Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus rhamnosus GG Dehydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Feed Solutions
2.3. Spray Drying
2.4. Electrostatic Spray Drying
2.5. Freeze Drying
2.6. Storage Conditions
2.7. Characterization Techniques
2.7.1. Particle Size Distribution
2.7.2. Particle Shape
2.7.3. Scanning Electron Microscopy (SEM)
2.7.4. Moisture Content (MC)
2.7.5. Water Activity (aw)
2.7.6. Glass Transition Temperature (Tg) Measurements
2.7.7. Reconstitution Time
- c(t): the normalized conductivity (-);
- κ(t): the conductivity at time t (µS/cm);
- κini (µS/cm): the initial conductivity;
- κfin (µS/cm): the final conductivity.
2.7.8. Cell Viability after the Drying Processes
2.7.9. Cell Viability during Storage
2.7.10. Statistical Analysis
3. Results and Discussion
3.1. Microparticle Characterisation
3.2. SEM Images of the Microparticles
3.3. MC, aw, and Tg Results
3.4. Reconstitution Time
3.5. Cell Viability after the Drying Processes
3.6. Cell Viability during 12 Weeks of Storage Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sohail, A.; Turner, M.S.; Coombes, A.; Bhandari, B. The Viability of Lactobacillus Rhamnosus GG and Lactobacillus Acidophilus NCFM Following Double Encapsulation in Alginate and Maltodextrin. Food Bioprocess Technol. 2013, 6, 2763–2769. [Google Scholar] [CrossRef]
- De Giulio, B.; Orlando, P.; Barba, G.; Coppola, R.; De Rosa, M.; Sada, A.; De Prisco, P.P.; Nazzaro, F. Use of Alginate and Cryo-Protective Sugars to Improve the Viability of Lactic Acid Bacteria after Freezing and Freeze-Drying. World J. Microbiol. Biotechnol. 2005, 21, 739–746. [Google Scholar] [CrossRef]
- Ying, D.Y.; Phoon, M.C.; Sanguansri, L.; Weerakkody, R.; Burgar, I.; Augustin, M.A. Microencapsulated Lactobacillus Rhamnosus GG Powders: Relationship of Powder Physical Properties to Probiotic Survival during Storage. J. Food Sci. 2010, 75, E588–E595. [Google Scholar] [CrossRef] [PubMed]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Sunny-Roberts, E.O.; Knorr, D. The Protective Effect of Monosodium Glutamate on Survival of Lactobacillus Rhamnosus GG and Lactobacillus Rhamnosus E-97800 (E800) Strains during Spray-Drying and Storage in Trehalose-Containing Powders. Int. Dairy J. 2009, 19, 209–214. [Google Scholar] [CrossRef]
- Kruis, W.; Frič, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia Coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Hussain, S.A.; Abdul Hamid, A.; Song, Y. Probiotics and Their Potential Preventive and Therapeutic Role for Cancer, High Serum Cholesterol, and Allergic and HIV Diseases. Biomed Res. Int. 2018, 2018, 3428437. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Aschenbrenner, M.; Kulozik, U.; Foerst, P. Role of Glassy State on Stabilities of Freeze-Dried Probiotics. J. Food Sci. 2011, 76, R152–R156. [Google Scholar] [CrossRef]
- Wang, X.; Xie, W.; Zhang, S.; Shao, Y.; Cai, J.; Cai, L.; Wang, X.; Shan, Z.; Zhou, H.; Li, J.; et al. Effect of Microencapsulation Techniques on the Stress Resistance and Biological Activity of Bovine Lactoferricin-Lactoferrampin-Encoding Lactobacillus reuteri. Foods 2022, 11, 3169. [Google Scholar] [CrossRef]
