Analytical Method Optimization of Tetrodotoxin and Its Contamination in Gastropods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagent and Materials
2.3. Preparation of Standard Solutions
2.4. Instrumentation Conditions
2.5. Sample Preparation
2.6. Preparation of Quality Control (QC) Samples
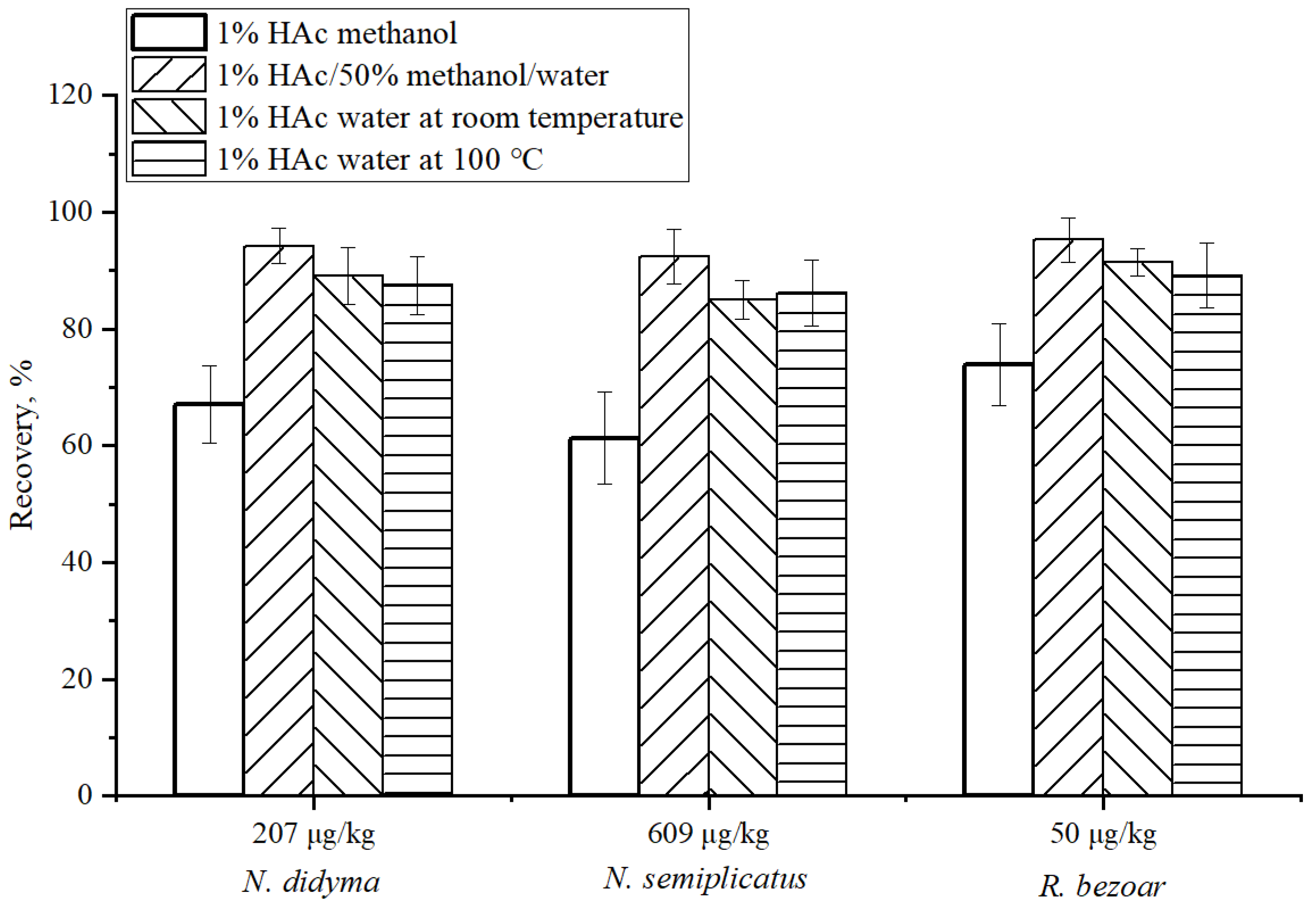
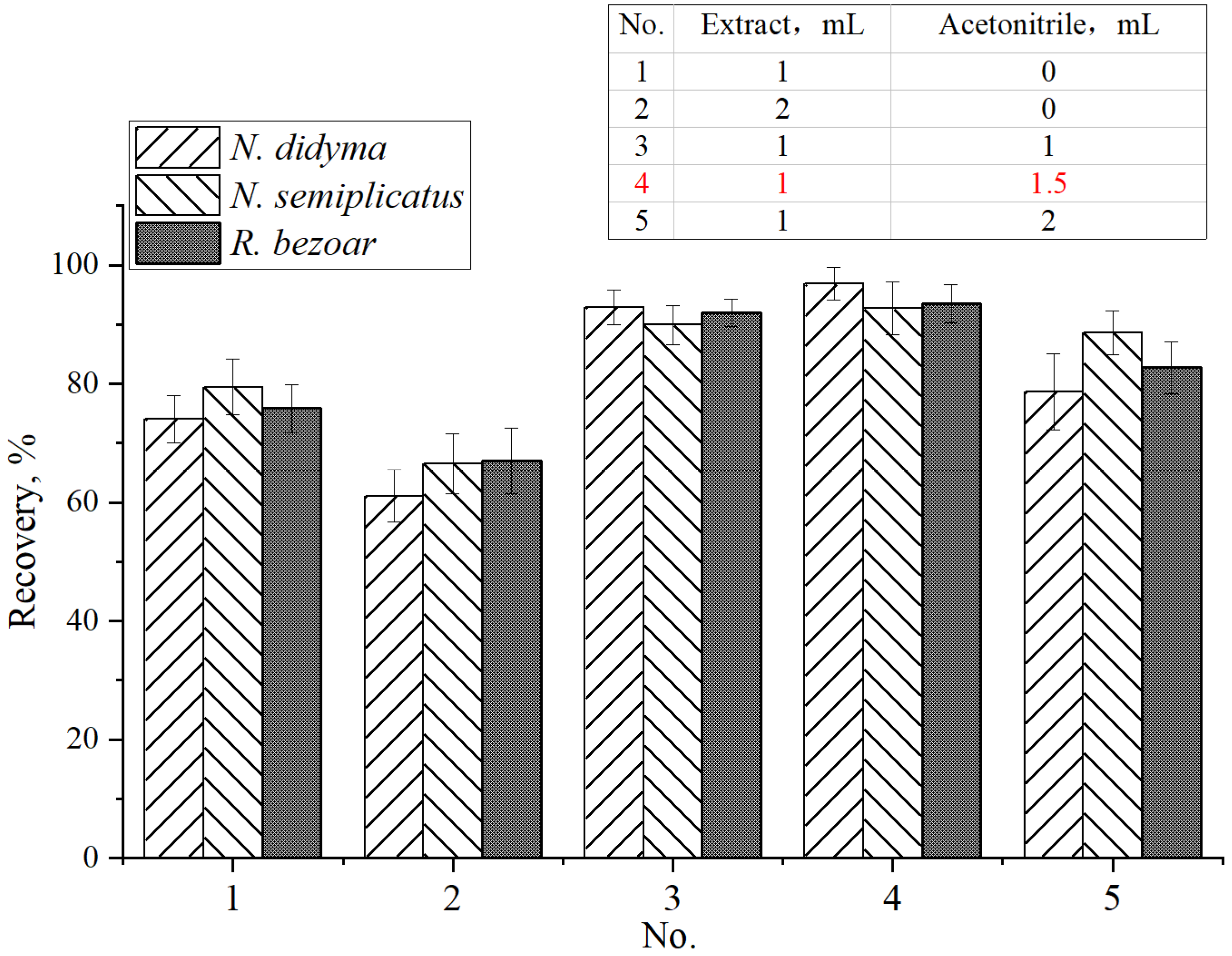
2.7. Condition Optimization of Sample Extraction
2.8. Calculation of Matrix Effects
2.9. Graph making and Statistical Analysis
3. Results and Discussion
3.1. Sample Extraction
3.2. Clean-Up by MCX Cartridge
3.2.1. Protein Precipitation before MCX Cartridge Clean-Up
3.2.2. TTX Eluting and the Treatment of Eluate
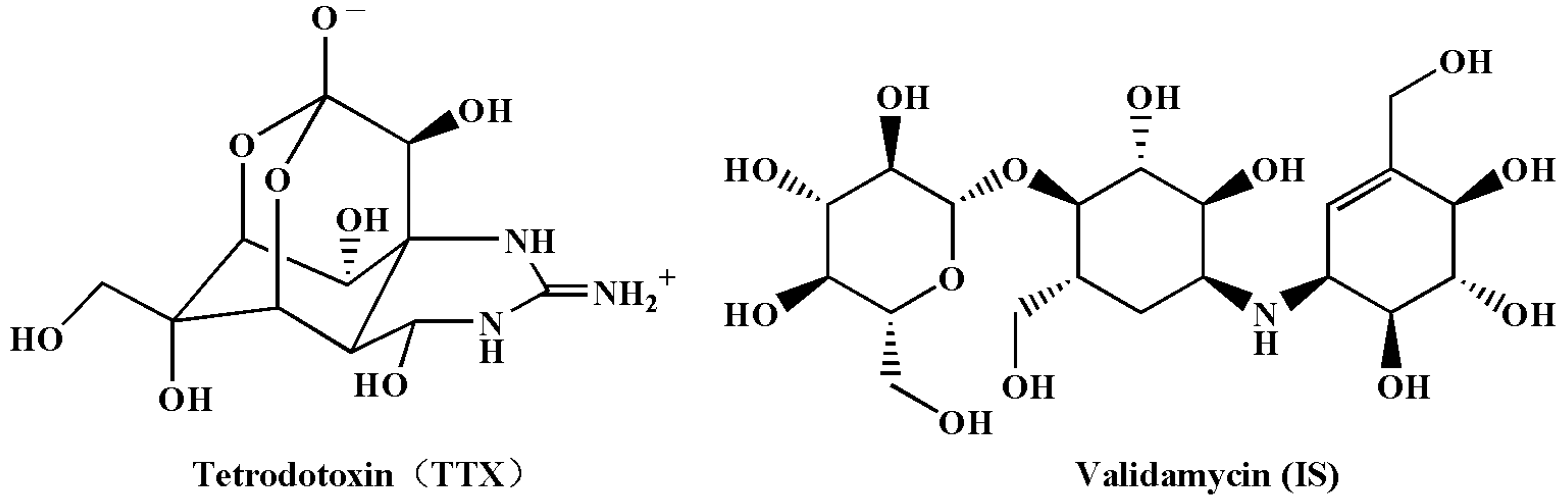
3.3. Screening of Internal Standard
3.4. Selection of Chromatographic Column
3.5. Method Validation
3.5.1. Limit of Detection (LOD) and Quantification (LOQ), and Linear Range
3.5.2. Recovery
3.5.3. Accuracy and Precision
3.6. Comparing with the Reported LC-MS/MS Methods
3.7. TTX in Gastropods
3.7.1. TTX Contaminations in Gastropods
3.7.2. Time Distribution of TTX Contaminations in N. didyma
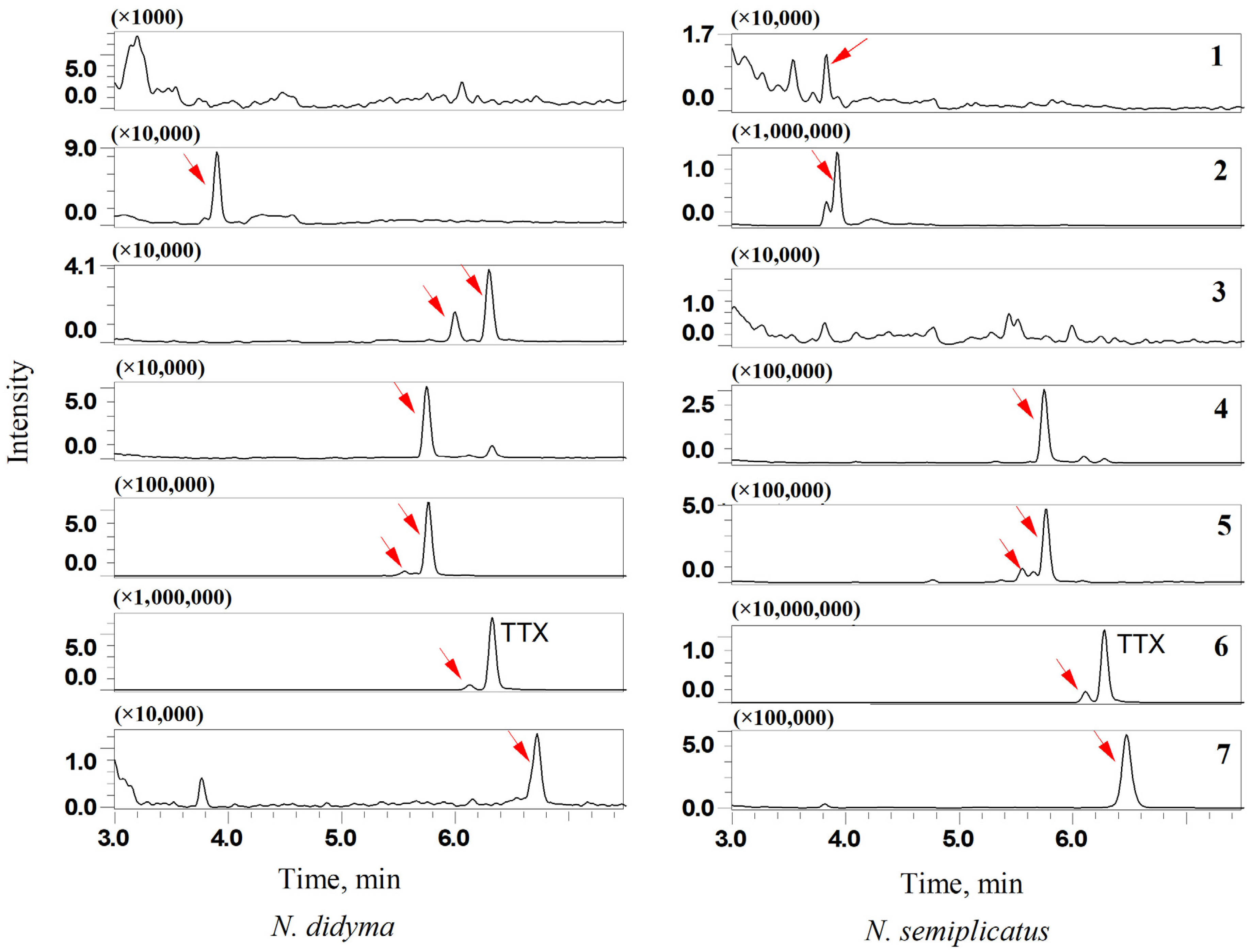
3.7.3. TTX Analogues in N. didyma and N. semiplicatus
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A global retrospective study on human cases of tetrodotoxin (TTX) poisoning after seafood consumption. Food Rev. Int. 2020, 36, 645–667. [Google Scholar] [CrossRef]
- Jen, H.C.; Lin, S.J.; Tsai, Y.H.; Chen, C.H.; Lin, Z.C.; Hwang, D.F. Tetrodotoxin poisoning evidenced by solid-phase extraction combining with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2008, 871, 95–100. [Google Scholar] [CrossRef]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, L.A.