Cadmium Exposure in Aquatic Products and Health Risk Classification Assessment in Residents of Zhejiang, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Inductively Coupled Plasma Mass Spectrometry
2.3. Food Consumption Data
2.4. Exposure Assessment
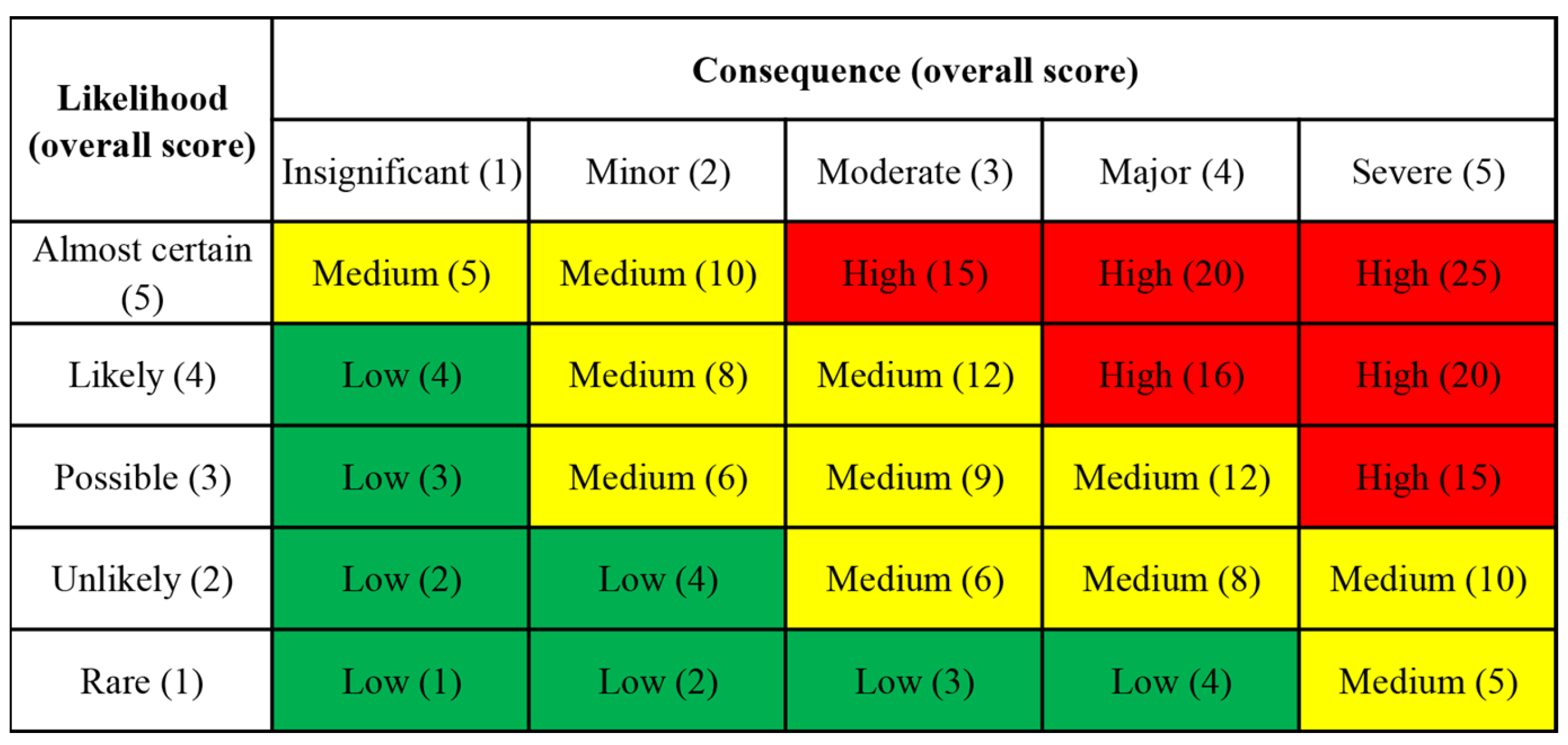
2.5. Health Risk Classification
2.6. Data Analysis
3. Results
3.1. Concentration of Cd in Aquatic Products
3.2. Exposure Assessment
3.2.1. Dietary Cd Exposure by Different Food Groups
3.2.2. Dietary Cd Exposure by Different Area
3.2.3. Cd Exposure by Age Subgroups
3.3. Health Risk Classification Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. J. Int. Assoc. Geochem. Cosmochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. The Role of Probiotics in the Amelioration of Cadmium Toxicity. Biol. Trace Elem. Res. 2020, 197, 440–444. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Kidney Cadmium Toxicity, Diabetes and High Blood Pressure: The Perfect Storm. Tohoku J. Exp. Med. 2017, 241, 65–87. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of Cadmium Exposure on the Immune System and Immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef]
- Mirkov, I.; Aleksandrov, A.P.; Ninkov, M.; Tucovic, D.; Kulas, J.; Zeljkovic, M.; Popovic, D.; Kataranovski, M. Immunotoxicology of cadmium: Cells of the immune system as targets and effectors of cadmium toxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 149, 112026. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Cullen, J.T.; Maldonado, M.T. Biogeochemistry of cadmium and its release to the environment. Met. Ions Life Sci. 2013, 11, 31–62. [Google Scholar] [PubMed]
- Evaluation of Certain Food Additive and Contaminants; World Health Organization Technical Report Series; Back Cover; World Health Organization: Geneva, Switzerland, 2011; pp. 1–226.
