Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits
Abstract
:1. Introduction
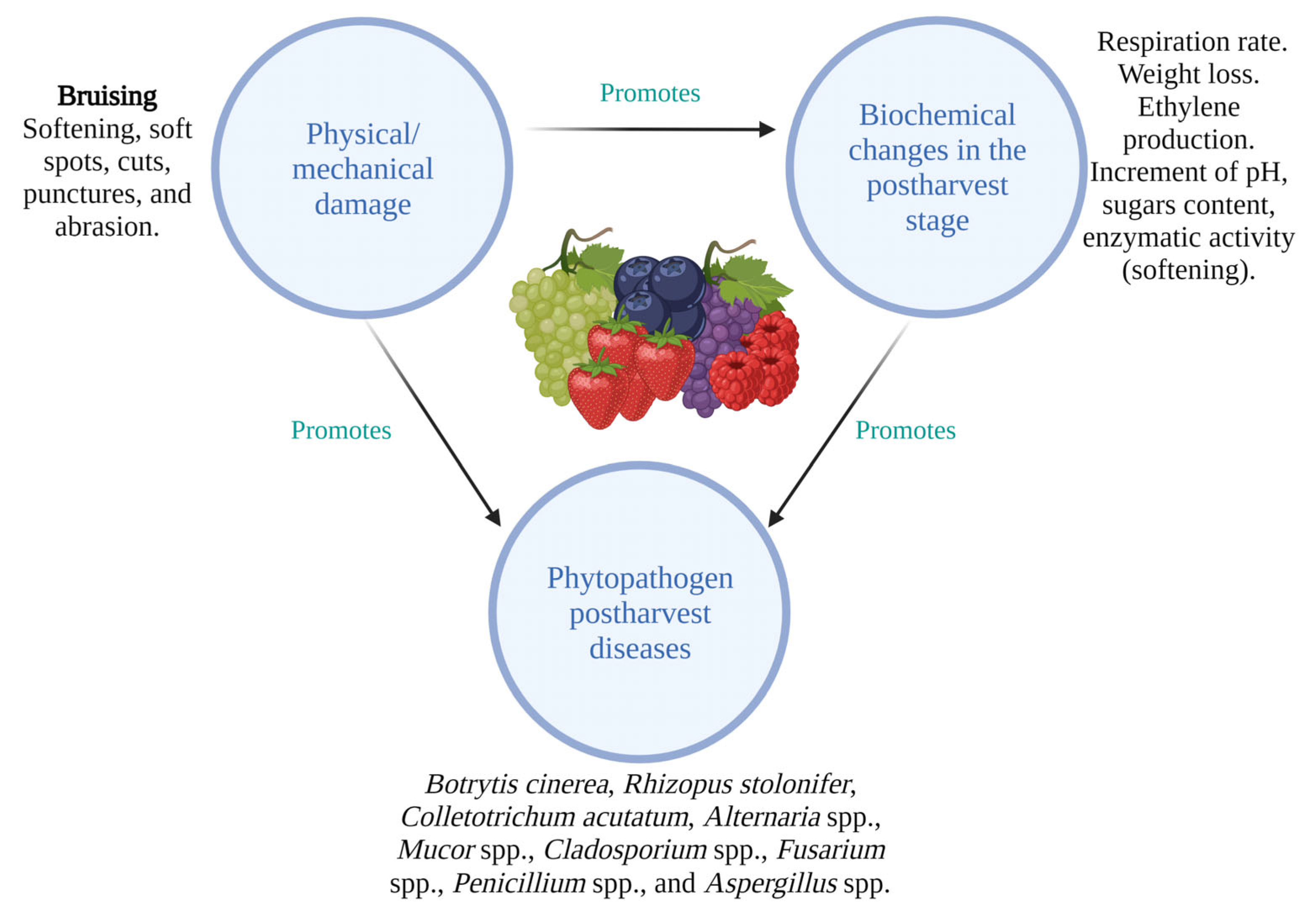
2. Main Factors Causing Postharvest Losses of Berries
2.1. Physical Factors
2.2. Biochemical Changes in Berries
2.3. Microbiological Agents
3. Brief View of Traditional Methods of Microbial Growth Control: Fungicides
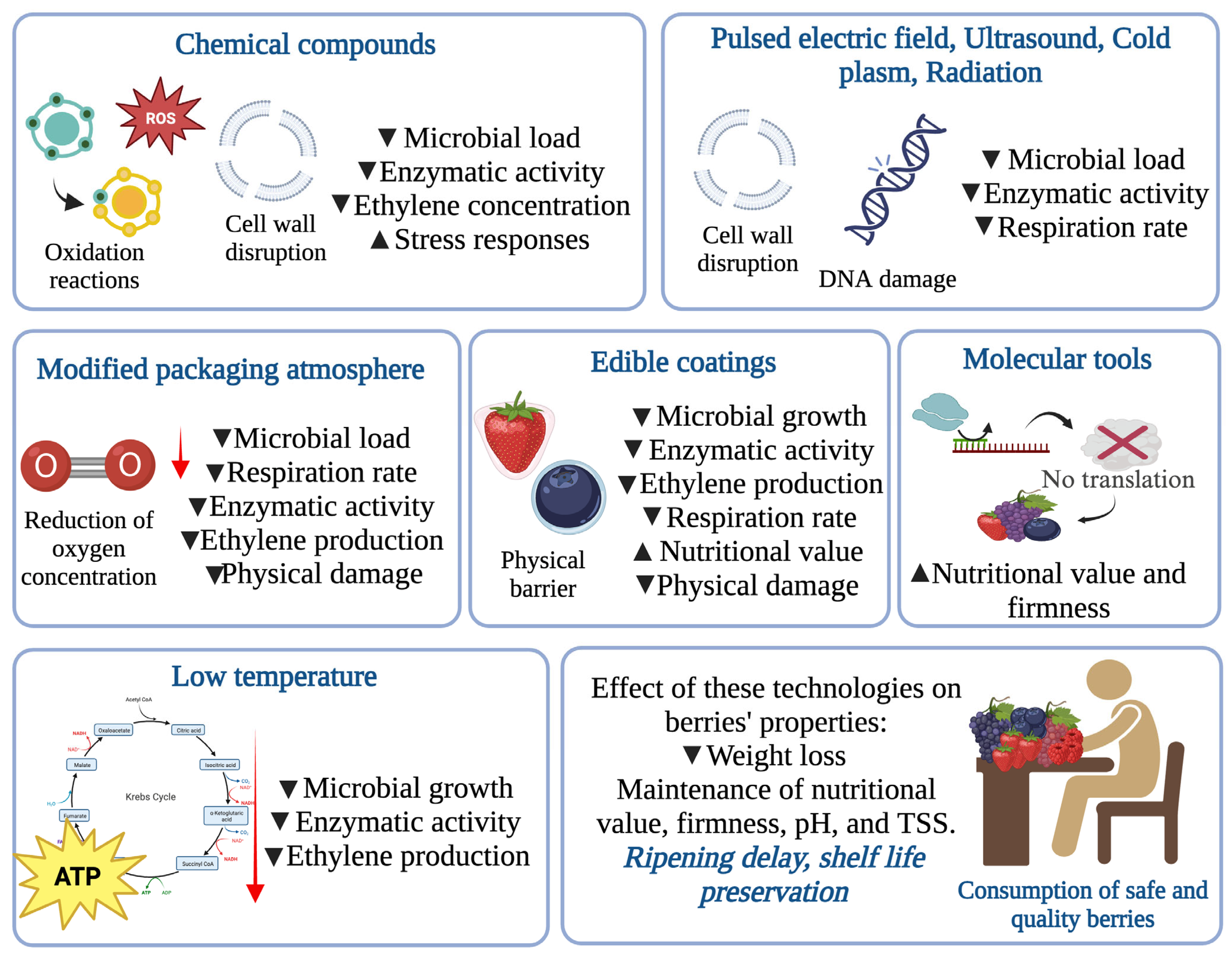
4. Sustainable Alternatives for the Postharvest Protection of Berries
4.1. Green Chemical Compounds
4.1.1. Ozone
4.1.2. Hydrogen Peroxide
4.1.3. Peracetic Acid (PAA)
| Berry | Preservation Technique | Storage Conditions | Main Result | Reference |
|---|---|---|---|---|
| Blueberries | Peracetic acid (PAA, 85 µL/L) | 1 °C/4 weeks | Inhibition of Botrytis cinerea maintaining the quality parameters of the fruit during the storage. | [41] |
| Blackberries and grapes | Ozone (18 mg O3/L for 10 min) | 4 °C/ 20 days | Reduced fungal decay and loss of weight along with storage. | [47] |
| Strawberries, raspberries, and blueberries | Ozone (13 mg/m3 for 16 h at 1 ± 0.5 °C) and MAP (10 kPa O2 and 40 kPa CO2) | 4 °C/15 days | The treatment did not affect the quality parameters of the fruits. In the case of blueberries, it protected the total and individual content of anthocyanins. | [6] |
| Strawberries | γ-irradiation (2 kGy, at 0.5 kGy/min) | 4 °C/15 days | The antioxidant activity increased in comparison with untreated fruits. | [7] |
| Strawberries | γ-irradiation (2 kGy) | 4 °C/14 days | Decreased the proliferation of molds and yeasts; sensory and physicochemical scores were not affected in comparison to the non-treated. | [8] |
| Goji berry | γ-irradiation (10 kGy, at 2.6 kGy/h) | 5 °C/50 days | Irradiation increased antioxidant activity by almost 30% in comparison with untreated fruits. | [24] |
| Blueberries | Cold plasma (4 kV/10 min) | 25 °C/10 days | Reduced decay, maintaining the quality and anthocyanin content of the fruits during storage. | [57] |
| Blueberries | Cold plasma (45 kV/50 s), ultraviolet (UV-C, 2.76 kJ/m2), or aqueous ozone (0.3 mg/L/5 min) | 20 °C/8 days | Cold plasma was the most effective treatment in the maintenance of the quality parameters, inhibiting the fungal decay and the growth of the microflora. | [58] |
