A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods
Abstract
:1. Introduction
2. Functions of Lipid Species
3. Lipidomics
4. Lipidomics Workflow
4.1. Induction of Adduct Formation
4.2. Mass Spectrometry Analysis
4.2.1. HPLC-MS and Shotgun MS
4.2.2. Scanning Modes in MS Analysis
4.3. Bioinformatics and Statistical Analysis
5. Validation of Lipidomics Analysis
6. Mass Spectrometry-Based Imaging Techniques
7. Lipidomics in Plant-Based Foods
7.1. TAGs
7.2. PLs
8. Lipidomics in Animal-Based Foods
8.1. TAGs
8.2. PLs
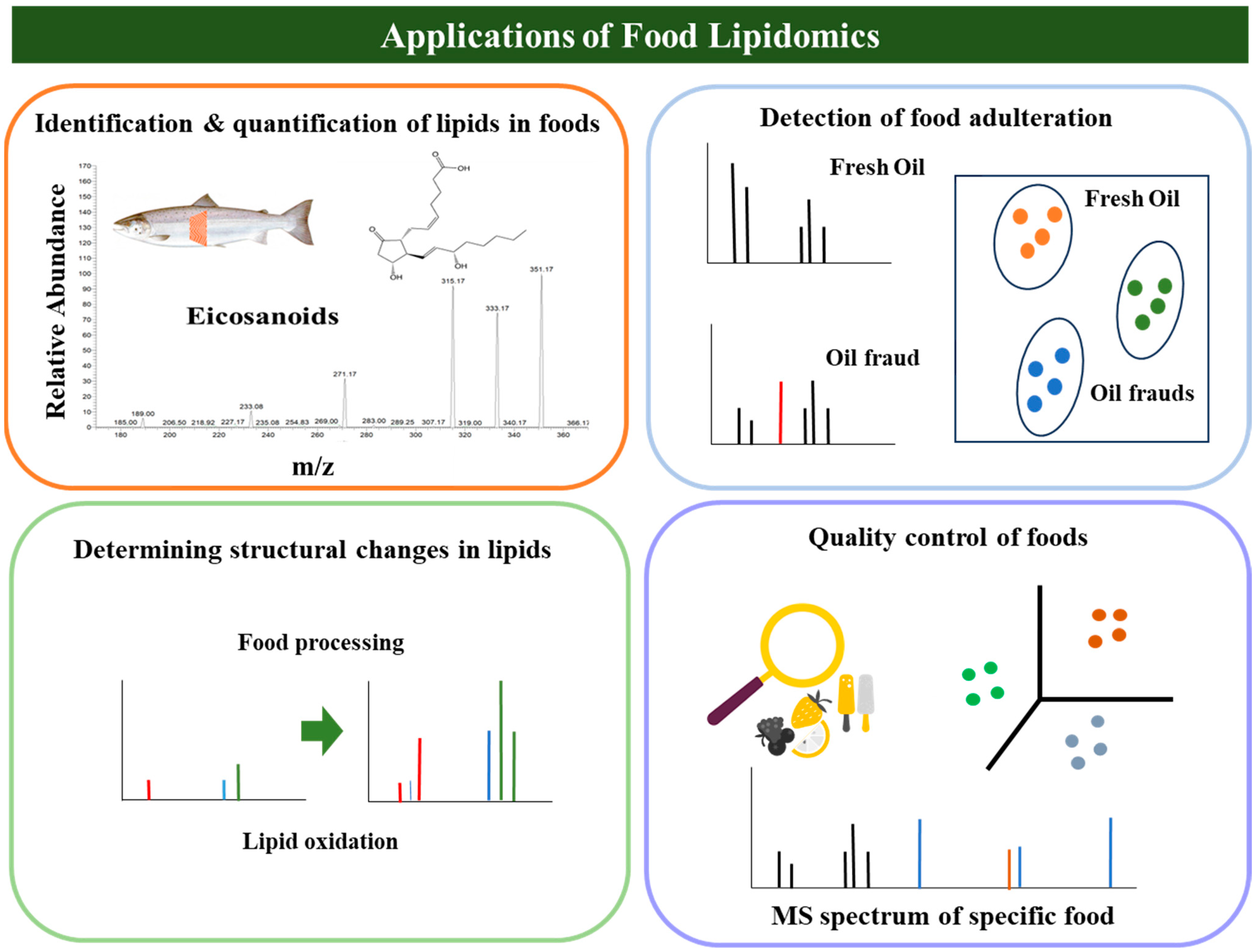
9. Use of Mass Spectrometry-Based Lipidomics in Food Processing
10. Application of Lipidomics in Detecting Food Adulteration
11. Assessment of Lipid Oxidation Using Lipidomics
12. Recent Important Advances in MS-Based Lipidomics
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wei, F.; Dong, X.Y.; Xiang, J.Q.; Quek, S.Y.; Wang, X. Lipidomics in food science. Curr. Opin. Food Sci. 2017, 16, 80–87. [Google Scholar] [CrossRef]
- Yeo, J.D.; Parrish, C.C. Mass spectrometry-based lipidomics in the characterization of individual triacylglycerol (TAG) and phospholipid (PL) species from marine sources and their beneficial health effects. Rev. Fish. Sci. Aquac. 2022, 30, 81–100. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodríguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. TrAC Trends Anal. Chem. 2009, 28, 263–278. [Google Scholar] [CrossRef]
- Navas-Iglesias, N.; Carrasco-Pancorbo, A.; Cuadros-Rodríguez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. TrAC Trends Anal. Chem. 2009, 28, 393–403. [Google Scholar] [CrossRef]
- Lottenberg, A.M.; Afonso, M.S.; da Lavrador, M.S.; Machado, R.M.; Nakandakare, E.R. The role of dietary fatty acids in the pathology of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 1027–1040. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Jiang, S.; Hu, W.; Li, T.; Di, S.; Wang, D.; Yang, Y. A global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Prog. Lipid Res. 2017, 66, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Fainberg, H.P.; Sebert, S.; Budge, H.; Symonds, M.E. Maternal health and eating habits: Metabolic consequences and impact on child health. Trends Mol. Med. 2015, 21, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Białek, M.; Białek, A.; Czauderna, M. Conjugated Linoleic Acid Isomers Affect Profile of Lipid Compounds and Intensity of Their Oxidation in Heart of Rats with Chemically Induced Mammary Tumors—Preliminary Study. Nutrients 2019, 11, 2032. [Google Scholar] [CrossRef]
- Zagrodzki, A.B.A.T.P. Conjugated Linoleic Acids in Diet of Female Rats Inhibit the Breast Cancer Formation in Their Offspring. J. Food Nutr. Res. 2014, 53, 39–50. [Google Scholar]
- Białek, A.; Tokarz, A.; Zagrodzki, P. Conjugated linoleic acids decrease the breast cancer risk in DMBA-treated rats. Drug Res. 2015, 72, 1163–1176. [Google Scholar]
- Li, Y.-J.; Kanaji, N.; Wang, X.-Q.; Sato, T.; Nakanishi, M.; Kim, M.; Michalski, J.; Nelson, A.J.; Farid, M.; Basma, H.; et al. Prostaglandin E2 switches from a stimulator to an inhibitor of cell migration after epithelial-to-mesenchymal transition. Prostaglandins Other Lipid Mediat. 2015, 116–117, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R. Advances in lipid analysis/lipidomics—Analyses of phospholipids by recent application of mass spectrometry. In Handbook of Neurochemistry and Molecular Neurobiology; Tettamanti, G., Goracci, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–20. [Google Scholar]
