Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Composition Analysis
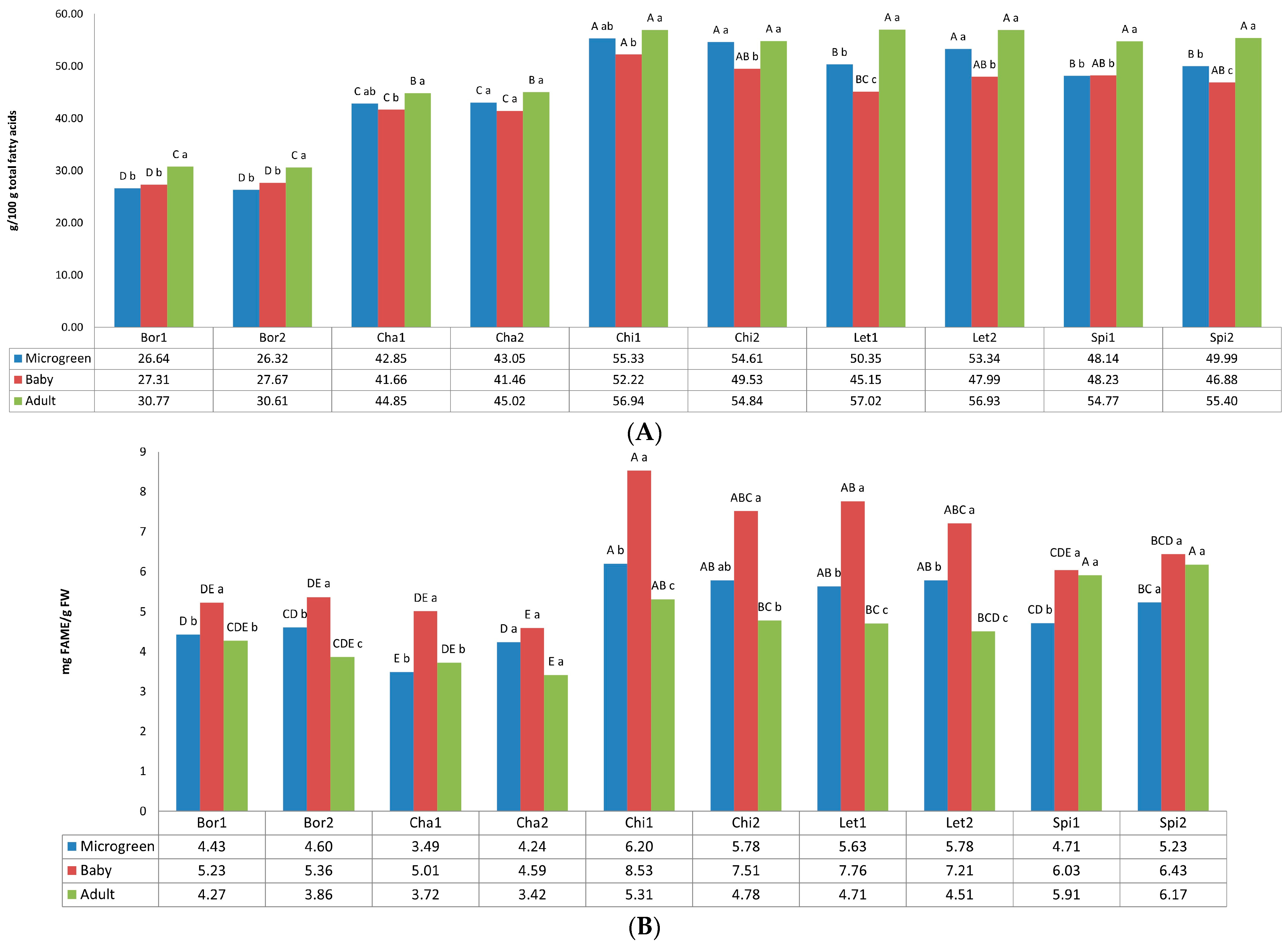
2.3.1. Fatty Acids
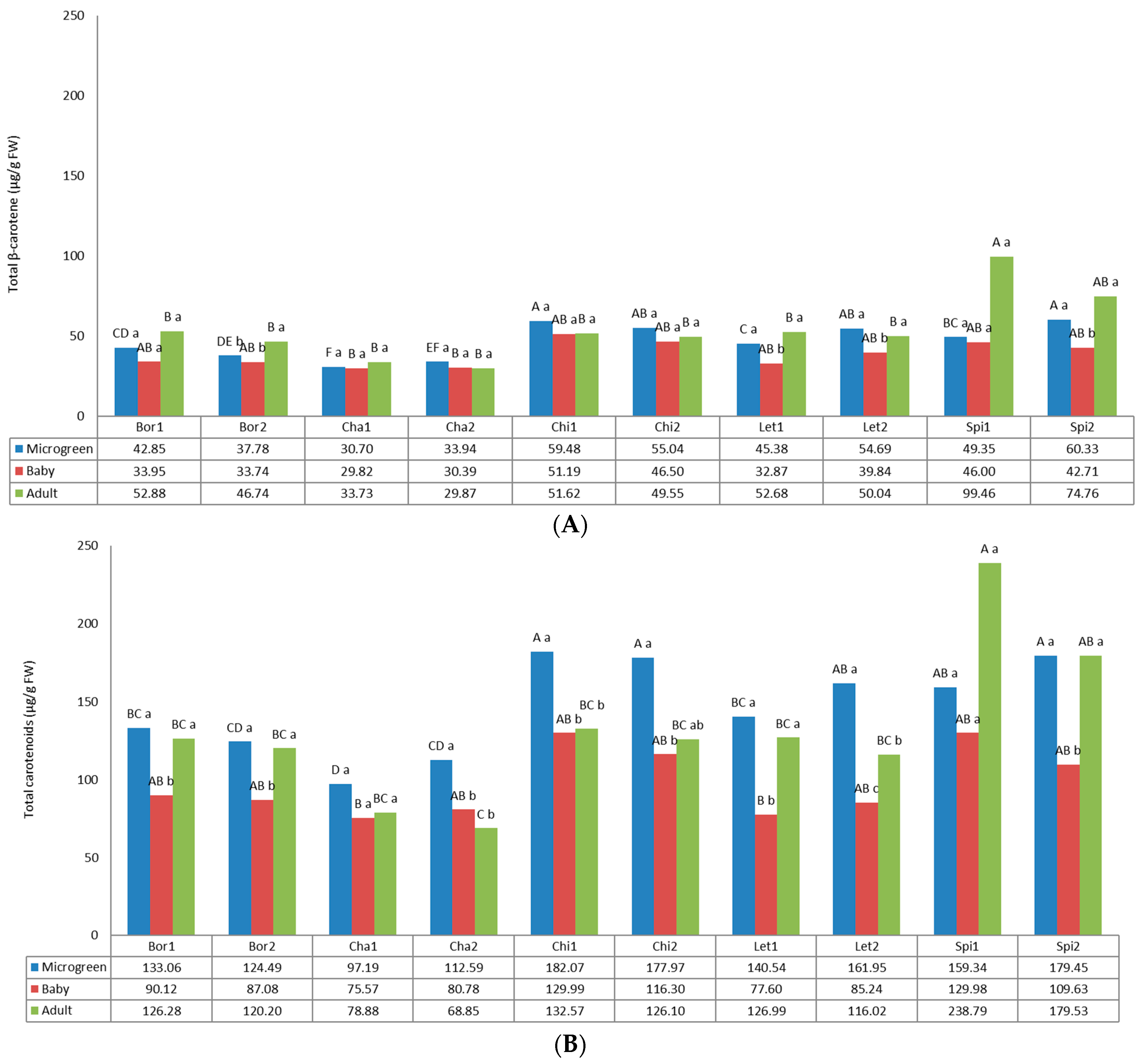
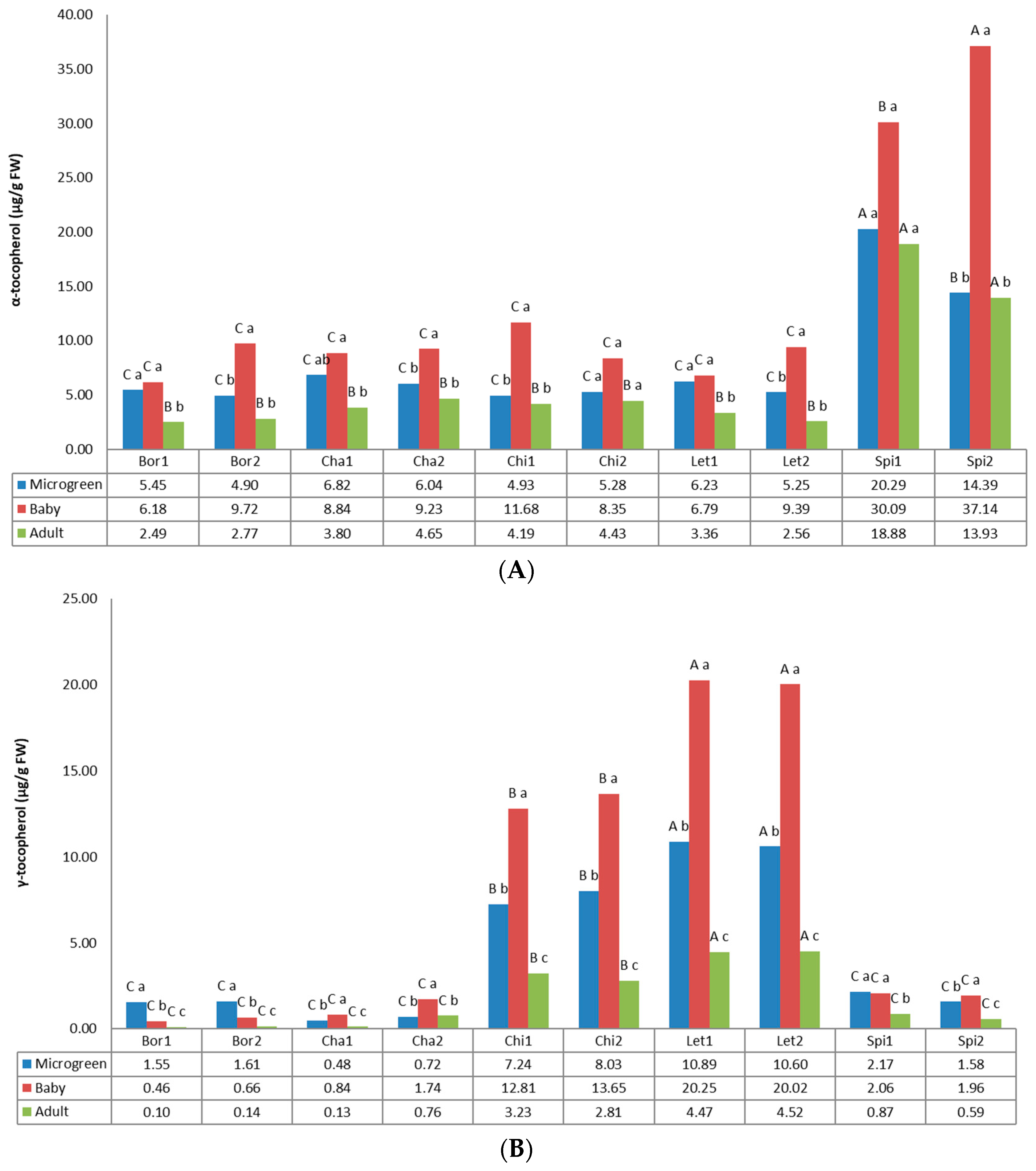
2.3.2. Carotenoids and Tocopherols
2.3.3. Vitamin C
2.3.4. Total Polyphenols
2.3.5. Antioxidant Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acids
3.2. Carotenoids
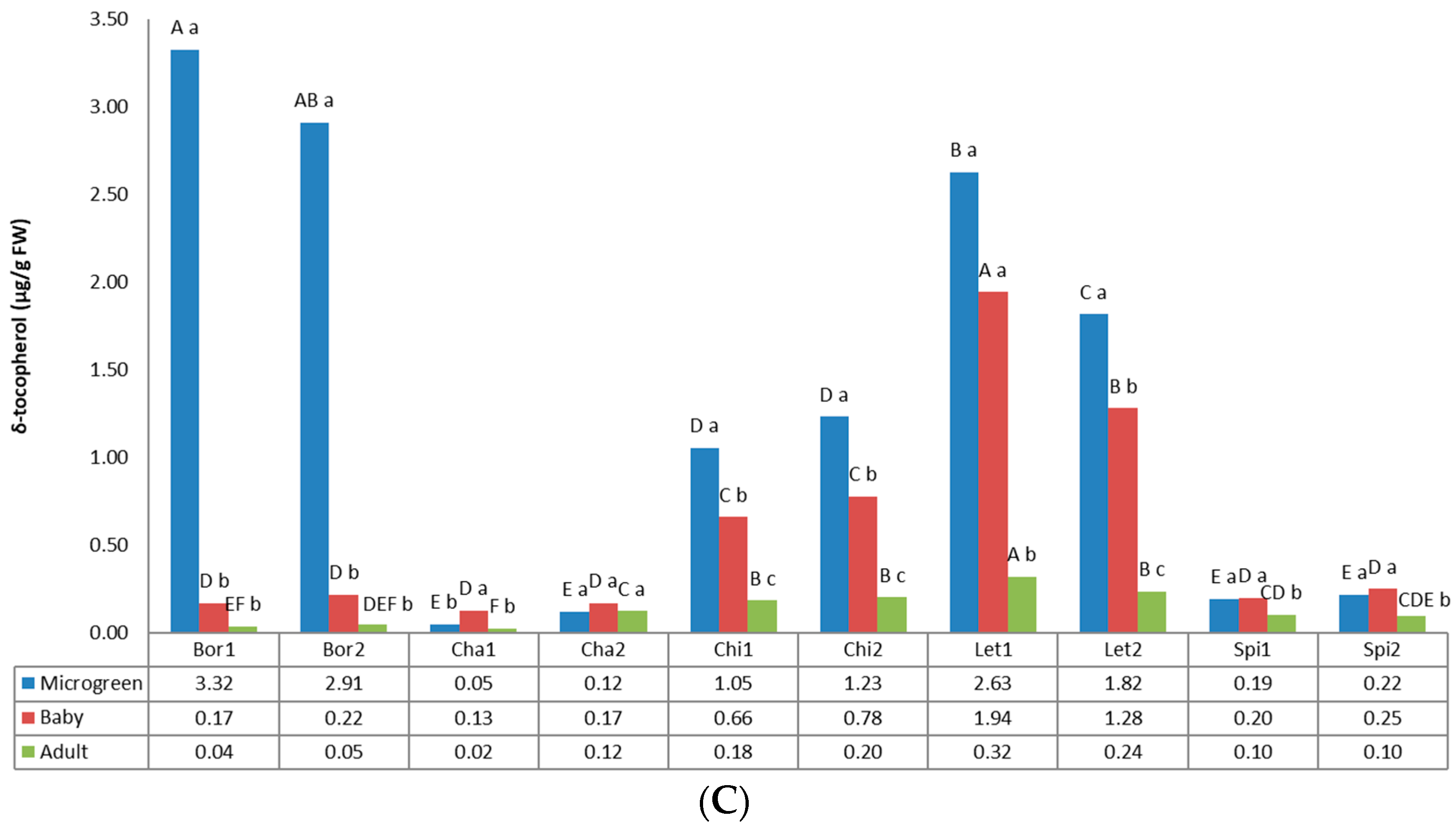