- European Food Safety Authority. Cadmium in food: Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2009, 7, 980. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2018, 50, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef]
- Mhungu, F.; Chen, K.; Wang, Y.; Liu, Y.; Zhang, Y.; Pan, X.; Cheng, Y.; Liu, Y.; Zhang, W. Probabilistic Risk Assessment of Dietary Exposure to Cadmium in Residents of Guangzhou, China-Young Children Potentially at a Health Risk. Int. J. Environ. Res. Public Health 2022, 19, 9572. [Google Scholar] [CrossRef]
- Qin, Y.; Tao, Y. Pollution status of heavy metals and metalloids in Chinese lakes: Distribution, bioaccumulation and risk assessment. Ecotoxicol. Environ. Saf. 2022, 248, 114293. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, X.; Gu, Y.; Wang, R.; Xie, P.; Liang, M.; Ming, H.; Su, J. Comprehensive large-scale investigation and assessment of trace metal in the coastal sediments of Bohai Sea. Mar. Pollut. Bull. 2018, 129, 126–134. [Google Scholar] [CrossRef]
- Schmidt, L.; Novo, D.L.R.; Druzian, G.T.; Landero, J.A.; Caruso, J.; Mesko, M.F.; Flores, E.M.M. Influence of culinary treatment on the concentration and on the bioavailability of cadmium, chromium, copper, and lead in seafood. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2021, 65, 126717. [Google Scholar] [CrossRef]
- Lao, Y.M.; Qu, C.L.; Zhang, B.; Jin, H. Development and validation of single-step microwave-assisted digestion method for determining heavy metals in aquatic products: Health risk assessment. Food Chem. 2023, 402, 134500. [Google Scholar] [CrossRef]
- Bonsignore, M.; Manta, D.S.; Mirto, S.; Quinci, E.M.; Ape, F.; Montalto, V.; Gristina, M.; Traina, A.; Sprovieri, M. Bioaccumulation of heavy metals in fish, crustaceans, molluscs and echinoderms from the Tuscany coast. Ecotoxicol. Environ. Saf. 2018, 162, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, L.; An, M.; Wang, T.; Li, M.; Yu, Y. Heavy metals in marine food web from Laizhou Bay, China: Levels, trophic magnification, and health risk assessment. Sci. Total Environ. 2022, 841, 156818. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shi, Y.; Wang, M.; Ran, M.; Wang, Y.; Tian, L.; Ye, H.; Han, F. A baseline study on the distribution characteristics and health risk assessment of cadmium in edible tissues of the swimming crabs (Portunus trituberculatus) from Shanghai, China. Mar. Pollut. Bull. 2022, 185, 114253. [Google Scholar] [CrossRef]
- Zhejiang Provincial Bureau of Statistics. Available online: http://tjj.zj.gov.cn/ (accessed on 1 July 2023).
