Preliminary Study of Pepper Types Based on Multielement Content Combined with Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, Chemicals and Material
2.2. Sample Preparation and Chemical Analyses
2.3. Figures of Merit of the Analytical Method
2.4. Data Treatment and Statistical Methods
3. Results
3.1. Method Characterization
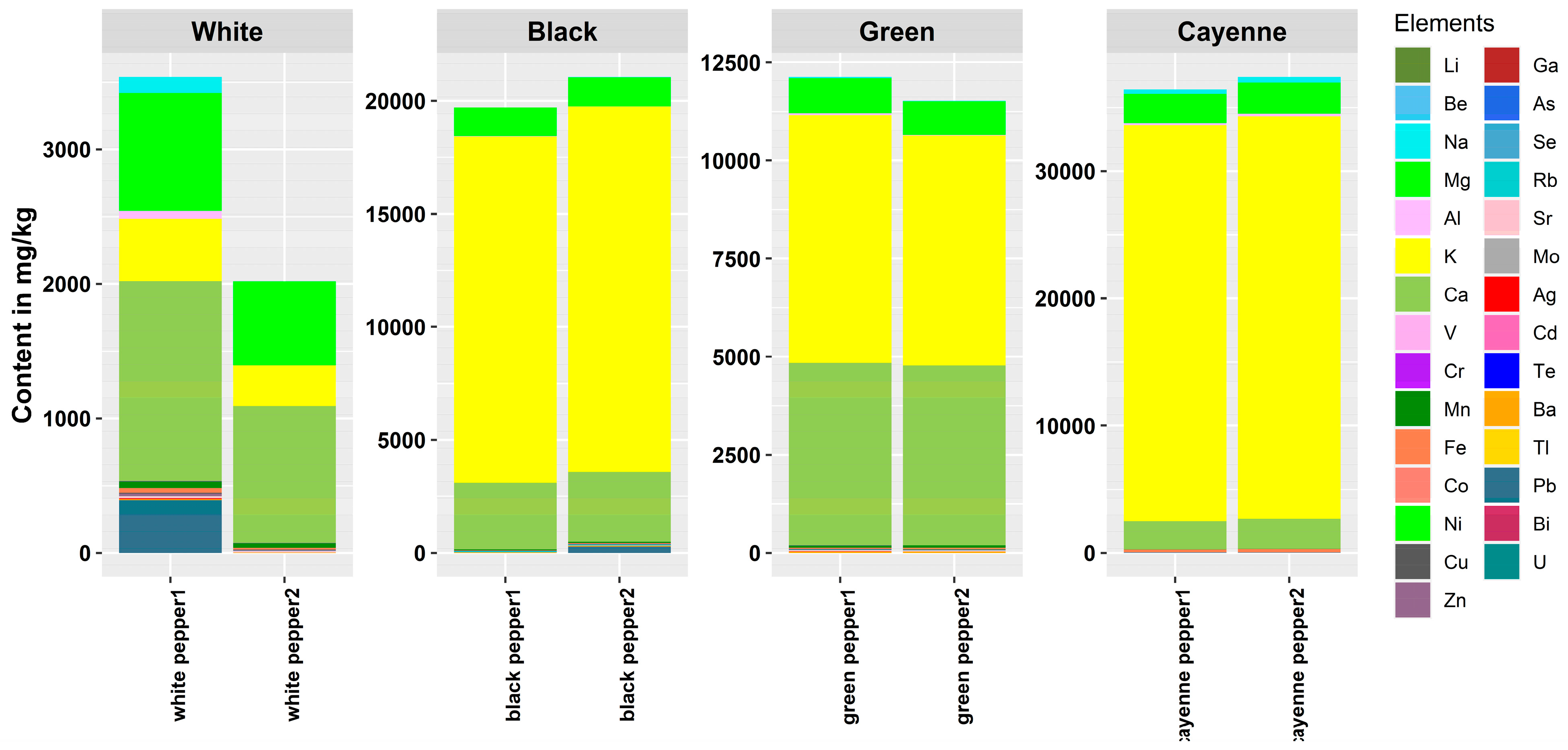
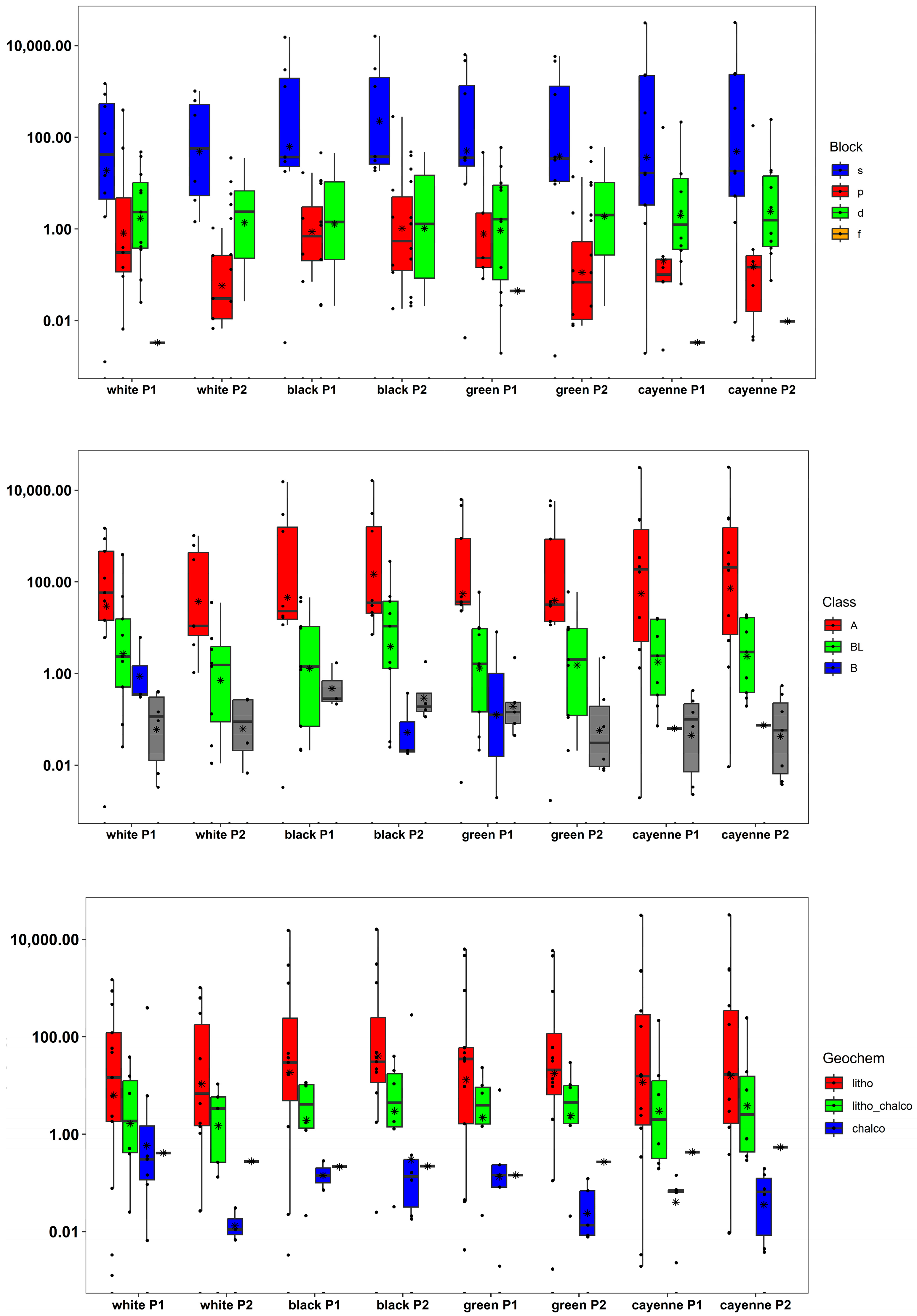
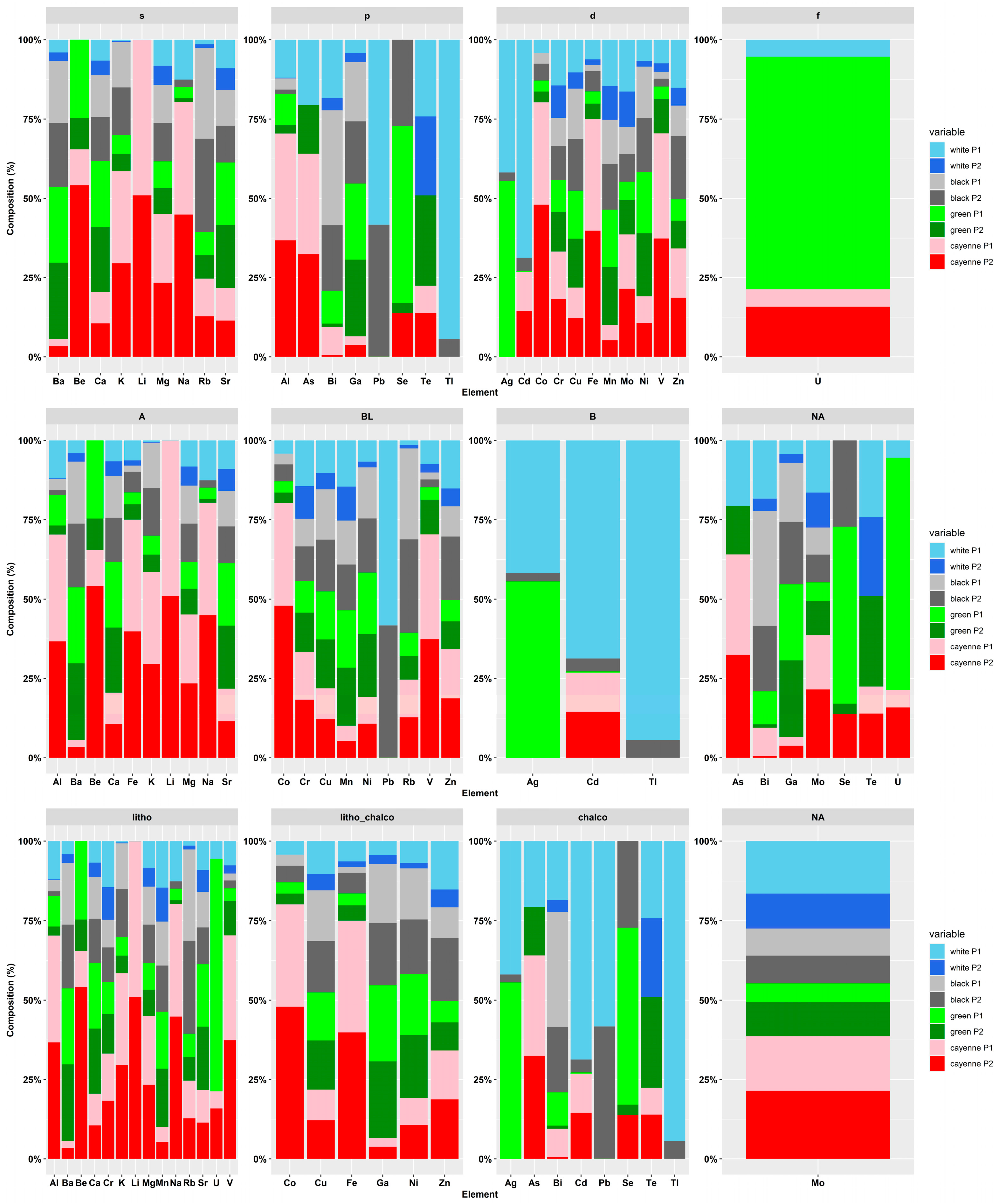
3.2. Elemental Composition of Peppers
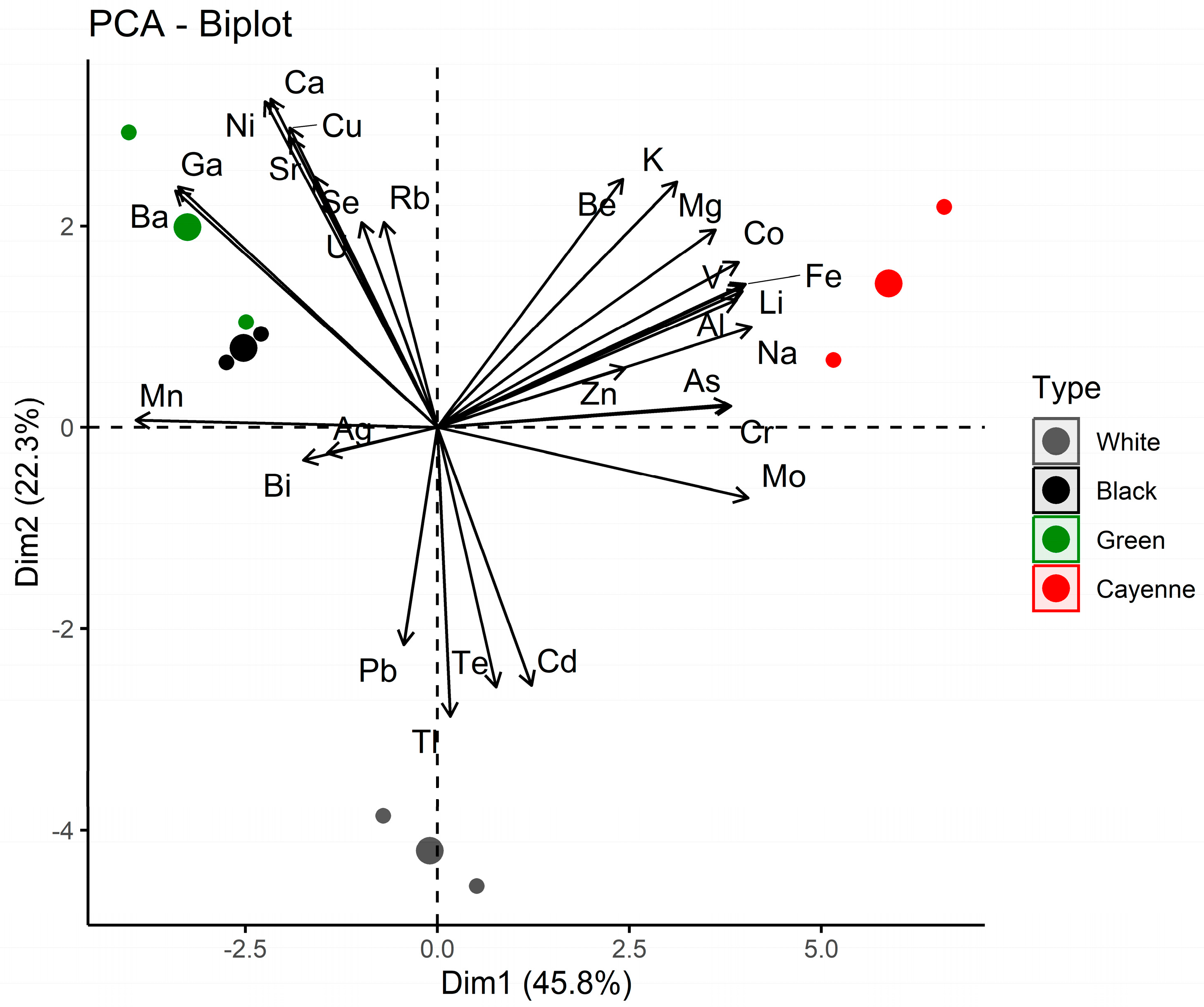
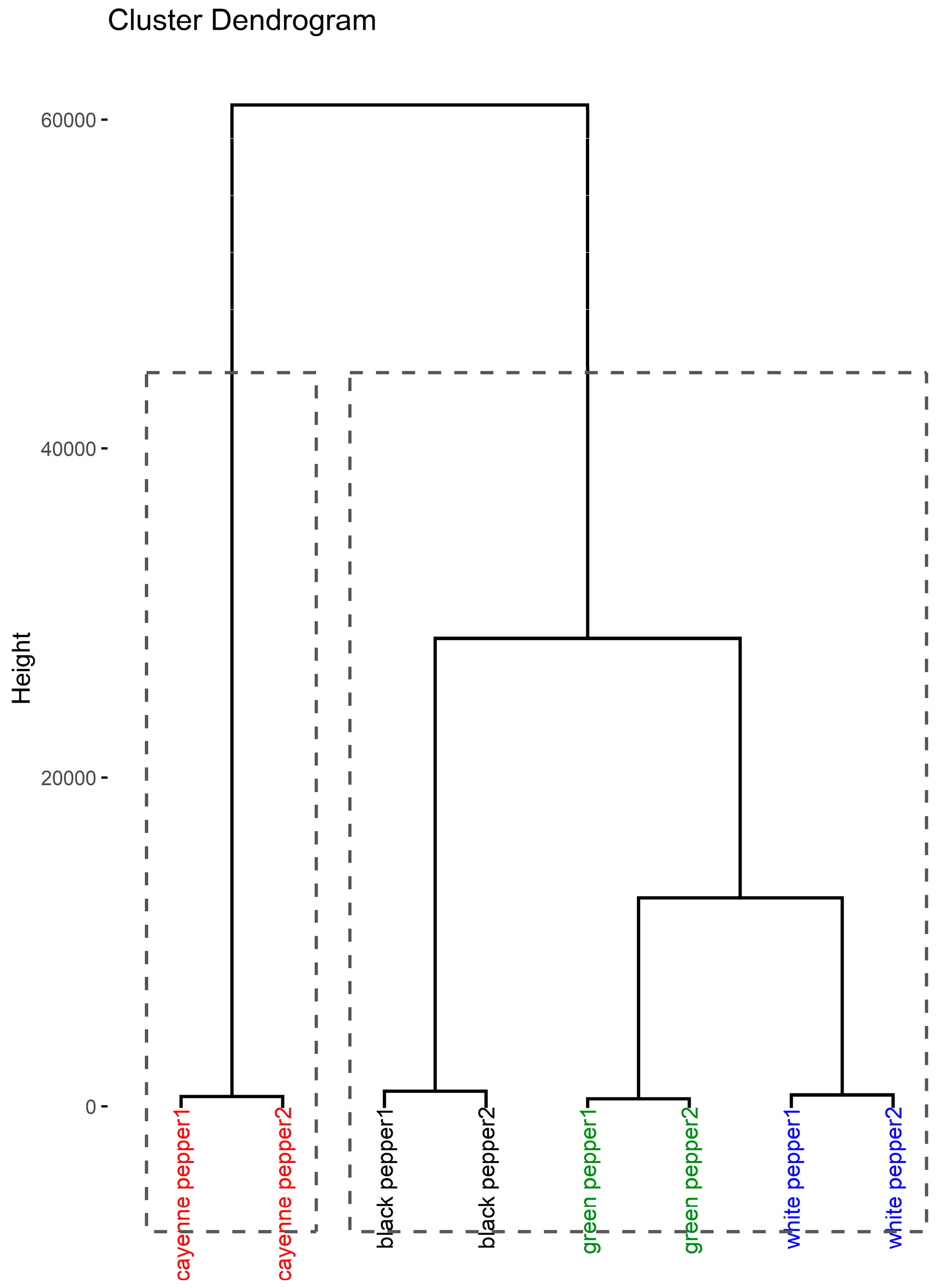