3.3. Tocopherols
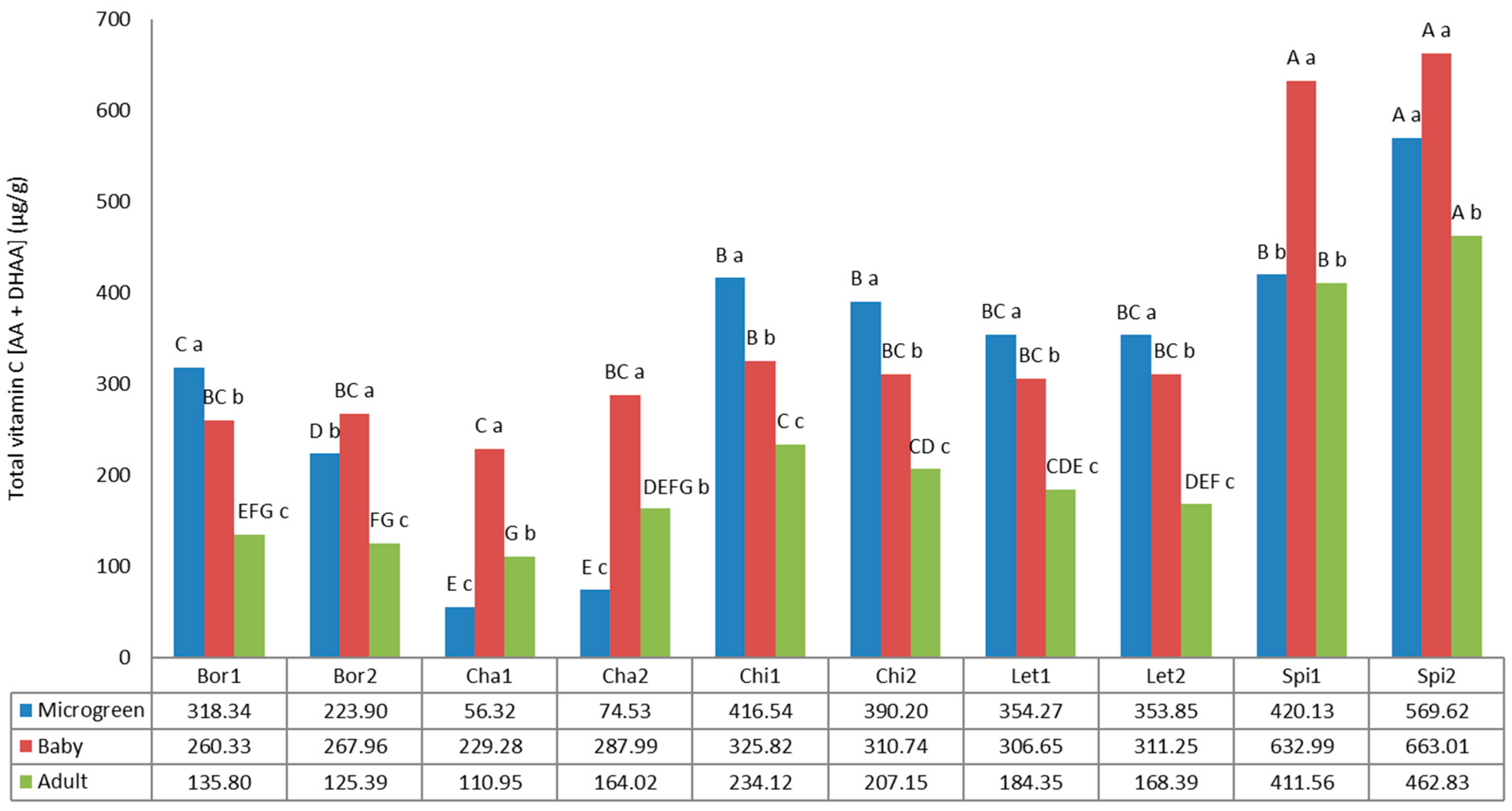
3.4. Vitamin C
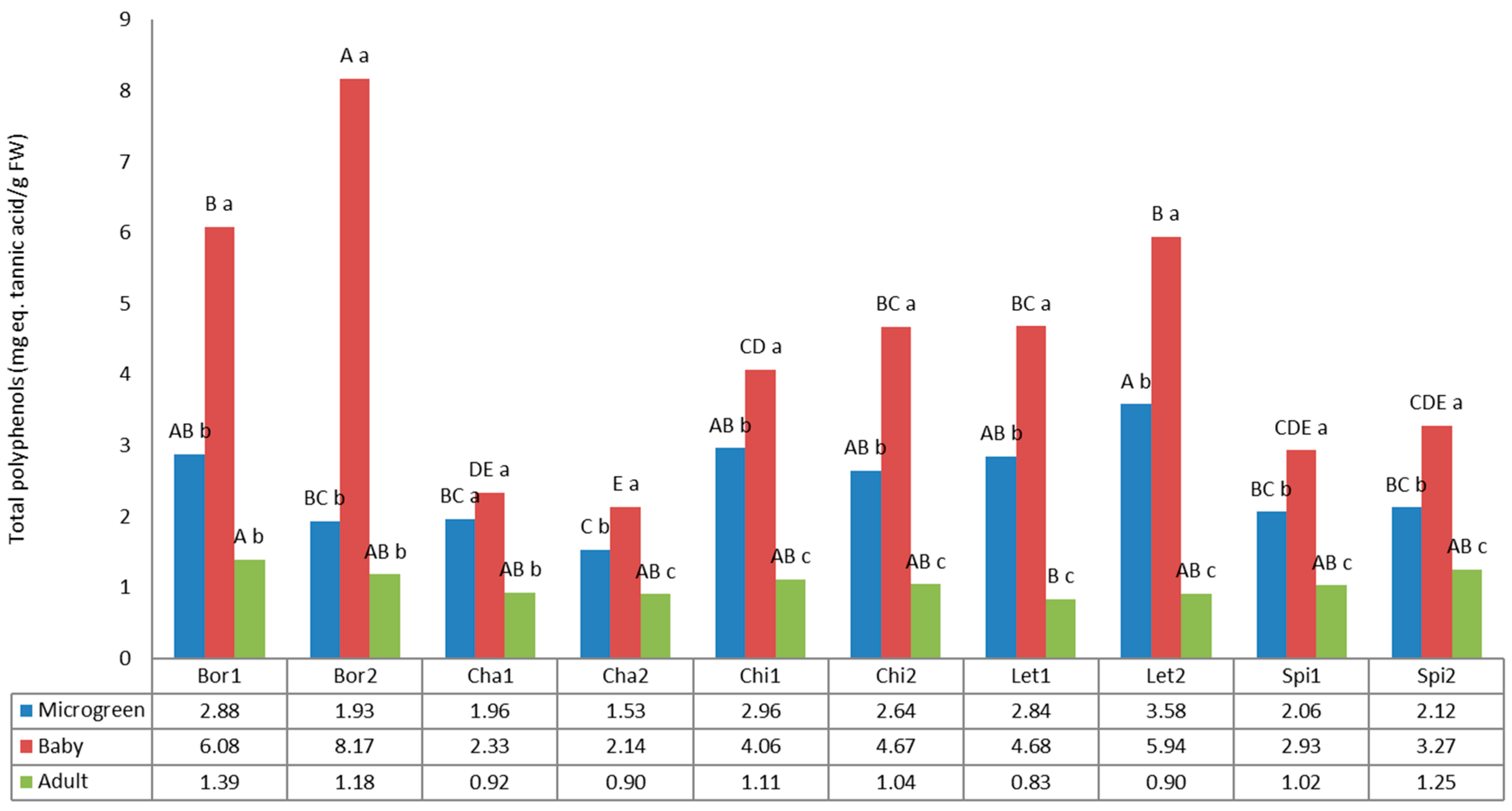
3.5. Total Polyphenols
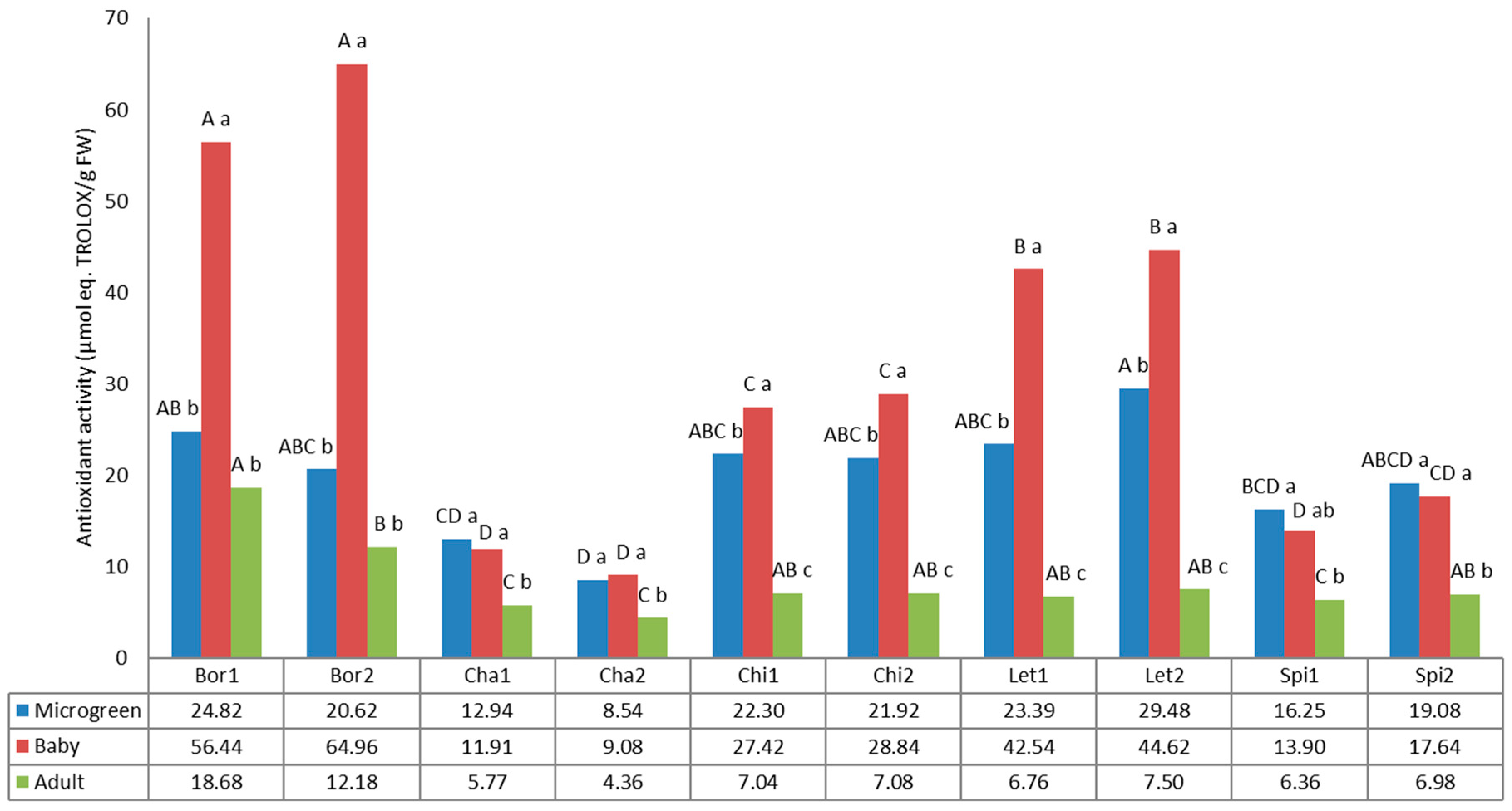
3.6. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizvi, A.; Sharma, M.; Saxena, S. Microgreens: A Next Generation Nutraceutical for Multiple Disease Management and Health Promotion. Genet. Resour. Crop Evol. 2023, 70, 311–332. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Pollock, R.L. The effect of green leafy and cruciferous vegetable intake on the incidence of cardiovascular disease: A meta-analysis. JRSM Cardiovasc. Dis. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Golden, D.L.; Chen, H.; Register, T.C.; Gugger, E.T. A diet rich in green and yellow vegetables inhibits atherosclerosis in mice. J. Nutr. 2006, 136, 1886–1889. [Google Scholar] [CrossRef]
- Chen, G.-C.; Koh, W.P.; Yuan, J.M.; Qin, L.Q.; van Dam, R.M. Green leafy and cruciferous vegetable consumption and risk of type 2 diabetes: Results from the Singapore Chinese Health Study and meta-analysis. Br. J. Nutr. 2018, 119, 1057–1067. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food. Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, T.; Singh, S.P.; Bhardwaj, A.; Srivastava, D.; Kumar, R. Prospects of microgreens as budding living functional food: Breeding and biofortification through OMICS and other approaches for nutritional security. Front. Genet. 2023, 14, 1053810. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Specialty Greens Pack a Nutritional Punch. AgResearch Magazine, January 2014. Available online: https://agresearchmag.ars.usda.gov/2014/jan/greens (accessed on 18 July 2023).