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of tetrodotoxin from the grey side-gilled sea slug—Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Costa, P.R.; Giraldez, J.; Rodrigues, S.M.; Leao, J.M.; Pinto, E.; Solino, L.; Gago-Martinez, A. High levels of tetrodotoxin (TTX) in trumpet shell Charonia lampas from the Portuguese coast. Toxins 2021, 13, 250. [Google Scholar] [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campas, M.; Ozogul, F. An updated review of tetrodotoxin and its peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef]
- Abal, P.; Louzao, M.C.; Antelo, A.; Alvarez, M.; Cagide, E.; Vilarino, N.; Vieytes, M.R.; Botana, L.M. Acute oral toxicity of tetrodotoxin in mice: Determination of lethal dose 50 (LD50) and no observed adverse effect level (NOAEL). Toxins 2017, 9, 75. [Google Scholar] [CrossRef]
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain; Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752. [Google Scholar]
- Hu, C.; Zhang, Y.; Zhou, Y.; Xiang, Y.J.; Liu, Z.F.; Wang, Z.H.; Feng, X.S. Tetrodotoxin and its analogues in food: Recent updates on sample preparation and analytical methods since 2012. J. Agric. Food Chem. 2022, 70, 12249–12269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. A gold nanoparticle-based lateral flow immunosensor for ultrasensitive detection of tetrodotoxin. Analyst 2020, 145, 2143–2151. [Google Scholar] [CrossRef]
- Man, C.N.; Noor, N.M.; Harn, G.L.; Lajis, R.; Mohamad, S. Screening of tetrodotoxin in puffers using gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Tooru, M. Fluorometric determination of tetrodotoxin by high performance liquid chromatography. Agric. Biol. Chem. 1985, 49, 3077–3080. [Google Scholar]