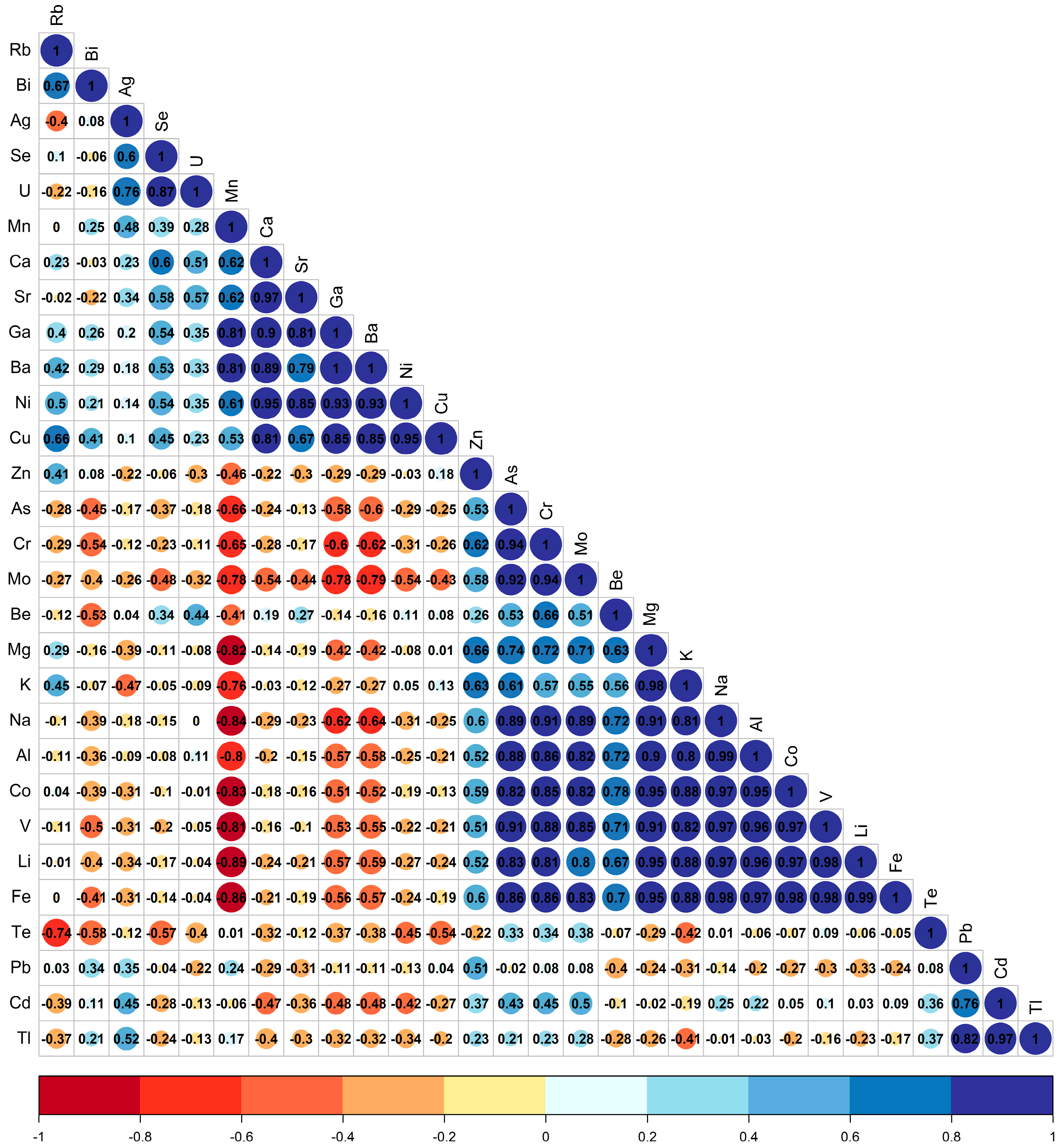
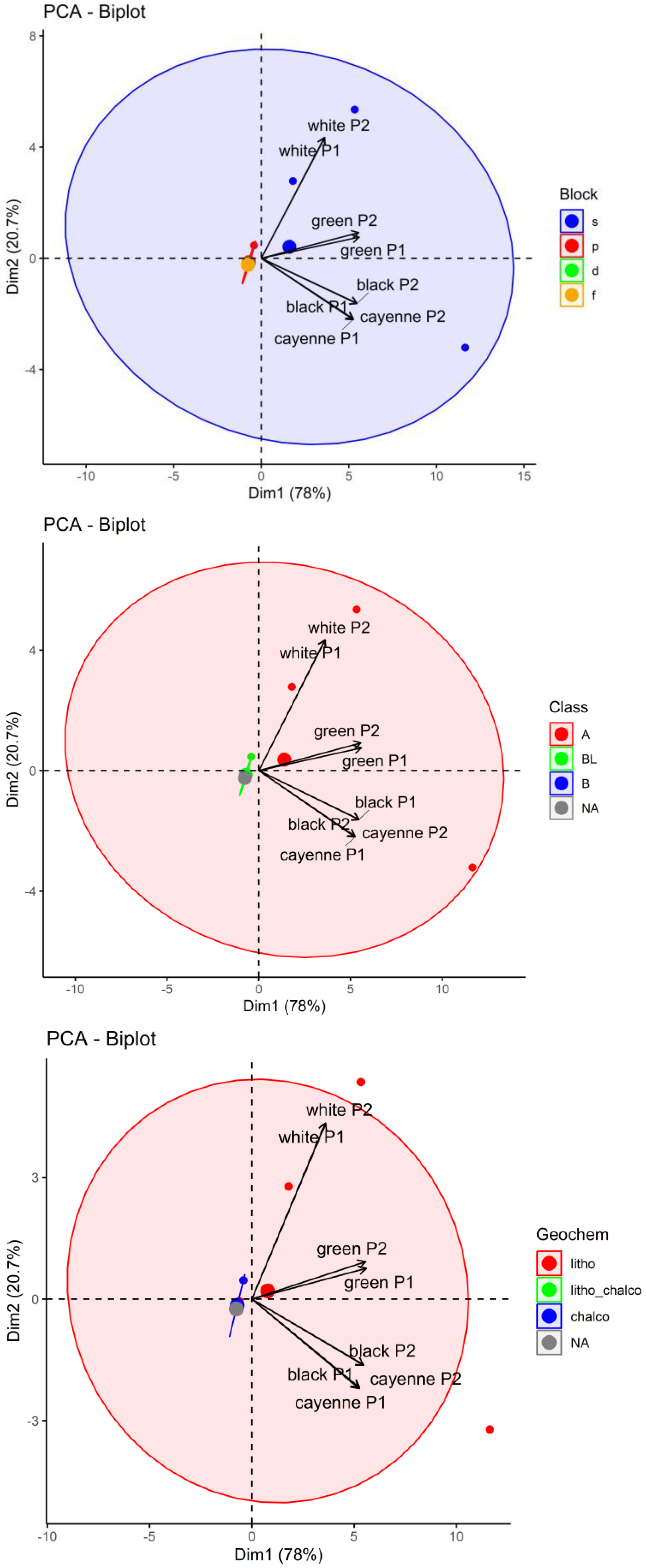
3.3. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammouti, B.; Dahmani, M.; Yahyi, A.; Ettouhami, A.; Messali, M.; Asehraou, A.; Bouyanzer, A.; Warad, I.; Touzani, R. Black Pepper, the “King of Spices”: Chemical composition to applications. Arab. J. Chem. Environ. Res. 2019, 6, 12–56. [Google Scholar]
- Berke, T.G.; Shien, S.C. Woodhead Publishing Series in Food Science, Technology and Nutrition. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing-Elsevier: Sawston, UK, 2012; Volume 1. [Google Scholar]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Aziz, N.S.; Sofian-Seng, N.S.; Mohd Razali, N.S.; Lim, S.J.; Mustapha, W.A.A. A review on conventional and biotechnological approaches in white pepper production. J. Sci. Food Agric. 2019, 99, 2665–2676. [Google Scholar] [CrossRef]
- Nunes Pinto Paracampo, N.P.; Abreu, L.F.; Lemos, O.F.; Both, J.P.C.L. Quality of black pepper produced in northeastern Pará. Rev. Agric. Neotrop. 2022, 9, 7020–7027. [Google Scholar] [CrossRef]
- Ishida, M.L.; Greene, V.; King, T.; Greenberg, J.; Luker, J.; Oglesby, D.; Sheridan, R.; Trodden, J. Regulatory policies for heavy metals in spices—A New York approach. J. Regul. Sci. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Manousi, N.; Isaakidou, E.; Zachariadis, G.A. An Inductively Coupled Plasma Optical Emission Spectrometric Method for the Determination of Toxic and Nutrient Metals in Spices after Pressure-Assisted Digestion. Appl. Sci. 2022, 12, 534. [Google Scholar] [CrossRef]
- Krause, M.; Vieira, L.V.; Cunha, T.P.; Brandão, G.P.; Filgueirasa, P.R.; Carneiro, W.D. Multi-Element Evaluation in Black