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant potentiality and mineral content of summer season leafy greens: Comparison at mature and microgreen stages using chemometric. Agric Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Johnson, S.A.; Prenni, J.E.; Heuberger, A.L.; Isweiri, H.; Chaparro, J.M.; Newman, S.E.; Uchanski, M.E.; Omerigic, H.M.; Michell, K.A.; Bunning, M.; et al. Comprehensive evaluation of metabolites and minerals in 6 microgreen species and the influence of maturity. Curr. Dev. Nutr. 2021, 5, nzaa180. [Google Scholar] [CrossRef] [PubMed]
- Enthoven, L.; Van den Broeck, G. Local food systems: Reviewing two decades of research. Agric. Syst. 2021, 193, 103226. [Google Scholar] [CrossRef]
- Stein, A.J.; Santini, F. The sustainability of “local” food: A review for policy-makers. Rev. Agric. Food Environ. Stud. 2022, 103, 77–89. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Zeven, A. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Gibson, R.W. A review of perceptual distinctiveness in landraces including an analysis of how its roles have been overlooked in plant breeding for low-input farming systems. Econ. Bot. 2009, 63, 242–255. [Google Scholar] [CrossRef]
- Corrado, G.; Rao, R. Towards the Genomic Basis of Local Adaptation in Landraces. Diversity 2017, 9, 51. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sarli, G.; Logozzo, G.; Zeuli, P.S. Landraces in Inland areas of the Basilicata region, Italy: Monitoring and perspectives for on farm conservation. Genet. Resour. Crop Evol. 2012, 59, 701–716. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- ISO-12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. International Organization for Standarization: Geneva, Switzerland, 2015.
- Blanco, M.; Ripoll, G.; Casasus, I.; Bertolín, J.R.; Joy, M. Carotenoids and tocopherol in plasma and subcutaneous fat colour to trace forage-feeding in growing steers. Livest. Sci. 2019, 219, 104–110. [Google Scholar] [CrossRef]
- Bertolín, J.; Joy, M.; Rufino-Moya, P.; Lobón, S.; Blanco, M. Simultaneous determination of carotenoids, tocopherols, retinol, and cholesterol in ovine lyophilised samples of milk, meat, and liver and in unprocessed/raw samples of fat. Food Chem. 2018, 257, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1-57881-072-8. [Google Scholar]
- Chauveau-Duriot, B.; Doreau, M.; Noziere, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Zufiaurre, R.; Díaz, A. Improved UPLC-UV Method for the Quantification of Vitamin C in Lettuce Varieties (Lactuca sativa L.) and Crop Wild Relatives (Lactuca spp.). J. Vis. Exp. 2020, 160, e61440. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage; FAO/IAEA Working Document; IAEA: Vienna, Austria, 2000; Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:33048138 (accessed on 18 July 2023).
- Rufino-Moya, P.J.; Bertolín, J.R.; Blanco, M.; Lobón, S.; Joy, M. Fatty acid profile, secondary compounds and antioxidant activities in the fresh forage, hay and silage of sainfoin (Onobrychis viciifolia) and sulla (Hedysarum coronarium). J. Sci. Food Agric. 2022, 102, 4736–4743. [Google Scholar] [CrossRef]
- Osipova, E.V.; Lantsova, N.V.; Chechetkin, I.R.; Mukhitova, F.K.; Hamberg, M.; Grechkin, A.N. Hexadecanoid pathway in plants: Lipoxygenase dioxygenation of (7Z,10Z,13Z)-hexadecatrienoic acid. Biochemistry 2010, 75, 708–716. [Google Scholar] [CrossRef]
- Mongrand, S.; Bessoule, J.J.; Cabantous, F.; Cassagne, C. The C(16:3)/C(18:3) fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry 1998, 49, 1049–1064. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Palmegiano, G.B.; Salamano, G. Quality and fatty acid content of borage (Borago officinalis) during the growth cycle. Ital. J. Food. Sci. 2004, 2, 177–183. [Google Scholar]
- Stahlër, K.; Quek, S.Y.; Miller, M.R. Investigation of -linolenic acid and stearidonic acid biosynthesis during a life cycle of Borago officinalis L. J. Am. Oil Chem. Soc. 2011, 88, 1715–1725. [Google Scholar] [CrossRef]