- Lafuente, C.; Calpe, J.; Musto, L.; Nazareth, T.d.M.; Dopazo, V.; Meca, G.; Luz, C. Preparation of Sourdoughs Fermented with Isolated Lactic Acid Bacteria and Characterization of Their Antifungal Properties. Foods 2023, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Kiep, J. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Ré, M.I. Microencapulsation of Spray Drying. Dry. Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Guerin, J.; Petit, J.; Burgain, J.; Borges, F.; Bhandari, B.; Perroud, C.; Desobry, S.; Scher, J.; Gaiani, C. Lactobacillus Rhamnosus GG Encapsulation by Spray-Drying: Milk Proteins Clotting Control to Produce Innovative Matrices. J. Food Eng. 2017, 193, 10–19. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of Drying Methods (Electrospraying, Freeze Drying and Spray Drying) on Survival and Viability of Microencapsulated Lactobacillus Rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M. Single and Double Layered Microencapsulation of Probiotics by Spray Drying and Spray Chilling. LWT Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus Casei LK-1 by Spray Drying Related to Its Stability and in Vitro Digestion. LWT Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.d.A.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; Flores, É.M.d.M.; da Silva, C.d.B.; de Menezes, C.R. Inulin, Hi-Maize, and Trehalose as Thermal Protectants for Increasing Viability of Lactobacillus Acidophilus Encapsulated by Spray Drying. LWT Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Arepally, D.; Goswami, T.K. Effect of Inlet Air Temperature and Gum Arabic Concentration on Encapsulation of Probiotics by Spray Drying. LWT Food Sci. Technol. 2019, 99, 583–593. [Google Scholar] [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Henkens, T.; Kiekens, S.; van den Broek, M.F.L.; Lebeer, S.; Kiekens, F. Enhancing the Viability of Lactobacillus Rhamnosus GG after Spray Drying and during Storage. Int. J. Pharm. 2017, 534, 35–41. [Google Scholar] [CrossRef]
- Xie, H.; Liao, Y.; Woo, M.W.; Xiong, H.; Zhao, Q. Whey Protein Hydrolysates as Prebiotic and Protective Agent Regulate Growth and Survival of Lactobacillus Rhamnosus CICC22152 during Spray/Freeze-Drying, Storage and Gastrointestinal Digestion. J. Sci. Food Agric. 2022, 103, 1237–1246. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Saxena, J.; Zisu, B. Electrostatic Spray Drying of High Oil Load Emulsions, Milk and Heat Sensitive Biomaterials. In Food Engineering Innovations Across the Food Supply Chain; Elsevier: Amsterdam, The Netherlands, 2022; pp. 237–246. [Google Scholar]