Pepper (Piper nigrum L.) According to the Processing. J. Braz. Chem. Soc. 2020, 31, 135–142. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Chen, R.; Li, Y.C.; Peng, Y.; Wen, X. Geographical origin discrimination of pepper (Capsicum annuum L.) based on multi-elemental concentrations combined with chemometrics. Food Sci. Biotechnol. 2019, 28, 1627–1635. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Zotos, A.; Thomatou, A.-A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), A Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11, 3705. [Google Scholar] [CrossRef]
- Gaya, U.I.; Ikechukwu, S.A. Heavy metal contamination of selected spices obtained from Nigeria. J. Appl. Sci. Environ. Manag. 2016, 20, 681–688. [Google Scholar] [CrossRef]
- Abukawsar, M.; Moshfekus, S.-E.-I.; Ahsan, A.; Rahim, M.; Bhuiyan, N.H.; Roy, S.K.; Ghosh, A.; Naher, S. Chemical, pharmacological and nutritional quality assessment of black pepper (Piper nigrum L.) seed cultivars. J. Food Biochem. 2018, 42, e12590–e12611. [Google Scholar] [CrossRef]
- Soylak, M.; Cihan, Z.; Yilmaz, E. Evaluation of trace element contents of some herbal plants and spices retailed in Kayseri, Turkey. Environ. Monit. Assess. 2012, 184, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Akbulut, M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2008, 106, 852–858. [Google Scholar] [CrossRef]
- Murugesan, M.; Surekar, B.; Mandal, S.; Pandey, B.; Oulkar, D. Determination of Trace Heavy Metals in Spices Using Single Quadrupole ICP-MS. Thermo Scientific-Application Note, 44476–44484. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-44476-icp-ms-trace-heavy-metals-spices-an44476-en.pdf (accessed on 15 June 2023).