- Montaner, C.; Zufiaurre, R.; Movila, M.; Mallor, C. Evaluation of Borage (Borago officinalis L.) Genotypes for Nutraceutical Value Based on Leaves Fatty Acids Composition. Foods 2022, 11, 16. [Google Scholar] [CrossRef]
- Saini, R.K.; Shang, X.M.; Ko, E.Y.; Choi, J.H.; Kim, D.; Keum, Y.S. Characterization of nutritionally important phytoconstituents in minimally processed ready-to-eat baby-leaf vegetables using HPLC–DAD and GC–MS. Food Meas. 2016, 10, 341–349. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.; Carvalho, A.; Sanchez-Mata, M.; Camara, M.; Tardio, J. Fatty acids profiles of some Spanish wild vegetables. Food Sci. Technol. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Vidrih, R.; Filip, S.; Hribar, J. Content of Higher Fatty Acids in Green Vegetables. Czech J. Food Sci. 2009, 27, 125–129. [Google Scholar] [CrossRef]
- Barcelo-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.; Brechany, E.Y.; Jackson, F.M.; Christie, W.W.; Stobart, A.K. Distribution and biosynthesis of stearidonic acid in leaves of Borago officinalis. Phytochemistry 1996, 43, 381–386. [Google Scholar] [CrossRef]
- He, M.; Ding, N.Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Štefelová, N.; Hron, K.; Doležalová, I.; Tarkowski, P. Preharvest Abiotic Stress Affects the Nutritional Value of Lettuce. Agronomy 2023, 13, 398. [Google Scholar] [CrossRef]
- Pereira, C.; Li, D.; Sinclair, A.J. The alpha-linolenic acid content of green vegetables commonly available in Australia. Int. J. Vitam. Nutr. Res. 2001, 71, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Vasileios, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and antioxidant activity of Cichorium spinosum L. leaves in relation to developmental stage. Food Chem. 2018, 239, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Importance of the ratio omega-6/omega-3 essential fatty acids: Evolutionary aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Haq, A.; Murkovic, M. Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (chicory) leaves. Eur. Food Res. Technol. 2019, 245, 365–374. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhe, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Lester, G.E.; Hallman, G.J.; Perez, J.A. γ-Irradiation Dose: Effects on Baby-Leaf Spinach Ascorbic Acid, Carotenoids, Folate, α- Tocopherol, and Phylloquinone Concentrations. J. Agric. Food. Chem. 2010, 58, 4901–4906. [Google Scholar] [CrossRef]
- Montefusco, A.; Semitaio, G.; Marrese, P.P.; Iurlaro, A.; De Caroli, M.; Piro, G.; Dalessandro, G.; Lenucci, M.S. Antioxidants in varieties of chicory (Cichorium intybus L.) and wild poppy (Papaver rhoeas L.) of Southern Italy. J. Chem. 2015, 2015, 923142. [Google Scholar] [CrossRef]
- Reif, C.; Arrigoni, E.; Schärer, H.; Nyström, L.; Hurrell, R.F. Carotenoid database of commonly eaten Swiss vegetables and their estimated contribution to carotenoid intake. J. Food Comp. Anal. 2013, 29, 64–72. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Chahdoura, H.; Chakroun, Y.; Cámara, M.; Fernandez-Ruiz, V.; Morales, P.; Mosbah, H.; Flamini, G.; Snoussi, M.; Majdoub, H. Wild edible Swiss chard leaves (Beta vulgaris L. var. cicla): Nutritional, phytochemical composition and biological activities. Food Res. Int. 2019, 119, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Gamba, M.; Raguindin, P.F.; Asllanaj, E.; Merlo, F.; Glisic, M.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Bioactive compounds and nutritional composition of Swiss chard (Beta vulgaris L. var. cicla and flavescens): A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of Crisphead ‘Iceberg’ and Romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8, 37–45. [Google Scholar] [CrossRef]
- Mou, B.; Ryder, E.J. Relationship between the nutritional value and the head structure of lettuce. Acta Hortic. 2004, 637, 361–367. [Google Scholar] [CrossRef]