- GB 5009.268–2016; National Food Safety Standard. Determination of Multi-Elements in Food. China Standards Press: Beijing, China, 2016. (In Chinese)
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Ping, Z.P.; Ping, L.Z.; Lei, Z.; Dong, L.A.; Yan, S.; Ling, Y.; Ning, L. Methodology and application for health risk classification of chemicals in foods based on risk matrix. Biomed. Environ. Sci. BES 2014, 27, 912–916. [Google Scholar] [CrossRef]
- GEMS/Food-EUROS. Reliable Evaluation of Low-Level Contamination of Food. In Proceedings of the Second Workshop GEM/Food-EUROS, Kulmbach, Germany, 26–27 May 1995. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 106 Pt A, 430–445. [Google Scholar] [CrossRef]
- Ferrari, P.; Arcella, D.; Heraud, F.; Cappé, S.; Fabiansson, S. Impact of refining the assessment of dietary exposure to cadmium in the European adult population. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 687–697. [Google Scholar] [CrossRef]
- Zhao, X.; Shao, Y.; Ma, L.; Shang, X.; Zhao, Y.; Wu, Y. Exposure to Lead and Cadmium in the Sixth Total Diet Study—China, 2016–2019. China CDC Wkly. 2022, 4, 176–179. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Wang, H.; Cui, L.; Zhang, Z.; Guo, J.; Liu, S.; Cui, W. Contamination and health risk assessment of lead, arsenic, cadmium, and aluminum from a total diet study of Jilin Province, China. Food Sci. Nutr. 2020, 8, 5631–5640. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liu, Y.; Liang, B.; Zhou, H.; Li, Y.; Zhang, Y.; Huang, J.; Yu, C.; Chen, K. An Assessment of Dietary Exposure to Cadmium in Residents of Guangzhou, China. Int. J. Environ. Res. Public Health 2018, 15, 556. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, C.; Zhou, H.; Tong, W.; Wang, Y. Dietary exposure assessment of cadmium of adults in Jiangxi Province in 2016. J. Hyg. Res. 2020, 49, 836–839. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, L.; Liu, G.; Zhang, H.; Zhang, J.; Jiang, J.; Xiao, Y.; Bai, W.; Jiao, R.; Huang, W. Dietary exposure to cadmium of Shenzhen adult residents from a total diet study. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, J.; Zhu, Y.; Li, Y.; Ma, W.; Zhang, C.; Li, X.; Wu, M.; Wang, H.; Kauffman, A.E.; et al. Cancer risk and disease burden of dietary cadmium exposure changes in Shanghai residents from 1988 to 2018. Sci. Total Environ. 2020, 734, 139411. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Yu, B.; Dong, K.F.; Ma, D.; Xiao, G.; Zhang, C. Heavy metals in aquatic products and the health risk assessment to population in China. Environ. Sci. Pollut. Res. Int. 2020, 27, 22708–22719. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Z.; Pan, X.D. Exposure assessment of heavy metals (Cd, Hg, and Pb) by the intake of local foods from Zhejiang, China. Environ. Geochem. Health 2014, 36, 765–771. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, Z.; Xiaona, H.; Wei, M. Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicol. Environ. Saf. 2012, 81, 55–64. [Google Scholar] [CrossRef]
- Fu, L.; Lu, X.; Niu, K.; Tan, J.; Chen, J. Bioaccumulation and human health implications of essential and toxic metals in freshwater products of Northeast China. Sci. Total Environ. 2019, 673, 768–776. [Google Scholar] [CrossRef]