| Strawberries | Electron beam irradiation (2 kGy, 70 cm/min) | 4 °C/14 days | Guaranteed microbial safety for up to 7 days and improved the physicochemical and sensorial properties of the coated fruits. | [59] |
| Strawberries, blackberries, and raspberries | Biodegradable packaging of gelatin- carboxymethylcellulose added with avocado peel extracts. | 25–28 °C/6 days | Protected the fruit from fungal growth during storage. | [60] |
| Blueberries | Biodegradable packaging based on polyvinyl pyrrolidone and carboxymethyl cellulose added with bacterial cellulose and guar gum. | 21 °C/15 days | Maintained the color and structure of the fruits after the storage period. | [61] |
| Blackberries and raspberries | Biodegradable packaging of poly (lactic acid) added with cyclodextrin and thymol. | 4 °C/ 21 days | Prolonged shelf life by one more compared with commercial clamshell packaging, this means 21 days. | [62] |
4.1.4. Organic Acids
4.2. Bioactive Compounds
4.2.1. Essential Oils (EOs)
4.2.2. Plant Extracts
4.3. Physical Methods
4.3.1. Controlled Atmosphere (CA) and Modified Atmosphere Packaging (MAP)
4.3.2. Low Temperature
4.3.3. Ultraviolet (UV) Irradiation
4.3.4. Pulsed Electric Field (PEF)
4.3.5. Cold Plasma (CP)
4.3.6. Ionized Irradiation
4.3.7. Ultrasound (US)
4.3.8. Edible Coatings
4.4. Biocontrol Agents (BCAs)
4.5. Molecular Tools to Improve Berry Preservation
5. Role of Artificial Intelligence (AI) in the Postharvest Protection of Berries
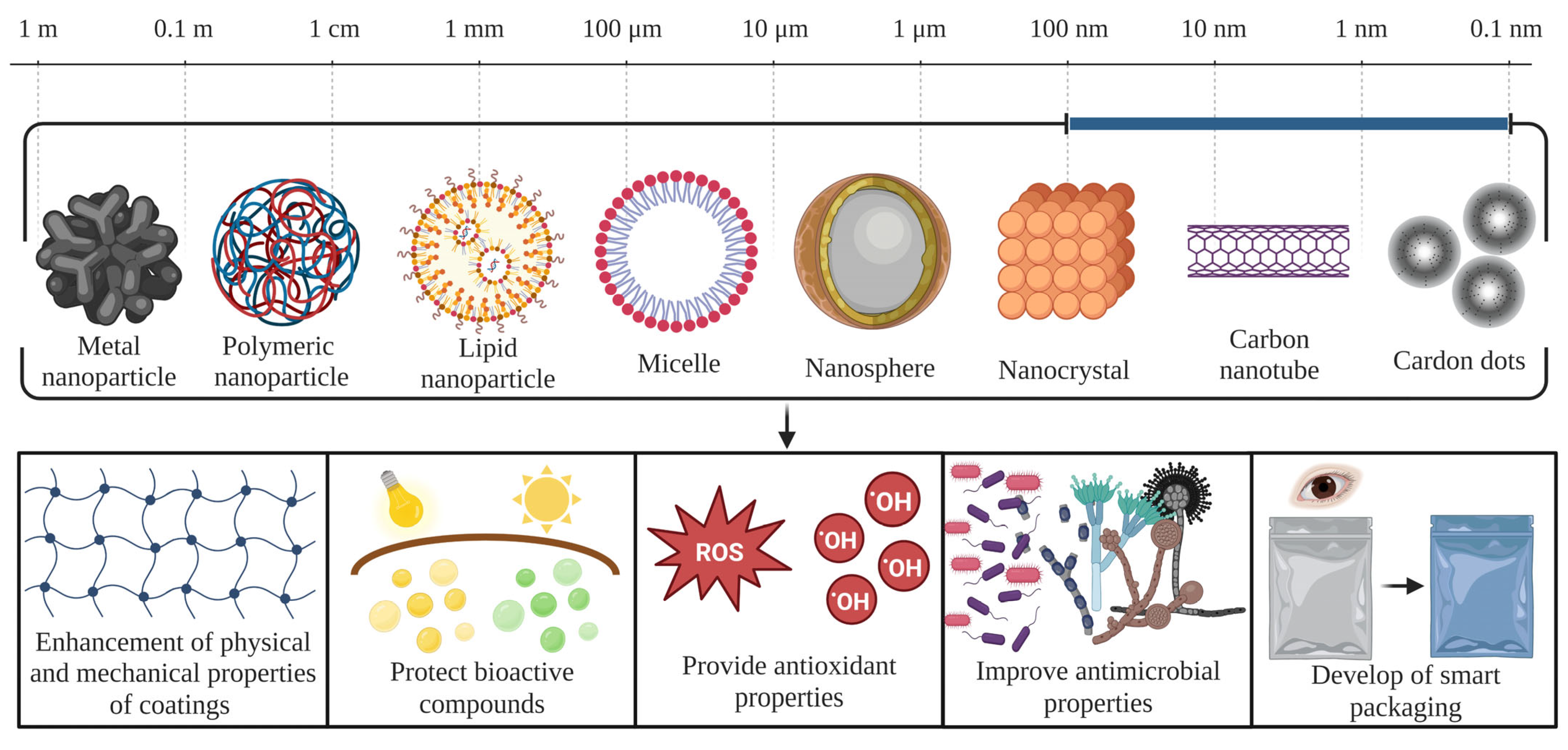
6. Nanotechnology Applied to Postharvest Protection of Berries
7. Current State and Challenges in the Implementation of Sustainable Alternatives at the Industrial Scale for Berry Protection
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elik, A.; Yanik, D.K.; Istanbullu, Y.; Guzelsoy, N.A. Strategies to reduce post-harvest losses for fruits and vegetables. Int. J. Sci. Technol. Res. 2019, 5, 29–39. [Google Scholar] [CrossRef]