- Godzien, J.; Ciborowski, M.; Martínez-Alcázar, M.P.; Samczuk, P.; Kretowski, A.; Barbas, C. Rapid and reliable identification of phospholipids for untargeted metabolomics with LC-ESI-QTOF-MS/MS. J. Proteome Res. 2015, 14, 3204–3216. [Google Scholar] [CrossRef]
- Pi, J.; Wu, X.; Yang, S.; Zeng, P.; Feng, Y. Rapid identification of erythrocyte phospholipids in Sprague–Dawley rats by ultra high performance liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2015, 38, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wu, T.; Guo, H.; Xu, J.; Chen, J.; Tao, N.; Wang, X.; Zhong, J. Lipid profile migration during the tilapia muscle steaming process revealed by a transactional analysis between MS data and lipidomics data. NPJ Sci. Food 2021, 5, 30. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Chingin, K.; Xiong, J.; Fang, X.; Chen, H. Ambient mass spectrometry for food science and industry. Tr. Anal. Chem. 2018, 107, 99–115. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. BBA-Mol. Cell Biol. Lipids 2000, 1486, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Violante, S.; IJlst, L.; te Brinke, H.; Koster, J.; de Almeida, I.T.; Wanders, R.J.A.; Ventura, F.V.; Houten, S.M. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. BBA-Mol. Cell Biol. Lipids 2013, 1831, 1467–1474. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Mass spectrometry: From proteomics to metabolomics and lipidomics. Chem. Soc. Rev. 2009, 38, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Kofeler, H.C.; Fauland, A.; Rechberger, G.N.; Trotzmuller, M. Mass spectrometry based lipidomics: An overview of technological platforms. Metabolites 2012, 2, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.K.; Ewing, A.G.; Winograd, N. Single-cell lipidomics: Characterizing and imaging lipids on the surface of individual Aplysia californica neurons with cluster secondary ion mass spectrometry. Anal. Chem. 2013, 85, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Caprioli, R.M. Matrix precoated targets for direct lipid analysis and imaging of tissue. Anal. Chem. 2013, 85, 2907–2912. [Google Scholar] [CrossRef]
- Kasuga, K.; Suga, T.; Mano, N. Bioanalytical insights into mediator lipidomics. J. Pharm. Biomed. Anal. 2015, 113, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Godzien, J.; Ciborowski, M.; Whiley, L.; Legido-Quigley, C.; Ruperez, F.J.; Barbas, C. In-vial dual extraction liquid chromatography coupled to mass spectrometry applied to streptozotocin-treated diabetic rats. Tips and pitfalls of the method. J. Chromatogr. A 2013, 1304, 52–60. [Google Scholar] [CrossRef]
- Whiley, L.; Godzien, J.; Ruperez, F.J.; Legido-Quigley, C.; Barbas, C. In-vial dual extraction for direct LC-MS analysis of plasma for comprehensive and highly reproducible metabolic fingerprinting. Anal. Chem. 2012, 84, 5992–5999. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Z.; Karnes, H. Sensitivity enhancement in liquid chromatography/atmospheric pressure ionization mass spectrometry using derivatization and mobile phase additives. J. Chromatogr. B. 2005, 825, 98–110. [Google Scholar] [CrossRef]
- Mathis, J.A.; McCord, B.R. The analysis of high explosives by liquid chromatography/electrospray ionization mass spectrometry: Multiplexed detection of negative ion adducts. Rapid Commun. Mass Spectrom. 2005, 19, 99–104. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K. Adduct formation in ESI/MS by mobile phase additives. J. Am. Soc. Mass Spectrom. 2017, 28, 887–894. [Google Scholar] [CrossRef]