- Deng, X.; Mao, L.; Wu, Y.; Tan, Z.; Feng, W.; Zhang, Y. Pollution, risks, and sources of heavy metals in sediments from the urban rivers flowing into Haizhou Bay, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 38054–38065. [Google Scholar] [CrossRef]
- Dietz, R.; Riget, F.; Johansen, P. Lead, cadmium, mercury and selenium in Greenland marine animals. Sci. Total Environ. 1996, 186, 67–93. [Google Scholar] [CrossRef]
- de Almeida Rodrigues, P.; Ferrari, R.G.; Kato, L.S.; Hauser-Davis, R.A.; Conte-Junior, C.A. A Systematic Review on Metal Dynamics and Marine Toxicity Risk Assessment Using Crustaceans as Bioindicators. Biol. Trace Elem. Res. 2022, 200, 881–903. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Rodrigues, P.; Ferrari, R.G.; Hauser-Davis, R.A.; Dos Santos, L.N.; Conte-Junior, C.A. Seasonal influences on swimming crab mercury levels in an eutrophic estuary located in southeastern Brazil. Environ. Sci. Pollut. Res. Int. 2020, 27, 3473–3482. [Google Scholar] [CrossRef] [PubMed]
- Raknuzzaman, M.; Ahmed, K.; Islam, S.; Mamun, H.A.; Tokumura, M.; Sekine, M.; Masunaga, S. Trace metal contamination in commercial fish and crustaceans collected from coastal area of Bangladesh and health risk assessment. Environ. Sci. Pollut. Res. Int. 2016, 23, 17298–17310. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Tseng, L.C.; Chou, C.; Wang, L.; Souissi, S.; Hwang, J.S. Effects of cadmium exposure on antioxidant enzymes and histological changes in the mud shrimp Austinogebia edulis (Crustacea: Decapoda). Environ. Sci. Pollut. Res. Int. 2019, 26, 7752–7762. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Zhong, Z.; Chen, H.; Wang, X.; Wang, M.; Xu, Z.; Cao, L.; Lian, C.; Zhang, H.; et al. Biochemical and Metabolic Responses of the Deep-Sea Mussel Bathymodiolus Platifrons to Cadmium and Copper Exposure; Aquatic Toxicology: Amsterdam, The Netherlands, 2021; Volume 236, p. 105845. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.; Williams, D.J.; Moore, M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef]
- Cao, R.; Liu, Y.; Wang, Q.; Dong, Z.; Yang, D.; Liu, H.; Ran, W.; Qu, Y.; Zhao, J. Seawater acidification aggravated cadmium toxicity in the oyster Crassostrea gigas: Metal bioaccumulation, subcellular distribution and multiple physiological responses. Sci. Total Environ. 2018, 642, 809–823. [Google Scholar] [CrossRef]
- Alava, J.J.; Cheung, W.W.L.; Ross, P.S.; Sumaila, U.R. Climate change-contaminant interactions in marine food webs: Toward a conceptual framework. Glob. Chang. Biol. 2017, 23, 3984–4001. [Google Scholar] [CrossRef]
- Janković, S.; Stošić, M.; Miljaković, E.A.; Ćurčić, M.; Ćosić, D.; Đorđević, A.B.; Bulat, Z.; Antonijević, B. Cadmium dietary exposure assessment in the adult population and pre-school children in the Republic of Serbia. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2023, 40, 67–80. [Google Scholar] [CrossRef]
- Stein, J.; Schettler, T.; Wallinga, D.; Valenti, M. In harm’s way: Toxic threats to child development. J. Dev. Behav. Pediatr. JDBP 2002, 23, S13–S22. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Abete, M.C.; Tarasco, R.; Amorena, M. Lead, cadmium and chromium in raw and boiled portions of Norway lobster. Food Addit. Contam. Part B Surveill. 2014, 7, 267–272. [Google Scholar] [CrossRef] [PubMed]

| Food Type | N | Cd Concentration (mg/kg) | Food Consumption (g/day) | |||
|---|---|---|---|---|---|---|
| <LOD | Mean ± SD | Median | P97.5 | |||