- Romero, J.; Albertos, I.; Díez-Méndez, A.; Poveda, J. Control of postharvest diseases in berries through edible coatings and bacterial probiotics. Sci. Hortic. 2022, 304, 111326. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Pathirana, R. Sustainable agro-food systems for addressing climate change and food security. Agriculture 2022, 12, 1554. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Skóra, B.; Balawejder, M. Quality and antioxidant activity of highbush blueberry fruit coated with starch-based and gelatine-based film enriched with cinnamon oil. Food Control 2022, 138, 109015. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological and sensory quality of small berry fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Barkaoui, S.; Madureira, J.; Santos, P.M.P.; Margaça, F.M.A.; Miloud, N.B.; Mankai, M.; Boudhrioua, N.M.; Cabo Verde, S. Effect of ionizing radiation and refrigeration on the antioxidants of strawberries. Food Bioprocess Technol. 2020, 13, 1516–1527. [Google Scholar] [CrossRef]
- Barkaoui, S.; Mankai, M.; Miloud, N.B.; Kraïem, M.; Madureira, J.; Verde, S.C.; Boudhrioua, N. Effect of gamma radiation coupled to refrigeration on antioxidant capacity, sensory properties and shelf life of strawberries. LWT Food Sci. Technol. 2021, 150, 112088. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Wang, Y.; Haskell-Ramsay, C.; Lara Gallegos, J.; Lodge, J.K. Effects of chronic consumption of specific fruit (berries, cherries and citrus) on cognitive health: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2023, 77, 7–22. [Google Scholar] [CrossRef]
- Bilawal, A.; Ishfaq, M.; Gantumur, M.A.; Qayum, A.; Shi, R.; Fazilani, S.A.; Anwar, A.; Jiang, Z.; Hou, J. A review of the bioactive ingredients of berries and their applications in curing diseases. Food Biosci. 2021, 44, 101407. [Google Scholar] [CrossRef]
- Kumar, S.; Baghel, M.; Yadav, A.; Dhakar, M.K. Postharvest biology and technology of berries. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 349–370. ISBN 9783319768434. [Google Scholar]
- Huynh, N.K.; Wilson, M.D.; Eyles, A.; Stanley, R.A. Recent advances in postharvest technologies to extend the shelf life of blueberries (Vaccinium sp.), raspberries (Rubus idaeus L.) and blackberries (Rubus sp.). J. Berry Res. 2019, 9, 709–724. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT) Cultivos. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 10 May 2023).