- Jen, H.C.; Lin, S.J.; Lin, S.Y.; Huang, Y.W.; Liao, I.C.; Arakawa, O.; Hwang, D.F. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit. Contam. 2007, 24, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Hort, V.; Arnich, N.; Guerin, T.; Lavison-Bompard, G.; Nicolas, M. First detection of tetrodotoxin in bivalves and gastropods from the French mainland coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef]
- Dhanji-Rapkova, M.; Turner, A.D.; Baker-Austin, C.; Huggett, J.F.; Ritchie, J.M. Distribution of tetrodotoxin in Pacific oysters (Crassostrea gigas). Mar. Drugs 2021, 19, 84. [Google Scholar] [CrossRef]
- Ye, H.; Xi, Y.; Tian, L.; Huang, D.; Huang, X.; Shen, X.; Cai, Y.; Wangs, Y. Simultaneous determination of tetrodotoxin in the fresh and heat-processed aquatic products by high-performance liquid chromatography-tandem mass spectrometry. Foods 2022, 11, 925. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yan, Z.Y.; Wang, Y.; Jiang, T.; Wang, J.; Sun, X.M.; Guo, Y.M. Immunoaffinity chromatography purification and ultrahigh performance liquid chromatography tandem mass spectrometry determination of tetrodotoxin in marine organisms. J. Agric. Food Chem. 2015, 63, 3129–3134. [Google Scholar] [CrossRef]
- Coleman, R.; Lemire, S.W.; Bragg, W.; Garrett, A.; Ojeda-Torres, G.; Hamelin, E.; Johnson, R.C.; Thomas, J. Development and validation of a high-throughput online solid phase extraction—Liquid chromatography—Tandem mass spectrometry method for the detection of tetrodotoxin in human urine. Toxicon 2016, 119, 64–71. [Google Scholar] [CrossRef]
- Reverte, L.; Rambla-Alegre, M.; Leonardo, S.; Belles, C.; Campbell, K.; Elliott, C.T.; Gerssen, A.; Klijnstra, M.D.; Diogene, J.; Campas, M. Development and validation of a maleimide-based enzyme-linked immunosorbent assay for the detection of tetrodotoxin in oysters and mussels. Talanta 2018, 176, 659–666. [Google Scholar] [CrossRef]