- Yeo, J.D.; Parrish, C.C. Evaluation of triacylglycerol (TAG) profiles and their contents in salmon muscle tissue using ESI-MS/MS spectrometry with multiple neutral loss scans. Food Chem. 2020, 324, 126816. [Google Scholar] [CrossRef]
- Koivusalo, M.; Haimi, P.; Heikinheimo, L.; Kostiainen, R.; Somerharju, P. Quantitative determination of phospholipid compositions by ESI-MS: Effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J. Lipid Res. 2001, 42, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Klink, F.E. Liquid Chromatography/Mass Spectrometry. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Wu, Z.; Shon, J.C.; Liu, K.H. Mass spectrometry-based lipidomics and its application to biomedical research. J. Lifestyle Med. 2014, 4, 17–33. [Google Scholar] [CrossRef]
- Kim, K.M.; Jung, B.H.; Lho, D.S.; Chung, W.Y.; Paeng, K.J.; Chung, B.C. Alteration of urinary profiles of endogenous steroids and polyunsaturated fatty acids in thyroid cancer. Cancer Lett. 2003, 202, 173–179. [Google Scholar] [CrossRef]
- Picotti, P.; Aebersold, R. Selected reaction monitoring–based proteomics: Workflows, potential, pitfalls and future directions. Nat. Methods 2012, 9, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, N.; Zhao, Y.; Wang, X.; Wang, M. Comparison of the fatty acid and triglyceride profiles of Big eye tuna (Thunnus obesus), Atlantic salmon (Salmo salar) and Bighead carp (Aristichthysnobilis) heads. Molecules 2019, 24, 3983. [Google Scholar] [CrossRef]
- Gang, K.Q.; Zhou, D.Y.; Lu, T.; Liu, Z.Y.; Zhao, Q.; Xie, H.K.; Song, L.; Shahidi, F. Direct infusion mass spectrometric identification of molecular species of glycerophospholipid in three species of edible whelk from Yellow Sea. Food Chem. 2018, 245, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, A.; Ament, Z.; West, J.A.; Stanley, E.; Griffin, J.L. Integration of metabolomics, lipidomics and clinical data using a machine learning method. BMC Bioinform. 2016, 17, 440. [Google Scholar] [CrossRef]
- Kind, T.; Okazaki, Y.; Saito, K.; Fiehn, O. LipidBlast templates as flexible tools for creating new in-silico tandem mass spectral libraries. Anal. Chem. 2014, 86, 11024–11027. [Google Scholar] [CrossRef]
- Herzog, R.; Schwudke, D.; Shevchenko, A. LipidXplorer: Software for quantitative shotgun lipidomics compatible with multiple mass spectrometry platforms. Curr. Protoc. Bioinform. 2013, 43, 14.12.1–14.12.30. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.E.; Lillis, P.G.; Lorenson, T.D.; Zumberge, J.E. Geochemically distinct oil families in the onshore and offshore Santa Maria basins, California. Am. Assoc. Pet. Geol. Bull. 2019, 103, 243–271. [Google Scholar] [CrossRef]
- Parchem, K.; Sasson, S.; Ferreri, C.; Bartoszek, A. Qualitative analysis of phospholipids and their oxidised derivatives—Used techniques and examples of their applications related to lipidomic research and food analysis. Free Radic. Res. 2019, 53, 1068–1100. [Google Scholar] [CrossRef]
- Gorrochategui, E.; Jaumot, J.; Tauler, R. ROIMCR: A powerful analysis strategy for LC-MS metabolomic datasets. BMC Bioinform. 2019, 20, 256. [Google Scholar] [CrossRef] [PubMed]
- Hartler, J.; Tharakan, R.; Köfeler, H.C.; Graham, D.R.; Thallinger, G.G. Bioinformatics tools and challenges in structural analysis of lipidomics MS/MS data. Brief. Bioinform. 2013, 14, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Checa, A.; Bedia, C.; Jaumot, J. Lipidomic data analysis: Tutorial, practical guidelines and applications. Anal. Chim. Acta 2015, 885, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ruckebusch, C.; Blanchet, L. Multivariate curve resolution: A review of advanced and tailored applications and challenges. Anal. Chim. Acta 2013, 765, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Smilowitz, J.T.; Fiehn, O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal. Chem. 2017, 89, 12360–12368. [Google Scholar] [CrossRef]