- Zamanpour, S.; Shakeri, G.; Afshari, A. Epidemiological evaluation of water- and outbreaks in the United States and Europe. J. Nutr. Fasting Health 2022, 10, 3. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Ferreira, N.C.A.; Fiocco, A.C.T.R.; Picone, C.S.F. Lactoferrin-Chitosan-TPP nanoparticles: Antibacterial action and axtension of strawberry shelf-life. Food Bioprocess Technol. 2023, 16, 135–148. [Google Scholar] [CrossRef]
- Santos, C.; de Araújo Gonçalves, M.; de Macedo, L.F.; Torres, A.H.F.; Marena, G.D.; Chorilli, M.; Trovatti, E. Green nanotechnology for the development of nanoparticles based on alginate associated with essential and vegetable oils for application in fruits and seeds protection. Int. J. Biol. Macromol. 2023, 232, 123351. [Google Scholar] [CrossRef]
- Lee, D.; Shayan, M.; Gwon, J.; Picha, D.H.; Wu, Q. Effectiveness of cellulose and chitosan nanomaterial coatings with essential oil on postharvest strawberry quality. Carbohydr. Polym. 2022, 298, 120101. [Google Scholar] [CrossRef]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetables: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef]
- Basak, J.K.; Madhavi, B.G.K.; Paudel, B.; Kim, N.E.; Kim, H.T. Prediction of total soluble solids and pH of strawberry fruits using RGB, HSV and HSL colour spaces and machine learning models. Foods 2022, 11, 2086. [Google Scholar] [CrossRef]
- FAO. The state of food and agriculture 2019: Moving forward on food loss and waste reduction. In Routledge Handbook of Religion and Ecology; FAO: Rome, Italy, 2019. [Google Scholar]
- King, E.S.; Noll, A.; Glenn, S.; Bolling, B.W. Refrigerated and frozen storage impact aronia berry quality. Food Prod. Process. Nutr. 2022, 4, 3. [Google Scholar] [CrossRef]
- DeVetter, L.W.; Yang, W.Q.; Takeda, F.; Chen, J. Harvesting Blueberries: A Guide to Machine Pick Blueberries for Fresh Market. 2022. Available online: https://s3.wp.wsu.edu/uploads/sites/2181/2022/02/FS368E.pdf (accessed on 15 August 2023).
- Mladenova, R.B.; Aleksieva, K.I.; Nacheva, I.B. Effect of gamma irradiation on antiradical activity of goji berry fruits (Lycium barbarum) evaluated by EPR spectroscopy. J. Radioanal. Nucl. Chem. 2019, 320, 569–575. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Mujumdar, A.S.; Yu, D. Advanced detection techniques using artificial intelligence in processing of berries. Food Eng. Rev. 2022, 14, 176–199. [Google Scholar] [CrossRef]
- Sanmartin, C.; Modesti, M.; Venturi, F.; Brizzolara, S.; Mencarelli, F.; Bellincontro, A. Postharvest water loss of wine grape: When, what and why. Metabolites 2021, 11, 318. [Google Scholar] [CrossRef]
- Horvitz, S. Postharvest handling of berries. In Postharvest Handling; Academic Press: Cambridge, MA, USA, 2017; pp. 107–123. [Google Scholar]
- Shrivastava, C.; Schudel, S.; Shoji, K.; Onwude, D.; da Silva, F.P.; Turan, D.; Paillart, M.; Defraeye, T. Digital twins for selecting the optimal ventilated strawberry packaging based on the unique hygrothermal conditions of a shipment from farm to retailer. Postharvest Biol. Technol. 2023, 199, 112283. [Google Scholar] [CrossRef]
- López-Cruz, R.; Sandoval-Contreras, T.; Iñiguez-Moreno, M. Plant Pigments: Classification, Extraction, and Challenge of Their Application in the Food Industry. Food Bioprocess Technol. 2023, 1–17. [Google Scholar] [CrossRef]
- López-Casado, G.; Sánchez-Raya, C.; Ric-Varas, P.D.; Paniagua, C.; Blanco-Portales, R.; Muñoz-Blanco, J.; Pose, S.; Matas, A.J.; Mercado, J.A. CRISPR/Cas9 editing of the polygalacturonase FaPG1 gene improves strawberry fruit firmness. Hortic. Res. 2023, 10, uhad011. [Google Scholar] [CrossRef]
- Jiménez-Bermúdez, S.; Redondo-Nevado, J.; Muñoz-Blanco, J.; Caballero, J.L.; López-Aranda, J.M.; Valpuesta, V.; Pliego-Alfaro, F.; Quesada, M.A.; Mercado, J.A. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 2002, 128, 751–759. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Bell, S.R.; Hernández Montiel, L.G.; González Estrada, R.R.; Gutiérrez Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT Food Sci. Technol. 2021, 149, 7–12. [Google Scholar] [CrossRef]
- Abd-Elkader, D.Y.; Salem, M.Z.M.; Komeil, D.A.; Al-Huqail, A.A.; Ali, H.M.; Salah, A.H.; Akrami, M.; Hassan, H.S. Post-harvest enhancing and Botrytis cinerea control of strawberry fruits using low cost and eco-friendly natural oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Jaworska, G.; Szarek, N.; Hanus, P. Effect of celeriac pulp maceration by Rhizopus sp. pectinase on juice quality. Molecules 2022, 27, 8610. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, K.M.S.; Ridgway, H.J.; Jaspers, M.V.; Jones, E.E. Influence of blueberry tissue type, wounding and cultivar on susceptibility to infection by Neofusicoccum species. J. Appl. Microbiol. 2022, 132, 3771–3782. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Electrospray coating of minimally processed strawberries and evaluation of the shelf-life quality properties. J. Food Process Eng. 2019, 42, e13082. [Google Scholar] [CrossRef]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Wang, F.; Obenland, D.; Xiao, C.L. Effects of peroxyacetic acid on postharvest diseases and quality of blueberries. Plant Dis. 2021, 105, 3231–3237. [Google Scholar] [CrossRef]
- Ezrari, S.; Lazraq, A.; El Housni, Z.; Radouane, N.; Belabess, Z.; Mokrini, F.; Tahiri, A.; Amiri, S.; Lahlali, R. Evaluating the sensitivity and efficacy of fungicides with different modes of action against Neocosmospora solani and Fusarium species, causing agents of citrus dry root rot. Arch. Phytopathol. Plant Prot. 2022, 55, 1117–1135. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.H.; Qiao, J.; Qu, W.; Wang, M.S.; Gao, X.; Zhang, C.; Brennan, C.S.; Qi, X. Improvement of betalains stability extracted from red dragon fruit peel by ultrasound-assisted microencapsulation with maltodextrin. Ultrason. Sonochem. 2022, 82, 105897. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.J.; Xiao, C.L. Postharvest use of natamycin to control Alternaria rot on blueberry fruit caused by Alternaria alternata and A. arborescens. Postharvest Biol. Technol. 2021, 172, 111383. [Google Scholar] [CrossRef]