- Xu, X.M.; Yu, X.W.; Lu, M.; Huang, B.F.; Ren, Y.P. Study of the matrix effects of tetrodotoxin and its content in cooked seafood by liquid chromatography with triple quadrupole mass spectrometry. J. Sep. Sci. 2015, 38, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Zhang, J.S.; Huang, B.F.; Han, J.L.; Chen, Q. Determination of ibotenic acid and muscimol in plasma by liquid chromatography-triple quadrupole mass spectrometry with bimolecular dansylation. J. Chromatogr. B 2020, 1146, 122128. [Google Scholar] [CrossRef]
- Han, C.; Zhang, X.; Li, L.; Chen, S.; Yan, Z.; Gao, X.; Chang, J. Analysis and evaluation of tetrodotoxin in coastal aquatic products of Zhejiang Province. J. Coastal Res. 2018, 83, 380–385. [Google Scholar] [CrossRef]
- Huang, H.N.; Lin, J.; Lin, H.L. Identification and quantification of tetrodotoxin in the marine gastropod Nassarius by LC-MS. Toxicon 2008, 51, 774–779. [Google Scholar] [CrossRef]
- Turner, A.D.; Boundy, M.J.; Rapkova, M.D. Development and single-laboratory validation of a liquid chromatography tandem mass spectrometry method for quantitation of tetrodotoxin in mussels and oysters. J. AOAC Int. 2017, 100, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Liang, Y.; Yang, S.; Jiang, F.; Yu, F.; Zhang, M.; Chang, W.; Wang, W.; Yu, C.; Liu, G.; et al. Simple and fast determination of tetrodotoxin in human plasma based on hydrophilic-interaction/ion-exchange mixed-mode solid phase extraction combined with liquid chromatography-tandem mass spectroscopy. J. Chromatogr. A 2022, 1684, 463567. [Google Scholar] [CrossRef]