| Freshwater fish | 1113 | 677 | 0.00988 ± 0.0325 | 0.00150 | 0.0937 | 16.5 ± 26.3 |
| Marine fish | 832 | 227 | 0.0417 ± 0.236 | 0.00636 | 0.258 | 15.2 ± 29.7 |
| Freshwater crustaceans | 2255 | 544 | 0.0940 ± 0.394 | 0.0130 | 0.550 | 3.91 ± 11.8 |
| Marine crustaceans | 3247 | 116 | 1.47 ± 2.41 | 0.604 | 8.290 | 9.23 ± 18.8 |
| Mollush | 2249 | 60 | 0.925 ± 1.56 | 0.390 | 5.330 | 1.83 ± 6.76 |
| Others | 660 | 63 | 0.180 ± 0.957 | 0.0230 | 1.200 | 0.588 ± 4.59 |
| Total | 10,356 | 1687 | 0.699 ± 1.681 | 0.0582 | 5.200 | 47.3 ± 51.6 |
| Food Type | Cd Exposure (mg/kg BW/month) | |||
|---|---|---|---|---|
| Mean ± SD | Median | P97.5 | Contribution (%) | |
| Freshwater fish | 0.0000891 ± 0.000143 | 0 | 0.000470 | 0.937 |
| Marine fish | 0.000360 ± 0.000672 | 0 | 0.00210 | 3.79 |
| Freshwater crustaceans | 0.000214 ± 0.000735 | 0 | 0.00200 | 2.25 |
| Marine crustaceans | 0.00786 ± 0.0176 | 0 | 0.0538 | 82.7 |
| Mollush | 0.000960 ± 0.00373 | 0 | 0.0113 | 10.1 |
| Others | 0.0000616 ± 0.000495 | 0 | 0.000766 | 0.648 |
| Total | 0.00951 ± 0.0189 | 0.000901 | 0.0589 | 100 |
| Area | N | Exposure (mg/kg BW/month) | ||
|---|---|---|---|---|
| Mean ± SD | Median | P97.5 | ||
| Hangzhou | 1715 | 0.00216 ± 0.00632 | 0.000171 | 0.0201 |
| Ningbo | 2718 | 0.0148 ± 0.0193 | 0.00800 | 0.0662 |
| Wenzhou | 2672 | 0.0182 ± 0.0220 | 0.01220 | 0.0753 |
| Jiaxing | 1890 | 0.00618 ± 0.0152 | 0.000562 | 0.0457 |
| Huzhou | 932 | 0.00441 ± 0.0105 | 0.000497 | 0.0295 |
| Shaoxing | 889 | 0.00567 ± 0.0111 | 0.000559 | 0.0352 |
| Jinhua | 2759 | 0.00213 ± 0.00828 | 0.0000534 | 0.0236 |
| Quzhou | 863 | 0.000254 ± 0.00156 | 0.00000 | 0.000933 |
| Zhoushan | 889 | 0.0229 ± 0.0250 | 0.0170 | 0.0852 |
| Taizhou | 1825 | 0.0182 ± 0.0275 | 0.0103 | 0.0827 |
| Lishui | 1761 | 0.0039 ± 0.0185 | 0.000130 | 0.0345 |
| Total | 18,913 | 0.00951 ± 0.0189 | 0.000900 | 0.0589 |
| Population Groups | N | Cd Exposure (mg/kg BW/month) | ||
|---|---|---|---|---|
| Mean ± SD | Median | P97.5 | ||
| 2–3 yr | 307 | 0.0239 ± 0.0504 c,d,e,f | 0.00312 | 0.152 |
| 4–11 yr | 1816 | 0.0161 ± 0.0315 c,e,f | 0.00141 | 0.0958 |
| 12–17 yr,male | 504 | 0.0105 ± 0.0205 a,b | 0.000698 | 0.0730 |
| 12–17 yr,female | 525 | 0.0107 ± 0.0198 a | 0.000757 | 0.0660 |
| ≥18 yr,male | 7868 | 0.00816 ± 0.0143 a,b | 0.000824 | 0.0493 |
| ≥18 yr,female | 7893 | 0.00864 ± 0.0158 a,b | 0.000901 | 0.0546 |
| General population | 18,913 | 0.00951 ± 0.0189 | 0.000901 | 0.0589 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Fang, H.; Pan, X.; Zhu, B.; Chen, J.; Wang, J.; Zhang, R.; Chen, L.; Qi, X.; Zhang, H. Cadmium Exposure in Aquatic Products and Health Risk Classification Assessment in Residents of Zhejiang, China. Foods 2023, 12, 3094. https://doi.org/10.3390/foods12163094
He Y, Fang H, Pan X, Zhu B, Chen J, Wang J, Zhang R, Chen L, Qi X, Zhang H. Cadmium Exposure in Aquatic Products and Health Risk Classification Assessment in Residents of Zhejiang, China. Foods. 2023; 12(16):3094. https://doi.org/10.3390/foods12163094
Chicago/Turabian StyleHe, Yue, Hangyan Fang, Xiaodong Pan, Bing Zhu, Jiang Chen, Jikai Wang, Ronghua Zhang, Lili Chen, Xiaojuan Qi, and Hexiang Zhang. 2023. "Cadmium Exposure in Aquatic Products and Health Risk Classification Assessment in Residents of Zhejiang, China" Foods 12, no. 16: 3094. https://doi.org/10.3390/foods12163094