- Tejasvi, T.; Maa, Y.; Gikanga, B.; Sakhnovsky, R.; Zhou, T. Electrostatic Spray Drying for Monoclonal Antibody Formulation. Int. J. Pharm. 2021, 607, 120942. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of Spray Drying, Freeze Drying and Convective Hot Air Drying for the Production of a Probiotic Orange Powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Simpson, P.J.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Intrinsic Tolerance of Bifidobacterium Species to Heat and Oxygen and Survival Following Spray Drying and Storage. J. Appl. Microbiol. 2005, 99, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, J.; Cano, A.; González-Martínez, C.; Chiralt, A. Disaccharide Incorporation to Improve Survival during Storage of Spray Dried Lactobacillus Rhamnosus in Whey Protein-Maltodextrin Carriers. J. Funct. Foods 2017, 37, 416–423. [Google Scholar] [CrossRef]
- Mishra, A.; Athmaselvi, K.A. Stress Tolerance and Physicochemical Properties of Encapsulation Processes for Lactobacillus Rhamnosus in Pomegranate (Punica Granatum L.) Fruit Juice. Food Sci. Biotechnol. 2016, 25, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.; Chotiko, A.; Chouljenko, A.; Sathivel, S. Viability of Lactobacillus Acidophilus NRRL B-4495 Encapsulated with High Maize Starch, Maltodextrin, and Gum Arabic. LWT Food Sci. Technol. 2018, 96, 642–647. [Google Scholar] [CrossRef]
- Azizi, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Microencapsulation of Lactobacillus Rhamnosus Using Sesame Protein Isolate: Effect of Encapsulation Method and Transglutaminase: Microencapsulated L. Rhamnosus Using Sesame Protein. Food Biosci. 2021, 41, 101012. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Gaiani, C.; Edorh, J.M.; Beaupeux, E.; Maudhuit, A.; Desobry, S. Impact of Matrices Composition and Processes on β-Galactosidase Encapsulation. J. Food Eng. 2023, 353, 111547. [Google Scholar] [CrossRef]
- Badin, R.; Burgain, J.; Desobry, S.; Bhandari, B.; Prakash, S.; Gaiani, C. Probing Maltodextrins Surface Properties by Atomic Force Microscopy: Interplay of Glass Transition and Reconstitution Properties. Food Hydrocoll. 2022, 132, 107853. [Google Scholar] [CrossRef]
- Mitchell, W.R.; Forny, L.; Althaus, T.O.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Mapping the Rate-Limiting Regimes of Food Powder Reconstitution in a Standard Mixing Vessel. Powder Technol. 2015, 270, 520–527. [Google Scholar] [CrossRef]
- Fournaise, T.; Burgain, J.; Perroud, C.; Scher, J.; Gaiani, C.; Petit, J. Impact of Formulation on Reconstitution and Flowability of Spray-Dried Milk Powders. Powder Technol. 2020, 372, 107–116. [Google Scholar] [CrossRef]
- Leylak, C.; Ozdemir, K.S.; Gurakan, G.C.; Begum, Z. Optimisation of Spray Drying Parameters for Lactobacillus Acidophilu s Encapsulation in Whey and Gum Arabic: Its Application in Yoghurt. Int. Dairy J. 2021, 112, 104865. [Google Scholar] [CrossRef]
- Obradović, N.; Volić, M.; Nedović, V.; Rakin, M.; Bugarski, B. Microencapsulation of Probiotic Starter Culture in Protein–Carbohydrate Carriers Using Spray and Freeze-Drying Processes: Implementation in Whey-Based Beverages. J. Food Eng. 2022, 321, 110948. [Google Scholar] [CrossRef]