- Tsujimura, K.; Yamanouchi, K. A rapid method for tetrodotoxin (TTX) determination by LC-MS/MS from small volumes of human serum, and confirmation of pufferfish poisoning by TTX monitoring. Food Addit. Contam. A 2015, 32, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.E.; Ahn, S.Y.; Son, I.S.; In, S.; Hong, R.S.; Kim, D.W.; Woo, S.H.; Moon, D.C.; Kim, S. Determination and validation of tetrodotoxin in human whole blood using hydrophilic interaction liquid chromatography-tandem mass spectroscopy and its application. Forensic Sci. Int. 2012, 217, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ochi, N. Simultaneous determination of ten paralytic shellfish toxins and tetrodotoxin in scallop and short-necked clam by ion-pair solid-phase extraction and hydrophilic interaction chromatography with tandem mass spectrometry. J. Chromatogr. A 2021, 1651, 462328. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, J.S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Liu, H.X.; Jin, Y.B.; Li, S.F.; Bi, X.; Chung, S.; Zhang, S.S.; Jiang, Y.Y. Separation, identification and quantification of tetrodotoxin and its analogs by LC-MS without calibration of individual analogs. Toxicon 2011, 57, 938–943. [Google Scholar] [CrossRef]
- Bane, V.; Hutchinson, S.; Sheehan, A.; Brosnan, B.; Barnes, P.; Lehane, M.; Furey, A. LC-MS/MS method for the determination of tetrodotoxin (TTX) on a triple quadruple mass spectrometer. Food Addit. Contam. Part A 2016, 33, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiu, J.; Tang, Y.; Xu, J.; Huang, S.; Liu, Y.; Ouyang, G. Rapid in vivo determination of tetrodotoxin in pufferfish (Fugu) muscle by solid-phase microextraction coupled to high-performance liquid chromatography tandem mass spectrometry. Talanta 2017, 171, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rey, V.; Botana, A.M.; Antelo, A.; Alvarez, M.; Botana, L.M. Rapid analysis of paralytic shellfish toxins and tetrodotoxins by liquid chromatography-tandem mass spectrometry using a porous graphitic carbon column. Food Chem. 2018, 269, 166–172. [Google Scholar] [CrossRef]