- El-Qudah, J.M. Identification and quantification of major carotenoids in some vegetables. Am. J. Appl. Sci. 2009, 6, 492–497. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Ceccanti, C.; Padilla, Y.G.; López-Galarza, S.; Calatayud, Á.; Conte, G.; Guidi, L. Effect of grafting on the production, physico-chemical characteristics and nutritional quality of fruit from pepper landraces. Antioxidants 2020, 9, 501. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Martínez-Cuenca, M.-R.; Marsal, J.I.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Calatayud, Á. Bioactive compounds and antioxidant capacity of valencian pepper landraces. Molecules 2021, 26, 1031. [Google Scholar] [CrossRef]
- Wagner, K.H.; Kamal-Eldin, A.; Elmadfa, I. Gamma- Tocopherol—An Underestimated Vitamin? Ann. Nutr. Metab. 2004, 48, 169–188. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Comp. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Thompson, M.D.; Cooney, R.V. The potential physiological role of γ-tocopherol in human health: A qualitative review. Nutr. Cancer 2020, 72, 808–825. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N. Z. J. Crop Hortic. Sci. 2016, 45, 119–129. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.S.; Dikshit, H.K.; Mishra, G.P.; Tontang, M.T.; Meena, N.L.; Kumar, R.R.; Ramesh, S.V.; Narwal, S.; Aski, M.; Thimmegowda, V.; et al. Evaluation of Growth Conditions, Antioxidant Potential, and Sensory Attributes of Six Diverse Microgreens Species. Agriculture 2023, 13, 676. [Google Scholar] [CrossRef]
- Corrado, G.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Zarrelli, A.; Giannini, P.; Ritieni, A.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Productive and Morphometric Traits, Mineral Composition and Secondary Metabolome Components of Borage and Purslane as Underutilized Species for Microgreens Production. Horticulturae 2021, 7, 211. [Google Scholar] [CrossRef]
- Tan, L.; Nuffer, H.; Feng, J.; Kwan, S.H.; Chen, H.; Tong, X.; Kong, L. Antioxidant properties and sensory evaluation of microgreens from commercial and local farms. Food Sci. Hum. Wellness 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
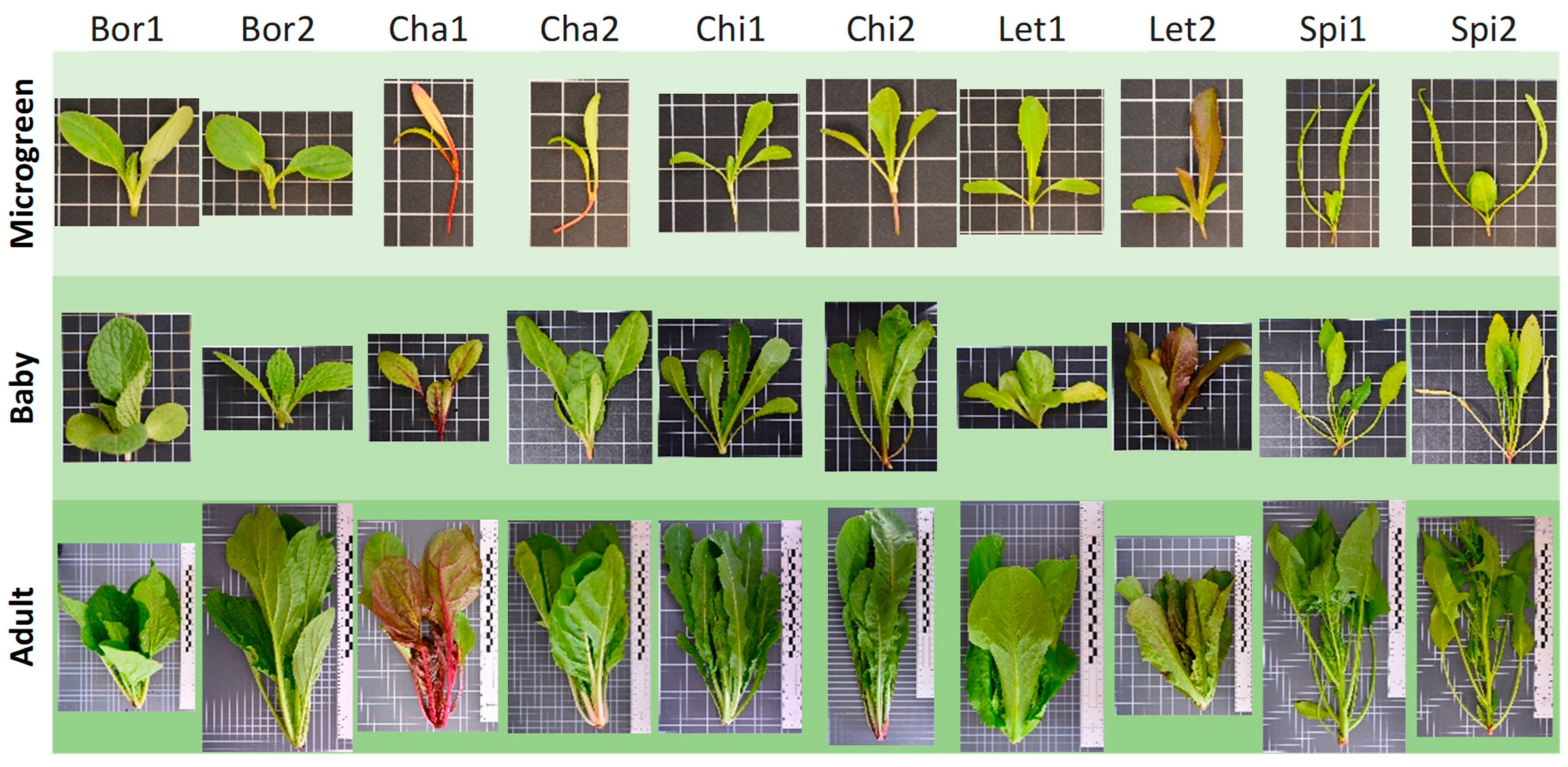
| Acronym | Species | Crop | Genebank Code | Plant Description (Differential Traits) |
|---|---|---|---|---|
| Bor1 | Borago officinalis L. | Borage | BGHZ3644 | Blue flowers |
| Bor2 | Borago officinalis L. | Borage | BGHZ5705 | White flowers |
| Cha1 | Beta vulgaris L. | Chard | BGHZ3760 | Green and red leaves with red leaf veins and petioles |
| Cha2 | Beta vulgaris L. | Chard | BGHZ7028 | Green leaves |
| Chi1 | Cichorium intybus L. | Chicory | BGHZ6528 | Green leaves |
| Chi2 | Cichorium intybus L. | Chicory | BGHZ6529 | Green leaves and basal anthocyanin pigmentation |
| Let1 | Lactuca sativa L. | Lettuce | BGHZ0366 | Green leaves |
| Let2 | Lactuca sativa L. | Lettuce | BGHZ7486 | Dark green and red leaves |
| Spi1 | Spinacia oleracea L. | Spinach | BGHZ6823 | Spineless seeds |
| Spi2 | Spinacia oleracea L. | Spinach | BGHZ0540 | Spiny seeds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallor, C.; Bertolín, J.R.; Paracuellos, P.; Juan, T. Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development. Foods 2023, 12, 3173. https://doi.org/10.3390/foods12173173
Mallor C, Bertolín JR, Paracuellos P, Juan T. Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development. Foods. 2023; 12(17):3173. https://doi.org/10.3390/foods12173173
Chicago/Turabian StyleMallor, Cristina, Juan Ramón Bertolín, Pablo Paracuellos, and Teresa Juan. 2023. "Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development" Foods 12, no. 17: 3173. https://doi.org/10.3390/foods12173173
APA StyleMallor, C., Bertolín, J. R., Paracuellos, P., & Juan, T. (2023). Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development. Foods, 12(17), 3173. https://doi.org/10.3390/foods12173173