- Nguyen, K.; Sanchez, C.L.; Brammer-Robbins, E.; Pena-Delgado, C.; Kroyter, N.; El Ahmadie, N.; Watkins, J.M.; Aristizabal-Henao, J.J.; Bowden, J.A.; Souders, C.L.; et al. Neurotoxicity assessment of QoI strobilurin fungicides azoxystrobin and trifloxystrobin in human SH-SY5Y neuroblastoma cells: Insights from lipidomics and mitochondrial bioenergetics. Neurotoxicology 2022, 91, 290–304. [Google Scholar] [CrossRef]
- Macías-Gallardo, F.; Barajas-Díaz, C.G.M.; Mireles-Arriaga, A.I.; Ozuna, C. Strawberry variety influences the effectiveness of postharvest treatment with gaseous ozone: Impact on the physicochemical, microbiological, and bioactive properties of the fruit. Processes 2023, 11, 346. [Google Scholar] [CrossRef]
- Jaramillo-Sánchez, G.; Contigiani, E.V.; Castro, M.A.; Hodara, K.; Alzamora, S.M.; Loredo, A.G.; Nieto, A.B. Freshness maintenance of blueberries (Vaccinium corymbosum L.) during postharvest using ozone in aqueous phase: Microbiological, structure, and mechanical issues. Food Bioprocess Technol. 2019, 12, 2136–2147. [Google Scholar] [CrossRef]
- Hasani, M.; Wu, F.; Warriner, K. Validation of a vapor-phase advanced oxidation process for inactivating Listeria monocytogenes, its surrogate Lactobacillus fructivorans, and spoilage molds associated with green or red table grapes. J. Food Sci. 2020, 85, 2645–2655. [Google Scholar] [CrossRef]
- Pagès, M.; Kleiber, D.; Violleau, F. Ozonation of three different fungal conidia associated with apple disease: Importance of spore surface and membrane phospholipid oxidation. Food Sci. Nutr. 2020, 8, 5292–5297. [Google Scholar] [CrossRef]
- Intarasit, S.; Saengnil, K. Transient production of H2O2 and NO induced by ascorbic acid coincides with promotion of antioxidant enzyme activity and reduction of pericarp browning of harvested longan fruit. Sci. Hortic. 2021, 277, 109784. [Google Scholar] [CrossRef]
- Heo, S.; Kim, S.; Kang, D. The role of hydrogen peroxide and peroxiredoxins throughout the cell cycle. Antioxidants 2020, 9, 280. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Brandão, T.R.S.; Silva, C.L.M. Assessment of the impact of hydrogen peroxide solutions on microbial loads and quality factors of red bell peppers, strawberries and watercress. Food Control 2012, 27, 362–368. [Google Scholar] [CrossRef]
- FDA. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=173.315 (accessed on 28 March 2023).
- Nicolau-Lapeña, I.; Abadias, M.; Bobo, G.; Aguiló-Aguayo, I.; Lafarga, T.; Viñas, I. Strawberry sanitization by peracetic acid washing and its effect on fruit quality. Food Microbiol. 2019, 83, 159–166. [Google Scholar] [CrossRef]
- Ao, X.W.; Eloranta, J.; Huang, C.H.; Santoro, D.; Sun, W.J.; Lu, Z.D.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- Pérez-Lavalle, L.; Carrasco, E.; Valero, A. Strategies for microbial decontamination of fresh blueberries and derived products. Foods 2020, 9, 1558. [Google Scholar] [CrossRef]
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Z.; Tu, S.; Chen, S.; Peng, J.; Tu, K. Effects of cold plasma, UV-C or aqueous ozone treatment on Botrytis cinerea and their potential application in preserving blueberry. J. Appl. Microbiol. 2019, 127, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Barkaoui, S.; Mankai, M.; Miloud, N.B.; Kraïem, M.; Madureira, J.; Verde, S.C.; Boudhrioua, N. E-beam irradiation of strawberries: Investigation of microbiological, physicochemical, sensory acceptance properties and bioactive content. Innov. Food Sci. Emerg. Technol. 2021, 73, 102769. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; von Borries-Medrano, E.; Aguilar-Méndez, M.A. Development of gelatin/carboxymethylcellulose active films containing Hass avocado peel extract and their application as a packaging for the preservation of berries. Int. J. Biol. Macromol. 2022, 206, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life 2019, 22, 100402. [Google Scholar] [CrossRef]
- Velázquez-Contreras, F.; García-Caldera, N.; Padilla de la Rosa, J.D.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Effect of PLA active packaging containing monoterpene-cyclodextrin complexes on berries preservation. Polymers 2021, 13, 1399. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C. Enhanced inactivation of Salmonella Typhimurium from blueberries by combinations of sodium dodecyl sulfate with organic acids or hydrogen peroxide. Food Res. Int. 2013, 54, 1553–1559. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Y.; Zhang, Z. The role of different natural organic acids in postharvest fruit quality management and its mechanism. Food Front. 2023, 1–17. [Google Scholar] [CrossRef]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2022, 375, 131810. [Google Scholar] [CrossRef]
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Song, K.B.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT Food Sci. Technol. 2017, 75, 742–750. [Google Scholar] [CrossRef]
- Magalhães Brandão, R.; Roberto Batista, L.; Elvis de Oliveira, J.; Bispo Barbosa, R.; Lee Nelson, D.; Graças Cardoso, M. In vitro and in vivo efficacy of poly(lactic acid) nanofiber packaging containing essential oils from Ocimum basilicum L. and Ocimum gratissimum L. against Aspergillus carbonarius and Aspergillus niger in table grapes. Food Chem. 2023, 400, 134087. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; Sarreal, S.B.L.; Hua, S.S.T.; Sambo, P.; McHugh, T.H. Increasing strawberry shelf-life with carvacrol and methyl cinnamate antimicrobial vapors released from edible films. Postharvest Biol. Technol. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Antunes Filho, S.; Dos Santos, M.S.; Dos Santos, O.A.L.; Backx, B.P.; Soran, M.L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of nanoparticles using plant extracts and essential oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Abu-Dieyeh, M. Novel Prosopis juliflora leaf ethanolic extract coating for extending postharvest shelf-life of strawberries. Food Control 2022, 133, 108641. [Google Scholar] [CrossRef]