- Patria, F.P.; Pekar, H.; Zuberovic-Muratovic, A. Multi-Toxin quantitative analysis of paralytic shellfish toxins and tetrodotoxins in bivalve mollusks with ultra-performance hydrophilic interaction LC-MS/MS—An in-house validation study. Toxins 2020, 12, 452. [Google Scholar] [CrossRef]
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef]
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef]
- Luo, X.; Yu, R.C.; Wang, X.J.; Zhou, M.J. Toxin composition and toxicity dynamics of marine gastropod Nassarius spp. collected from Lianyungang, China. Food Addit. Contam. A 2012, 29, 117–127. [Google Scholar] [CrossRef]




| TTXs | Precursor Ion (m/z) | Product Ion (m/z) a | Collision Energy (eV) |
|---|---|---|---|
| TTX | 320 | 302/162 | 23/37 |
| IS | 498 | 178/336 | 28/23 |
| 4,9-anhydro-5,6,11-trideoxyTTX | 254 | 236/162 | 23/37 |
| 5,6,11-trideoxyTTX | 272 | 254/162 | 23/37 |
| 11-norTTX-6(S)-ol and 11-norTTX-6(R)-ol | 290 | 272/162 | 23/37 |
| 4,9-anhydroTTX | 302 | 284/162 | 23/37 |
| 5-deoxyTTX and 11-deoxyTTX | 304 | 286/162 | 23/37 |
| 4-epiTTX | 320 | 302/162 | 23/37 |
| 11-oxoTTX | 336 | 318/162 | 23/37 |
| Matrix | Spiking Level (μg/kg) | Recovery(%) | Average (%) | RSD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| N. didyma | 1.5 | 81.2 | 87.4 | 81.6 | 80.6 | 93.8 | 73.4 | 83.0 | 8.4 |
| 44 | 92.2 | 90.8 | 98.9 | 92.7 | 85.3 | 90.7 | 91.8 | 4.7 | |
| 1500 | 90.5 | 96.2 | 95.0 | 88.9 | 98.6 | 85.8 | 92.5 | 5.3 | |
| H. tuba | 1.5 | 86.3 | 75.4 | 81.0 | 90.9 | 82.1 | 80.1 | 82.6 | 6.5 |
| 44 | 92.3 | 95.4 | 89.8 | 97.8 | 92.1 | 88.3 | 92.6 | 3.8 | |
| 1500 | 95.0 | 104.3 | 88.7 | 92.8 | 101.3 | 84.2 | 94.4 | 8.0 | |
| R. bezoar | 1.5 | 86.6 | 81.5 | 79.2 | 73.3 | 84.4 | 92.2 | 82.9 | 7.8 |
| 44 | 88.6 | 97.3 | 94.4 | 85.6 | 87.0 | 98.0 | 91.8 | 5.9 | |
