innovative Use of Propolis in the Production of Dipping Sauce Powder as a Functional Food to Mitigate Testicular Toxicity Induced by Cadmium Chloride: Technological and Biological Evidence" Foods 12, no. 16: 3069. https://doi.org/10.3390/foods12163069
APA StyleSheir, M. A., Serrapica, F., & Ahmed, R. A. (2023). An Innovative Use of Propolis in the Production of Dipping Sauce Powder as a Functional Food to Mitigate Testicular Toxicity Induced by Cadmium Chloride: Technological and Biological Evidence. Foods, 12(16), 3069. https://doi.org/10.3390/foods12163069