- Dhiman, A.; Prabhakar, P.K. Micronization in Food Processing: A Comprehensive Review of Mechanistic Approach, Physicochemical, Functional Properties and Self-Stability of Micronized Food Materials. J. Food Eng. 2021, 292, 110248. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of Bioactive Compounds Using Competitive Emerging Techniques: Electrospraying, Nano Spray Drying, and Electrostatic Spray Drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- M’be, C.U.; Scher, J.; Petit, J.; Amani, N.G.; Burgain, J. Relationship between Drying and Grinding Parameters and Physicochemical Properties of Hibiscus Sabdariffa Calyx Powders. Part. Sci. Technol. 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Damas, A.M.; Martins, P.; Rocha, F. Microencapsulation of β-Galactosidase with Different Biopolymers by a Spray-Drying Process. Food Res. Int. 2014, 64, 134–140. [Google Scholar] [CrossRef]
- González, A.; Martínez, M.L.; Paredes, A.J.; León, A.E.; Ribotta, P.D. Study of the Preparation Process and Variation of Wall Components in Chia (Salvia Hispanica L.) Oil Microencapsulation. Powder Technol. 2016, 301, 868–875. [Google Scholar] [CrossRef]
- Palzer, S.; Fowler, M. Generation of Specific Product Structures during Spray Drying of Food. Food Sci. Technol. 2014, 24, 44–45. [Google Scholar]
- Cui, T.; Chen, C.; Jia, A.; Li, D.; Shi, Y.; Zhang, M.; Bai, X.; Liu, X.; Liu, C. Characterization and Human Microfold Cell Assay of Fish Oil Microcapsules: Effect of Spray Drying and Freeze-Drying Using Konjac Glucomannan (KGM)-Soybean Protein Isolate (SPI) as Wall Materials. J. Funct. Foods 2021, 83, 104542. [Google Scholar] [CrossRef]
- Adhikari, B.; Howes, T.; Lecomte, D.; Bhandari, B.R. A Glass Transition Temperature Approach for the Prediction of the Surface Stickiness of a Drying Droplet during Spray Drying. Powder Technol. 2005, 149, 168–179. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular Injuries and Storage Stability of Spray-Dried Lactobacillus Rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Teixeira, P.; Castro, H.; Kirby, R.; Man, D. Evidence of Membrane Lipid Oxidation of Spray-Dried Lactobacillus Bulgaricus during Storage. Lett. Appl. Microbiol. 1996, 22, 34–38. [Google Scholar] [CrossRef]
- Dianawati, D.; Mishra, V.; Shah, N.P. Effect of Drying Methods of Microencapsulated Lactobacillus Acidophilus and Lactococcus Lactis ssp. cremoris on Secondary Protein Structure and Glass Transition Temperature as Studied by Fourier Transform Infrared and Differential Scanning Calorimetry. J. Dairy Sci. 2013, 96, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Vandenheuvel, D.; Meeus, J.; Lavigne, R.; Mooter, G. Van Den Instability of Bacteriophages in Spray-Dried Trehalose Powders Is Caused by Crystallization of the Matrix. Int. J. Pharm. 2014, 472, 202–205. [Google Scholar] [CrossRef]
- Ewa, S. The Influence of Trehalose—Maltodextrin and Lactose—Maltodextrin Matrices on Thermal and Sorption Properties of Spray-Dried b -Lactoglobulin—Vitamin D 3 Complexes. J. Therm. Anal. Calorim. 2013, 112, 429–436. [Google Scholar] [CrossRef]
- Mahmud, S.; Khan, S.; Khan, M.R.; Islam, J.; Sarker, U.K.; Hasan, G.M.M.A.; Ahmed, M. Viability and Stability of Microencapsulated Probiotic Bacteria by Freeze-Drying under in Vitro Gastrointestinal Conditions. J. Food Process. Preserv. 2022, 46, e17123. [Google Scholar] [CrossRef]
- Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Effect of Encapsulation Methods on the Physicochemical Properties and the Stability of Lactobacillus Plantarum (NCIM 2083) in Synbiotic Powders and in-Vitro Digestion Conditions. J. Food Eng. 2020, 283, 110033. [Google Scholar] [CrossRef]
- Broeckx, G.; Kiekens, S.; Jokicevic, K.; Byl, E.; Henkens, T.; Vandenheuvel, D.; Lebeer, S.; Kiekens, F. Effects of Initial Cell Concentration, Growth Phase, and Process Parameters on the Viability of Lactobacillus Rhamnosus GG after Spray Drying. Dry. Technol. 2020, 38, 1474–1492. [Google Scholar] [CrossRef]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.F.; Gigante, M.L. Microencapsulation of Lactobacillus Acidophilus La-5 by Spray-Drying Using Sweet Whey and Skim Milk as Encapsulating Materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Arepally, D.; Reddy, R.S.; Goswami, T.K. Function Encapsulation of Lactobacillus Acidophilus NCDC. Food Funct. 2020, 8694–8706. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation Mechanisms of Lactic Acid Starter Cultures Preserved by Drying Processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oluwatosin, S.O.; Tai, S.L.; Fagan-Endres, M.A. Sucrose, Maltodextrin and Inulin Efficacy as Cryoprotectant, Preservative and Prebiotic—Towards a Freeze Dried Lactobacillus Plantarum Topical Probiotic. Biotechnol. Reports 2022, 33, e00696. [Google Scholar] [CrossRef]
- Vaessen, E.M.J.; den Besten, H.M.W.; Esveld, E.D.C.; Schutyser, M.A.I. Accumulation of Intracellular Trehalose and Lactose in Lactobacillus Plantarum WCFS1 during Pulsed Electric Field Treatment and Subsequent Freeze and Spray Drying. LWT Food Sci. Technol. 2019, 115, 108478. [Google Scholar] [CrossRef]
- Farahmandi, K.; Rajab, S.; Tabandeh, F.; Shahraky, M.K.; Maghsoudi, A.; Ashengroph, M. Efficient Spray-Drying of Lactobacillus Rhamnosus PTCC 1637 Using Total CFU Yield as the Decision Factor. Food Biosci. 2021, 40, 100816. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, N.; Duan, M.; Woo, M.W.; Selomulya, C.; Chen, X.D. The Mechanisms of the Protective Effects of Reconstituted Skim Milk during Convective Droplet Drying of Lactic Acid Bacteria. Food Res. Int. 2015, 76, 478–488. [Google Scholar] [CrossRef]
- Siemons, I.; Vaessen, E.M.J.; Oosterbaan van Peski, S.E.; Boom, R.M.; Schutyser, M.A.I. Protective Effect of Carrier Matrices on Survival of Lactobacillus Plantarum WCFS1 during Single Droplet Drying Explained by Particle Morphology Development. J. Food Eng. 2021, 292, 110263. [Google Scholar] [CrossRef]
- Dima, P.; Stubbe, P.R.; Mendes, A.C.; Chronakis, I.S. Electric Field Charge Polarity Triggers the Organization and Promotes the Stability of Electrosprayed Probiotic Cells. Food Hydrocoll. 2023, 139, 108549. [Google Scholar] [CrossRef]
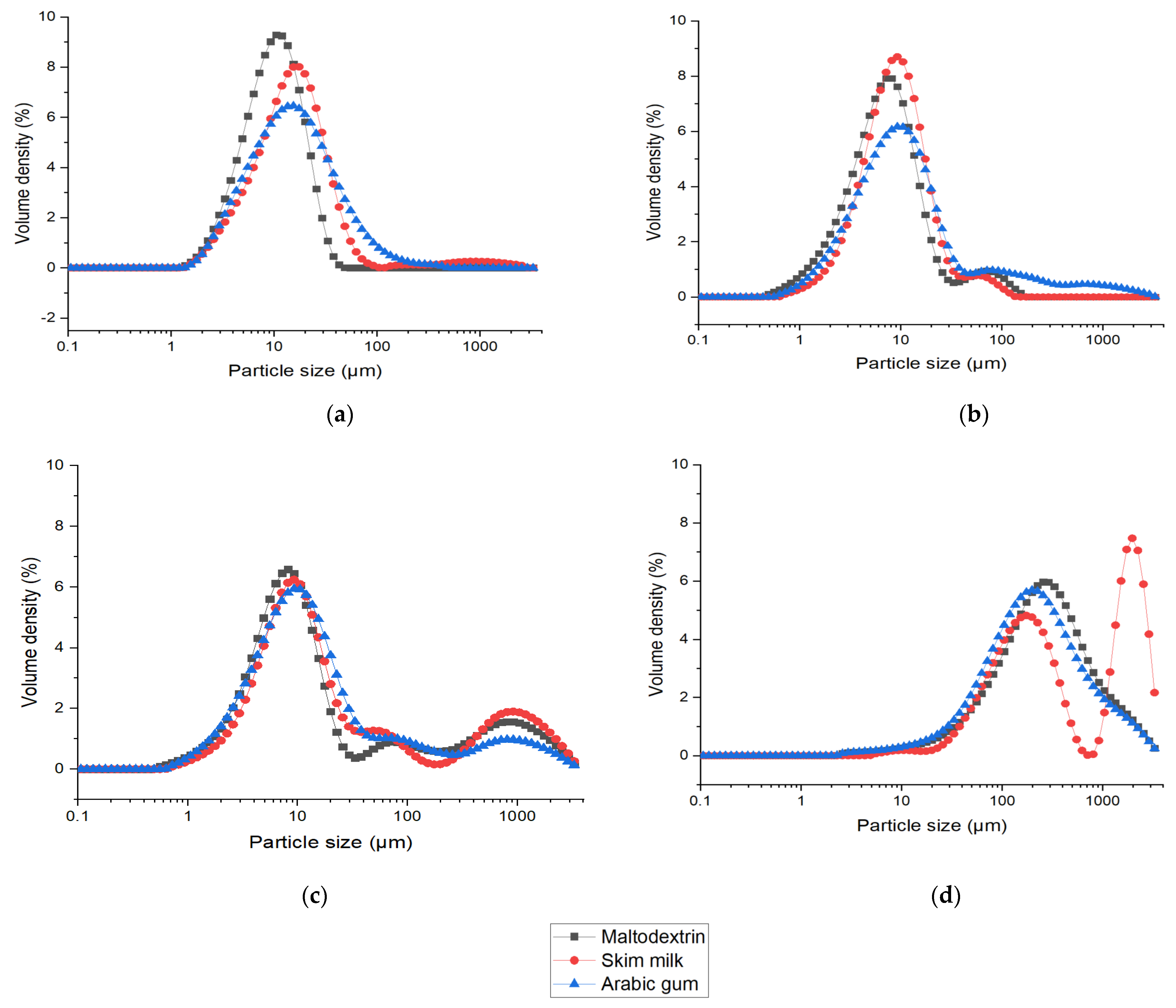


 ; ESD at 3 kV
; ESD at 3 kV  ; ESD at 12 kV
; ESD at 12 kV  ; FD
; FD  ) over storage period of twelve weeks with LGG concentration before drying (
) over storage period of twelve weeks with LGG concentration before drying ( ).
).