| 1500 | 92.0 | 95.8 | 105.8 | 86.4 | 92.0 | 88.8 | 93.5 | 7.3 | |
| Matrix | QC Levels (μg/kg) | Intra-Day (n = 6) | Inter-Days (3 days, n = 3) | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | ||
| N. didyma | 1.42 | 93.2 | 7.4 | 92.8 | 8.3 |
| 207 | 94.2 | 3.7 | 97.8 | 4.6 | |
| 2427 a | 98.8 | 4.0 | 92.4 | 5.8 | |
| N. semiplicatus | 559 | 97.4 | 4.6 | 92.0 | 5.3 |
| 1856 a | 95.5 | 5.5 | 92.2 | 5.3 | |
| 34,730 b | 91.8 | 6.0 | 93.6 | 7.2 | |
| Toxins | Matrix | Extraction | Clean-Up | LC Column | LOD (μg/kg) | Matrix Effects (%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| TTX | Gastropod Nassarius | 0.05 mol/L acetic acid, boiling, 30 min | Sep-Pak plus C18 | AQ-C18 | NM a | NM | NM | [26] |
| TTX and 4 analogues | Puffer fish | 0.05 mol/L acetic acid, boiling, 5 min | Sep-Pak C18, and activated charcoal | BEH Amide | 160 | NM | NM | [32] |
| TTX and 7 analogues | Puffer fish | 0.05 mol/L acetic acid | Cleanert ODS | HILIC Silia | 0.1 ng/mL | NM | 94.2–108.3 | [33] |
| TTX and 6 analogues | Cooked seafood | 2% formic acid/methanol; ultrasound-assisted extraction, 20 min, RT | MCX, 0.1 M HCl in methanol | BEH Amide | 5 | 80-110 | 66.9–89.2 | [23] |
| TTX and 7 analogues | Puffer fish and trumpet shell | 1% acetic acid, boiling, 8 min | Strata C18-E | ZIC HILIC | 410 | 82.1 | 61.17 | [34] |
| TTX and 3 analogues | Puffer fish | Water | Home-made SPME fibers | HILIC Silica | 2.3 | NM | NM | [35] |
| TTX and 4,9-anhydroTTX | Bivalve mollusks | 0.1M HCl; boiling, 5 min | PCOX clean-up, dichloromethane extraction and hypercarb cartridge | Hypercarb | 6.2–10.8 | >89.4 | 90–119 | [36] |
| TTX and 3 analogues | Bivalve mollusks | 1% acetic acid, boiling, 5 min | ENVI-Carb | BEH Amide | 7.84 | 45 | 68 | [37] |