- Fan, X.J.; Zhang, B.; Yan, H.; Feng, J.T.; Ma, Z.Q.; Zhang, X. Effect of lotus leaf extract incorporated composite coating on the postharvest quality of fresh goji (Lycium barbarum L.) fruit. Postharvest Biol. Technol. 2019, 148, 132–140. [Google Scholar] [CrossRef]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Sempere-Ferre, F.; Giménez-Santamarina, S.; Roselló, J.; Santamarina, M.P. Antifungal in vitro potential of Aloe vera gel as postharvest treatment to maintain blueberry quality during storage. LWT Food Sci. Technol. 2022, 163, 113512. [Google Scholar] [CrossRef]
- Dammak, I.; Lasram, S.; Hamdi, Z.; Ben Moussa, O.; Mkadmini Hammi, K.; Trigui, I.; Houissa, H.; Mliki, A.; Hassouna, M. In vitro antifungal and anti-ochratoxigenic activities of Aloe vera gel against Aspergillus carbonarius isolated from grapes. Ind. Crops Prod. 2018, 123, 416–423. [Google Scholar] [CrossRef]
- Ehtesham Nia, A.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Forney, C.F.; Jordan, M.A.; Pennell, K.M.; Fillmore, S. Controlled atmosphere storage impacts fruit quality and flavor chemistry of five cultivars of highbush blueberry (Vaccinium corymbosum). Postharvest Biol. Technol. 2022, 194, 112073. [Google Scholar] [CrossRef]
- Van de Velde, F.; Méndez-Galarraga, M.P.; Grace, M.H.; Fenoglio, C.; Lila, M.A.; Pirovani, M.É. Changes due to high oxygen and high carbon dioxide atmospheres on the general quality and the polyphenolic profile of strawberries. Postharvest Biol. Technol. 2019, 148, 49–57. [Google Scholar] [CrossRef]
- Yang, M.; Ban, Z.; Luo, Z.; Li, J.; Lu, H.; Li, D.; Chen, C.; Li, L. Impact of elevated O2 and CO2 atmospheres on chemical attributes and quality of strawberry (Fragaria × ananassa Duch.) during storage. Food Chem. 2020, 307, 125550. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Cameron, A.C.; Shirazi, A.; Dostal-Lange, D.L. Modified-atmosphere packaging of blueberry fruit: Effect of temperature on package O2 and CO2. J. Am. Soc. Hortic. Sci. 2019, 117, 436–441. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.J.; Opara, U.L. Modified Atmosphere Packaging Technology of Fresh and Fresh-cut Produce and the Microbial Consequences-A Review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Farber, J.N.; Harris, L.J.; Parish, M.E.; Beuchat, L.R.; Suslow, T.V.; Gorney, J.R.; Garrett, E.H.; Busta, F.F. Microbiological safety of controlled and modified atmosphere packaging of fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 142–160. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Lee, D.S. Modified atmosphere and moisture condensation in packaged fresh produce: Scientific efforts and commercial success. Postharvest Biol. Technol. 2023, 198, 112235. [Google Scholar] [CrossRef]
- Kaavya, R.; Pandiselvam, R.; Abdullah, S.; Sruthi, N.U.; Jayanath, Y.; Ashokkumar, C.; Chandra Khanashyam, A.; Kothakota, A.; Ramesh, S.V. Emerging non-thermal technologies for decontamination of Salmonella in food. Trends Food Sci. Technol. 2021, 112, 400–418. [Google Scholar] [CrossRef]
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of ultraviolet light treatment on microbiological safety and quality of fresh produce: An overview. Front. Nutr. 2022, 9, 871243. [Google Scholar] [CrossRef]
- Jaramillo Sánchez, G.; Contigiani, E.V.; Coronel, M.B.; Alzamora, S.M.; García-Loredo, A.; Nieto, A.B. Study of UV-C treatments on postharvest life of blueberries ‘O’Neal’ and correlation between structure and quality parameters. Heliyon 2021, 7, e07170. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Jin, T.Z.; Yu, Y.; Gurtler, J.B. Effects of pulsed electric field processing on microbial survival, quality change and nutritional characteristics of blueberries. LWT Food Sci. Technol. 2017, 77, 517–524. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Sun, R.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of dielectric barrier discharge cold plasma on the quality and phenolic metabolism in blueberries based on metabonomic analysis. Postharvest Biol. Technol. 2023, 197, 112208. [Google Scholar] [CrossRef]
- Wang, C.; Tao, Y.; Han, Y.; Zhang, R.; Li, L.; Gao, Y. Influences of subcellular Ca redistribution induced by γ irradiation on the fruit firmness of refrigerated blueberries. Postharvest Biol. Technol. 2023, 195, 112146. [Google Scholar] [CrossRef]
- FAO; WHO. High-Dose Irradiation: Wholesomeness of Food Irradiated with Doses above 10 kGy. Report of a Joint FAO/IAEA/WHO Study Group; FAO: Rome, Italy, 1999. [Google Scholar]
- Zhang, H.; Tsai, S.; Tikekar, R.V. Inactivation of Listeria innocua on blueberries by novel ultrasound washing processes and their impact on quality during storage. Food Control 2021, 121, 107580. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-pressure homogenization: Principles and applications beyond microbial inactivation. Food Eng. Rev. 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Song, H.; Asghari, M.; Zahedipour-Sheshglani, P.; Diao, E.; Xiang, X.; Liang, X.; Abdollahi Mandoulakani, B.; Qian, S. Investigation of pectolytic and PR genes expression, quality and phytochemical contents in organic and non-organic table grapes at harvest and during storage. Food Res. Int. 2023, 167, 112717. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Mohammadi Nafchi, A.; Salehabadi, A.; Oladzad-abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Gutiérrez-Corte, E. Effect of nano-edible coating based on beeswax solid lipid nanoparticles on strawberry’s preservation. Coatings 2020, 10, 253. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Picone, C.S.F. Antimicrobial activity of lactoferrin-chitosan-gellan nanoparticles and their influence on strawberry preservation. Food Res. Int. 2022, 159, 111586. [Google Scholar] [CrossRef] [PubMed]