- Zeiner, M.; Šoltić, M.; Nemet, I.; Juranović Cindrić, I. Multielement Determination in Turmeric (Curcuma longa L.) Using Different Digestion Methods. Molecules 2022, 27, 8392. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, A.N.; Mrmošanin, J.M.; Jovanović, S.Č.; Mitić, S.S.; Tošić, S.B.; Krstić, J.N.; Stojanović, G.S. Elemental analysis of culinary herbs and spices by ICP OES: Classification by chemometrics. Stud. UBB Chem. 2020, 2, 69–83. [Google Scholar] [CrossRef]
- Voica, C.; Nechita, C.; Iordache, A.M.; Roba, C.; Zgavarogea, R.; Ionete, R.E. ICP-MS Assessment of Essential and Toxic Trace Elements in Foodstuffs with Different Geographic Origins Available in Romanian Supermarkets. Molecules 2021, 26, 7081. [Google Scholar] [CrossRef]
- Potortì, A.G.; Bua, G.D.; Lo Turco, V.; Ben Tekaya, A.; Beltifa, A.; Ben Mansour, H.; Dugo, G.; Di Bella, G. Major, minor and trace element concentrations in spices and aromatic herbs from Sicily (Italy) and Mahdia (Tunisia) by ICP-MS and multivariate analysis. Food Chem. 2020, 313, 126094. [Google Scholar] [CrossRef]
- Savić, S.; Petrović, S.; Petronijević, M.; Cvetanović, A.; Petronijević, Ž. Determination of the mineral content of spices by ICP-AES. Adv. Tehnol. 2019, 8, 27–32. [Google Scholar]
- Hwang, I.M.; Moon, E.W.; Lee, H.W.; Jamila, N.; Kim, K.S.; Ha, J.H.; Kim, S.H. Discrimination of Chili Powder Origin Using Inductively Coupled Plasma–Mass Spectrometry (ICP-MS), Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES), and Near Infrared (NIR) Spectroscopy. Anal. Lett. 2019, 52, 932–947. [Google Scholar] [CrossRef]
- Tefera, M.; Chandravanshi, B.S. Assessment of metal contents in commercially available Ethiopian red pepper. Int. Food Res. J. 2018, 25, 989–1000. [Google Scholar]
- Altaf, M.A.; Hao, Y.; He, C.; Mumtaz, M.A.; Shu, H.; Fu, H.; Wang, Z. Physiological and Biochemical Responses of Pepper (Capsicum annuum L.) Seedlings to Nickel Toxicity. Front. Plant Sci. 2022, 13, 950392–950408. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998; Available online: https://apps.who.int/iris/handle/10665/41986 (accessed on 24 July 2023).
- Barata, L.M.; Andrade, E.H.; Ramos, A.R.; Lemos, O.F.; Setzer, W.N.; Byler, K.G.; Maia, J.G.; Silva, J.K. Secondary Metabolic Profile as a Tool for Distinction and Characterization of Cultivars of Black Pepper (Piper nigrum L.) Cultivated in Pará State, Brazil. Int. J. Mol. Sci. 2021, 22, 890. [Google Scholar] [CrossRef] [PubMed]
- Taharn, N.; Techawongstein, S.; Chanthai, S. Determination of major-to-trace elements in hot chilli and tomato varieties economically grown in the Northeast of Thailand by ICP-OES following microwave assisted digestion. Int. Food Res. J. 2014, 21, 517–522. [Google Scholar]
- Naccarato, A.; Furia, E.; Sindona, G.; Tagarelli, A. Multivariate class modeling techniques applied to multielement analysis for the verification of the geographical origin of chili pepper. Food Chem. 2016, 206, 217–222. [Google Scholar] [CrossRef]
- Gupta, K.K.; Bhattacharjee, S.; Kar, S.; Chakrabarty, S.; Thakur, P.; Bhattacharyya, G.; Srivastava, S.C. Mineral compositions of eight common spices. Commun. Soil Sci. Plant Anal. 2003, 34, 681–693. [Google Scholar] [CrossRef]
- Tokalıoğlu, S.; Dokan, F.K.; Köprü, S. ICP-MS multi-element analysis for determining the origin by multivariate analysis of red pepper flakes from three different regions of Turkey. LWT 2019, 103, 301–307. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals”—A meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Duffus, J.H. Errata “Heavy metals”—A meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 1357. [Google Scholar] [CrossRef]
- Appenroth, K.-J. What are “heavy metals” in Plant Sciences? Acta Physiol. Plant. 2010, 32, 615–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiner, M.; Fiedler, H.; Juranović Cindrić, I.; Nemet, I.; Toma, D.; Habinovec, I. Preliminary Study of Pepper Types Based on Multielement Content Combined with Chemometrics. Foods 2023, 12, 3132. https://doi.org/10.3390/foods12163132
Zeiner M, Fiedler H, Juranović Cindrić I, Nemet I, Toma D, Habinovec I. Preliminary Study of Pepper Types Based on Multielement Content Combined with Chemometrics. Foods. 2023; 12(16):3132. https://doi.org/10.3390/foods12163132
Chicago/Turabian StyleZeiner, Michaela, Heidelore Fiedler, Iva Juranović Cindrić, Ivan Nemet, Doris Toma, and Iva Habinovec. 2023. "Preliminary Study of Pepper Types Based on Multielement Content Combined with Chemometrics" Foods 12, no. 16: 3132. https://doi.org/10.3390/foods12163132
APA StyleZeiner, M., Fiedler, H., Juranović Cindrić, I., Nemet, I., Toma, D., & Habinovec, I. (2023). Preliminary Study of Pepper Types Based on Multielement Content Combined with Chemometrics. Foods, 12(16), 3132. https://doi.org/10.3390/foods12163132