| TTX | Scallop and short-necked clam | 1% acetic acid, boiling, 5 min | Ion-pair SPE | BEH Amide | 27.4 | 95/97 | 75.7–78.9 | [31] |
| TTX | Bivalve mollusks | 1% acetic acid, boiling, 5 min | ENVI-Carb | BEH Amide | 25 | NM | NM | [16] |
| TTX | Fresh and heat-processed aquatic products | 1% acetic acid/methanol; ultrasound-assisted extraction, 15 min, 40 °C | Immuneaffinity columns | BEH Amide | 0.2 | 81.7 | 90.5–107.2 | [19] |
| TTX and 8 analogues | Gastropod | 1% acetic acid/50% methanol/water; ultrasound-assisted extraction, 20 min, RT | MCX, 0.2% HCl/50% acetonitrile/water | BEH Amide | 0.5 | 86.9–92.2 | 82.6–94.4 | This study |
| Gastropods | Sample Numbers | Positive Numbers | Detection Rate (%) | Concentrations (μg/kg) |
|---|---|---|---|---|
| Hemifusus tuba | 30 | 0 | 0.0 | ND a |
| Reishia clavigera | 30 | 0 | 0.0 | ND |
| Rapana bezoar | 62 | 2 | 3.2 | ND~5.6 |
| Bullacta exarata | 30 | 2 | 6.7 | ND~4.8 |
| Margarites pupillus | 16 | 2 | 12.5 | ND~41.2 |
| Nassarius semiplicatus | 8 | 8 | 100.0 | 82.9~34,730 |
| Neverita didyma | 565 | 193 | 34.2 | ND~2327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-L.; Zhang, L.; Zhou, P.-P.; Xu, J.-J.; Pan, X.-D.; Cao, P.; Xu, X.-M. Analytical Method Optimization of Tetrodotoxin and Its Contamination in Gastropods. Foods 2023, 12, 3103. https://doi.org/10.3390/foods12163103
Han J-L, Zhang L, Zhou P-P, Xu J-J, Pan X-D, Cao P, Xu X-M. Analytical Method Optimization of Tetrodotoxin and Its Contamination in Gastropods. Foods. 2023; 12(16):3103. https://doi.org/10.3390/foods12163103
Chicago/Turabian StyleHan, Jian-Long, Lei Zhang, Ping-Ping Zhou, Jiao-Jiao Xu, Xiao-Dong Pan, Pei Cao, and Xiao-Min Xu. 2023. "Analytical Method Optimization of Tetrodotoxin and Its Contamination in Gastropods" Foods 12, no. 16: 3103. https://doi.org/10.3390/foods12163103