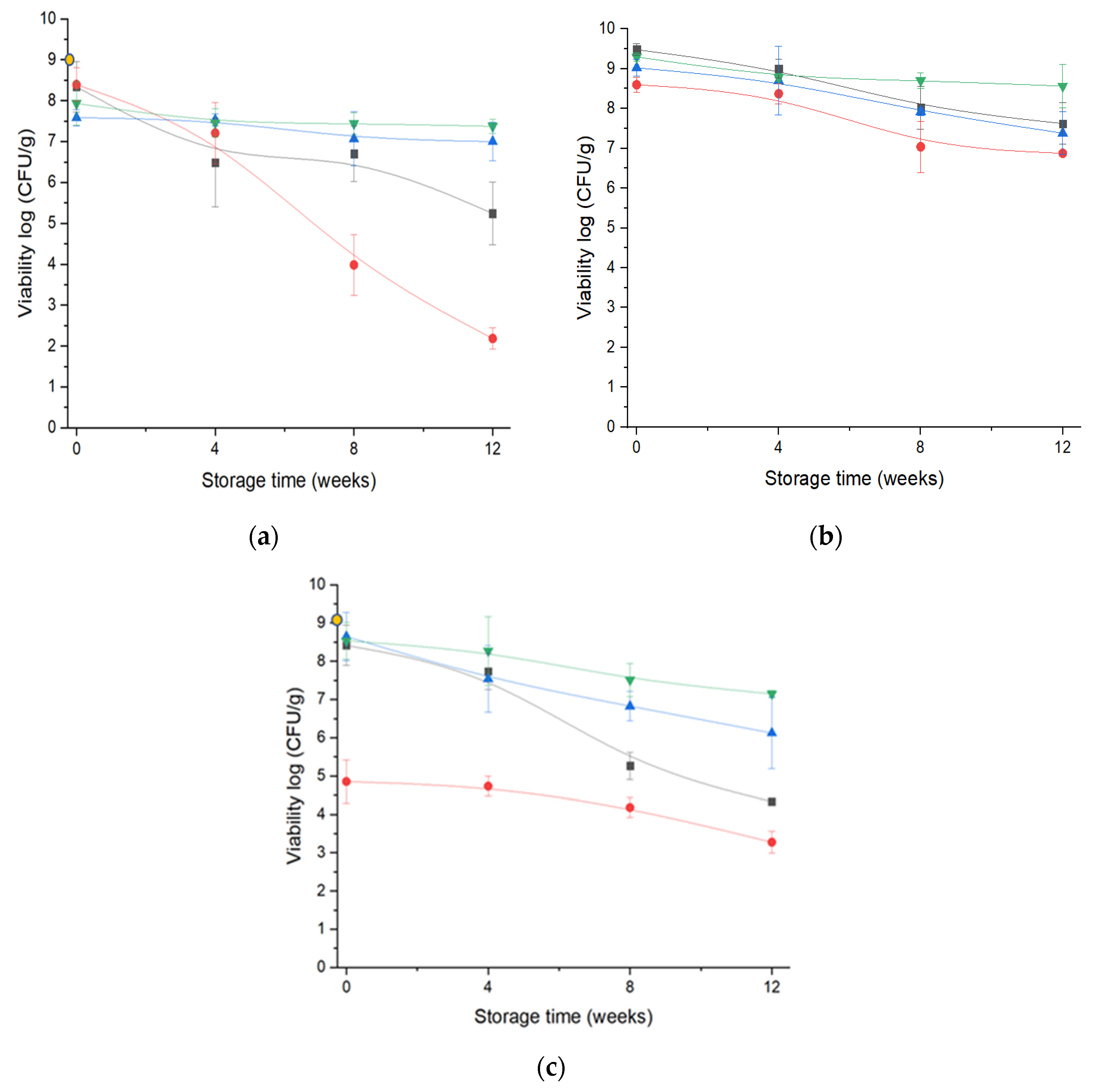
 ; ESD at 3 kV
; ESD at 3 kV  ; ESD at 12 kV
; ESD at 12 kV  ; FD
; FD  ) over storage period of twelve weeks with LGG concentration before drying (
) over storage period of twelve weeks with LGG concentration before drying ( ).
).