- Alaş, M.Ö.; Doǧan, G.; Yalcin, M.S.; Ozdemir, S.; Genç, R. Multicolor emitting carbon dot-reinforced pva composites as edible food packaging films and coatings with antimicrobial and UV-blocking properties. ACS Omega 2022, 7, 29967–29983. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carrillo, J.G.; Orta-Zavalza, E.; González-Rodríguez, S.E.; Montoya-Torres, C.; Sepúlveda-Ahumada, D.R.; Ortiz-Rivera, Y. Evaluation of the effectivity of reuterin in pectin edible coatings to extend the shelf-life of strawberries during cold storage. Food Packag. Shelf Life 2021, 30, 100760. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Priyadarshi, R.; Han, S. Cellulose nanofiber-based coating film integrated with nitrogen-functionalized carbon dots for active packaging applications of fresh fruit. Postharvest Biol. Technol. 2022, 186, 111845. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Huang, P.; Li, H.; Liu, Y.; Zhang, Y.; Hu, X.; Deng, S.; Liu, Y.; Qin, W. Use of heat-shock and edible coating to improve the postharvest preservation of blueberries. Foods 2023, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Han, Z.; Zhao, C.; Jiang, Q.; Tang, Y.; Li, Y.; Cheng, Z. Preparation and characterization of Aloe vera polysaccharide-based packaging film and its application in blueberry preservation. Prog. Org. Coat. 2023, 177, 107445. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Influence of polysaccharide-based edible coatings as carriers of prebiotic fibers on quality attributes of ready-to-eat fresh blueberries. J. Sci. Food Agric. 2018, 98, 2587–2597. [Google Scholar] [CrossRef]
- Poonia, A.; Dhewa, T. Edible Food Packaging: Applications, Innovations and Sustainability; Springer: Singapore, 2022; ISBN 9789811623820. [Google Scholar]
- Pinto, C.A.; Moreira, S.A.; Fidalgo, L.G.; Inácio, R.S.; Barba, F.J.; Saraiva, J.A. Effects of high-pressure processing on fungi spores: Factors affecting spore germination and inactivation and impact on ultrastructure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 553–573. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; Jiang, S.; He, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L.; Tostrud, L.; Costanzo, E.; Coe, C.L.; Serlin, R.C.; Ward, S.E.; Zhang, Y. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J. Pain Symptom Manag. 2018, 55, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Diaz, I.F.; Mena-Violante, H.G.; Hernandez-Lauzardo, A.N.; Oyoque-Salcedo, G.; Oregel-Zamudio, E.; Angoa-Perez, M.V. Postharvest control of rhizopus stolonifer on blackberry (Rubus fruticosus) by blackberry native crop bacteria. Rev. la Fac. Cienc. Agrar. 2019, 51, 306–317. [Google Scholar]
- Zhang, X.; Gao, Z.; Zhang, X.; Bai, W.; Zhang, L.; Pei, H. Control effects of Bacillus siamensis G-3 volatile compounds on raspberry postharvest diseases caused by Botrytis cinerea and Rhizopus stolonifer. Biol. Control 2020, 141, 104135. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Zeng, J.; Shao, Y. Mechanisms of action of the yeast Debaryomyces nepalensis for control of the pathogen Colletotrichum gloeosporioides in mango fruit. Biol. Control 2018, 123, 111–119. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Korsten, L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 2010, 53, 122–128. [Google Scholar] [CrossRef]
- Cai, J.; Mo, X.; Wen, C.; Gao, Z.; Chen, X.; Xue, C. FvMYB79 positively regulates strawberry fruit softening via transcriptional activation of FvPME38. Int. J. Mol. Sci. 2022, 23, 101. [Google Scholar] [CrossRef]
- Gopi, V.; Samruban, J. Biotechnology approaches enhancing improved post harvest technology of fruit crops. In Recent Advances in Agricultural and Allied Sciences; 2020; pp. 12–34. ISBN 9788194563198. Available online: https://www.researchgate.net/profile/Gopi-Venkatachalapathy/publication/371491441_Chapter_-2_2_BIOTECHNOLOGY_APPROACHES_ENHANCING_IMPROVED_POST_HARVEST_TECHNOLOGY_OF_FRUIT_CROPS/links/6486bfdab3dfd73b777f847b/Chapter-2-2-BIOTECHNOLOGY-APPROACHES-ENHANCING-IMPROVED-POST-HARVEST-TECHNOLOGY-OF-FRUIT-CROPS.pdf (accessed on 15 August 2023).
- Sonwani, E.; Bansal, U.; Alroobaea, R.; Baqasah, A.M.; Hedabou, M. An artificial intelligence approach toward food spoilage detection and analysis. Front. Public Health 2022, 9, 816226. [Google Scholar] [CrossRef]
- Palumbo, M.; Cozzolino, R.; Laurino, C.; Malorni, L.; Picariello, G.; Siano, F.; Stocchero, M.; Cefola, M.; Corvino, A.; Romaniello, R.; et al. Rapid and non-destructive techniques for the discrimination of ripening stages in Candonga Strawberries. Foods 2022, 11, 1534. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An overview of antimicrobial properties of carbon nanotubes-based nanocomposites. Adv. Pharm. Bull. 2022, 12, 449–465. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial nanomaterials for food packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H. Polymeric nanomaterials for efficient delivery of antimicrobial agents. Pharmaceutics 2021, 13, 2108. [Google Scholar] [CrossRef]
- Rabia, E.; Tuga, B.; de Ondarza, J.; Ramos, S.M.; Lam, E.; Hrapovic, S.; Liu, Y.; Sunasee, R. Carboxylated cellulose nanocrystals decorated with varying molecular weights of poly(diallyldimethylammonium chloride) as sustainable antibacterial agents. Polymers 2023, 15, 865. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Rezaei, Z. Carbon quantum dots-based antifungal coating film for active packaging application of avocado. Food Packag. Shelf Life 2022, 33, 100878. [Google Scholar] [CrossRef]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Cellulose nanofiber-based multifunctional films integrated with carbon dots and anthocyanins from Brassica oleracea for active and intelligent food packaging applications. Int. J. Biol. Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of fungicides and factors affecting their fate and removal efficacy: A review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]

| Berry | Coating Composition | Nanoparticle Wall Materials | Bioactive Compound | Method to Obtain the Particles | Coating Technique | Storage Conditions | Significant Result | Reference |
|---|---|---|---|---|---|---|---|---|
| Strawberry | Xanthan gum and propylene glycol | Beeswax solid lipid nanoparticles | -- | Homogenization | Dipping | 4 °C/21 days | Coatings increased the shelf life of strawberries stored in refrigeration. | [98] |
| Carboxymethylcellulose | Lactoferrin, chitosan, and gellan solutions. | -- | Homogenization | Dipping | 25 °C, 50% RH 6 days | Carboxymethylcellulose enhances the adhesion of particles to the fruits. | [99] | |
| Sodium alginate | -- | ZnO suspensions | Ultrasonic homogenization | Dipping | 20 days at 1 °C and 95% RH | Higher antioxidant and superoxide dismutase activity, the lowest peroxidase activity, and received the highest-ranked sensory attributes. | [9] | |
| Particle nanoemulsion | Sodium alginate | Tea tree and cucumber seed oil | Dispersion by stirring | Brushing | 25 °C/18 days | Inhibition of microbial growth and delaying fruit maturation, indicating its potential for prolonging the shelf life of fresh food. | [17] | |
| Sodium alginate | Bagasse cellulose nanocrystals and chitosan nanofibers | Oregano essential oil | High-pressure homogenization. | Dipping | 25 °C/9 days | Coating retained desired moisture, respiration rate, stiffness, firmness, and appearance properties of strawberries due to its gas barrier properties, resulting from the entangled matrix structure. | [18] | |
| Carboxymethylcellulose | Lactoferrin, chitosan and tripolyphosphate (TTP) | – | Ionic cross-linking | Dipping | 25 °C, 50% RH for 6 days | Applied to strawberries, the nanoparticles delayed the ripening and degradation of the fruit. Additionally, the antimicrobial properties of lactoferrin and chitosan were intensified by the ionic cross-linking with TPP. | [16] | |
| Polyvinyl alcohol (PVA) | -- | Carbon dots from carob molasses | Hydrothermal process | Dipping | 4 °C/12 days | Coating extended shelf life by reducing fungal development and spoilage, as well as moisture loss. | [100] | |
| Pectin (3%) from orange peels | – | Reuterin and lemon essential oil | Stirring | Dipping | 4 °C/31 days | Coatings can avoid fungal spoilage without quality reduction | [101] | |
| Cellulose nanofibers | -- | Carbon dots from glucose and urea | Hydrothermal process/Nitrogen-doped | Dipping | 25 °C/2 days | Inhibited fungal growth on the fruit surface and controlled microbial growth. | [102] | |
| Blueberry | Konjac glucomannan/low acyl gellan gum | β-cyclodextrin | Thymol | Spray-drying | Atomization | 25 °C/14 days | The combination of heat treatment at 45 °C/60 min and coatings maintained the level of ascorbic acid, total anthocyanin, total acid, and soluble solids and improved the aroma of the coated fruit during the storage. | [103] |
| Starch and gelatin | -- | Cinnamon essential oil | -- | Dipping | 4 °C/10 days | Inhibited the growth of molds and yeasts and reduced the ROS level and the activity of superoxide dismutase and catalase by 82, 56, and 63%, respectively, in comparison to uncoated fruits. | [4] | |
| β-hydroxy-β-methylbutyrate calcium and nanocellulose | -- | Aloe vera | -- | Dipping | 4 °C/15 days | Improved the resistance to external forces and reduced the respiration rate, weight loss, and relative electrical conductivity of the fruit, which significantly delayed softening, decomposition, and consumption of total soluble solids and titratable acidity during storage | [104] | |
| Chitosan | A. vera | -- | Dipping | 5 °C/25 days | Reduced microbiological growth and water loss levels by 50 and 42%, respectively, in comparison to uncoated blueberries. Uncoated fruits showed mold contamination after 2nd day of storage (2.0 ± 0.32 Log CFU/g), whereas coated fruits after the 9th day reached 1.3 ± 0.35 Log CFU/g. | [105] | ||
| Grape | Chitosan | -- | Lemongrass essential oil | -- | Dipping | 4 and 25 °C/7 days | Reduced the microbial development on the surface of the fruits and inhibited Salmonella growth, maintaining the sensorial properties. | [66] |
| Poly(lactic acid) (PLA) | -- | Ocimum basilicum L. and Ocimum gratissimum L. essential oil | -- | Nanofibers by solution blow spinning | 25 °C/10 days | Reduction between 10 and 12% in comparison to the control and preserved the organoleptic, sensory, and nutritional properties of the fruits. | [67] |
| Technique | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Chemical compounds |
|
| [6,13] |
| Modified atmosphere packaging |
|
| [6] |
| Low temperature |
|
| [4,108] |
| Ultraviolet (UV) irradiation |
|
| [84,85] |
| Pulsed electric field |
|
| [87,88] |
| Cold plasma |
|
| [19,89] |
| Ionized irradiation |
|
| [7,8] |
| Ultrasound |
|
| [93,95] |
| Edible coatings |
|
| [96,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iñiguez-Moreno, M.; González-González, R.B.; Flores-Contreras, E.A.; Araújo, R.G.; Chen, W.N.; Alfaro-Ponce, M.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits. Foods 2023, 12, 3159. https://doi.org/10.3390/foods12173159
Iñiguez-Moreno M, González-González RB, Flores-Contreras EA, Araújo RG, Chen WN, Alfaro-Ponce M, Iqbal HMN, Melchor-Martínez EM, Parra-Saldívar R. Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits. Foods. 2023; 12(17):3159. https://doi.org/10.3390/foods12173159
Chicago/Turabian StyleIñiguez-Moreno, Maricarmen, Reyna Berenice González-González, Elda A. Flores-Contreras, Rafael G. Araújo, Wei Ning Chen, Mariel Alfaro-Ponce, Hafiz M. N. Iqbal, Elda M. Melchor-Martínez, and Roberto Parra-Saldívar. 2023. "Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits" Foods 12, no. 17: 3159. https://doi.org/10.3390/foods12173159
APA StyleIñiguez-Moreno, M., González-González, R. B., Flores-Contreras, E. A., Araújo, R. G., Chen, W. N., Alfaro-Ponce, M., Iqbal, H. M. N., Melchor-Martínez, E. M., & Parra-Saldívar, R. (2023). Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits. Foods, 12(17), 3159. https://doi.org/10.3390/foods12173159