| Drying Process | Matrices | Particle Size | Particle Shape at d50 µm | aw | Moisture Content % | Reconstitution Time (s) to Reach 85% | Glass Transition (Tg) °C | Cell Viability after Drying (log CFU/g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| d50 (µm) | Span | Aspect Ratio | Sphericity | |||||||
| SD | Maltodextrin | 10.1 ± 0.6 a | 3.1 ± 0.9 a | 0.58 ± 0.02 c | 0.87 ± 0.01 | 0.20 ± 0.01 d | 3.90 ± 0.42 cd | 110 ± 56 ab | 104.31 ± 6.88 d | 4.49 ± 0.06 a |
| Skim Milk | 14.3 ± 0.4 a | 2.2 ± 0.2 a | 0.60 ± 0.01 d | 0.75 ± 0.13 | 0.22 ± 0.03 d | 3.83 ± 0.09 cd | 285 ± 35 de | 62.70 ± 0.55 c | 8.57 ± 0.18 bc | |
| Arabic gum | 15.4 ± 1.1 a | 3.8 ± 0.9 a | 0.60 ± 0.01 d | 0.82 ± 0.01 b | 0.47 ± 0.03 e | 9.05 ± 1.48 e | 485 ± 77 f | 51.15 ± 0.77 ab | 8.39 ± 0.40 bc | |
| ESD at 3 kV | Maltodextrin | 7.1 ± 0.6 a | 3.1 ± 0.9 a | 0.45 ± 0.01 a | 0.90 ± 0.01 b | 0.11 ± 0.07 c | 3.27 ± 0.29 bcd | 95 ± 7 ab | 101.90 ± 6.20 d | 8.64 ± 0.62 bc |
| Skim Milk | 13.3 ± 7.5 a | 2.2 ± 0.1 a | 0.61 ± 0.02 e | 0.82 ± 0.01 b | 0.11 ± 0.05 c | 2.97 ± 0.47 abcd | 135 ± 7 abc | 54.03 ± 5.79 abc | 9.02 ± 0.21 c | |
| Arabic gum | 10.0 ± 0.1 a | 7.6 ± 4.7 b | 0.58 ± 0.01 c | 0.85 ± 0.04 b | 0.10 ± 0.07 bc | 4.23 ± 0.54 d | 264 ± 35 cde | 48.64 ± 0.69 ab | 7.58 ± 0.19 b | |
| ESD at 12 kV | Maltodextrin | 9.8 ± 0.2 a | 76.1 ± 3.5 d | 0.52 ± 0.01 b | 0.88 ± 0.01 b | 0.13 ± 0.04 c | 2.35 ± 0.58 abcd | 62 ± 7 ab | 107.06 ± 1.03 d | 8.53 ± 0.48 bc |
| Skim Milk | 11.6 ± 1.1 a | 70.8 ± 11.6 d | 0.63 ± 0.01 e | 0.82 ± 0.01 b | 0.05 ± 0.01 ab | 2.15 ± 0.15 abcd | 180 ± 56 bcd | 52.08 ± 3.95 abc | 9.29 ± 0.10 c | |
| Arabic gum | 10.8 ± 0.8 a | 21.8 ± 19.8 c | 0.66 ± 0.01 f | 0.84 ± 0.02 b | 0.11 ± 0.01 c | 3.98 ± 0.06 cd | 362 ± 5 ef | 43.89 ± 2.50 a | 7.93 ± 0.53 bc | |
| FD | Maltodextrin | 323.0 ± 97.6 b | 4.1 ± 0.2 a | 0.62 ± 0.01 e | 0.70 ± 0.01 a | 0.03 ± 0.05 a | 0.98 ± 0.06 a | 15 ± 1 a | 100.81 ± 7.20 d | 8.45 ± 0.52 bc |
| Skim Milk | 301.0 ± 7.1 b | 5.7 ± 3.1 ab | 0.63 ± 0.01 e | 0.68 ± 0.01 a | 0.04 ± 0.04 a | 1.96 ± 0.05 abc | 24 ± 4 a | 58.85 ± 1.37 bc | 9.5 ± 0.14 c | |
| Arabic gum | 261.5 ± 78.5 b | 3.9 ± 0.6 a | 0.62 ± 0.01 e | 0.67 ± 0.01 a | 0.03 ± 0.04 a | 1.50 ± 0.06 ab | 104 ± 55 bcd | 49.52 ± 3.70 ab | 8.34 ± 0.62 bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaprakash, P.; Gaiani, C.; Edorh, J.-M.; Borges, F.; Beaupeux, E.; Maudhuit, A.; Desobry, S. Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus rhamnosus GG Dehydration. Foods 2023, 12, 3117. https://doi.org/10.3390/foods12163117
Jayaprakash P, Gaiani C, Edorh J-M, Borges F, Beaupeux E, Maudhuit A, Desobry S. Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus rhamnosus GG Dehydration. Foods. 2023; 12(16):3117. https://doi.org/10.3390/foods12163117
Chicago/Turabian StyleJayaprakash, Preethi, Claire Gaiani, Jean-Maxime Edorh, Frédéric Borges, Elodie Beaupeux, Audrey Maudhuit, and Stéphane Desobry. 2023. "Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus rhamnosus GG Dehydration" Foods 12, no. 16: 3117. https://doi.org/10.3390/foods12163117