- Momin, A.A.; Park, H.; Portz, B.J.; Haynes, C.A.; Shaner, R.L.; Kelly, S.L.; Jordan, I.K.; Merrill, A.H., Jr. A method for visualization of ‘omic’ datasets for sphingolipid metabolism to predict potentially interesting differences. J. Lipid Res. 2011, 52, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Hornemann, T.; Penno, A.; Rütti, M.F.; Ernst, D.; Kivrak-Pfiffner, F.; Rohrer, L.; von Eckardstein, A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 2009, 284, 26322–26330. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Gupta, S.D.; Gable, K.; Niranjanakumari, S.; Moitra, P.; Eichler, F.; Brown, R.H., Jr.; Harmon, J.M.; Dunn, T.M. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. USA 2009, 106, 8186–8191. [Google Scholar] [CrossRef]
- Gode, D.; Volmer, D.A. Lipid imaging by mass spectrometry—A review. Analyst 2013, 138, 1289–1315. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M.; Pitt, A.R. Oxidative lipidomics coming of age: Advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox Signal 2015, 22, 1646–1666. [Google Scholar] [CrossRef]
- Shimizu, Y.; Satou, M.; Hayashi, K.; Nakamura, Y.; Fujimaki, M.; Horibata, Y.; Ando, H.; Watanabe, T.; Shiobara, T.; Chibana, K.; et al. Matrix-assisted laser desorption/ionization imaging mass spectrometry reveals changes of phospholipid distribution in induced pluripotent stem cell colony differentiation. Anal. Bioanal. Chem. 2017, 409, 1007–1016. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Zaima, N. Application of mass spectrometry imaging for visualizing food components. Foods 2020, 9, 575. [Google Scholar] [CrossRef]
- Goto-Inoue, N.; Sato, T.; Morisasa, M.; Igarashi, Y.; Mori, T. Characterization of metabolite compositions in wild and farmed red sea bream (Pagrus major) using mass spectrometry imaging. J. Agric. Food Chem. 2019, 67, 7197–7203. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Furukawa, T.; Takeda, S.; Hatta, H.; Zaima, N. Unique distribution of diacyl-, alkylacyl-, and alkenylacyl-phosphatidylcholine species visualized in pork chop tissues by matrix-assisted laser desorption/ionization-mass spectrometry imaging. Foods 2020, 9, 205. [Google Scholar] [CrossRef]
- Enomoto, H.; Takeda, S.; Hatta, H.; Zaima, N. Tissue-specific distribution of sphingomyelin species in pork chop revealed by matrix-assisted laser desorption/ionization-imaging mass spectrometry. J. Food Sci. 2019, 84, 1758–1763. [Google Scholar] [CrossRef]
- Thazar-Poulot, N.; Miquel, M.; Fobis-Loisy, I.; Gaude, T. Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies. Proc. Natl. Acad. Sci. USA 2015, 112, 4158–4163. [Google Scholar] [CrossRef]
- Yang, Y.; Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotechnol. 2018, 49, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Zhu, D.; Pang, X.; Liu, Y.; Frew, R.; Chen, G. Lipidomics profiling of goat milk, soymilk and bovine milk by UPLC-Q-exactive orbitrap mass spectrometry. Food Chem. 2017, 224, 302–309. [Google Scholar] [CrossRef]
- Li, M.; Butka, E.; Wang, X. Comprehensive quantification of triacylglycerols in soybean seeds by electrospray ionization mass spectrometry with multiple neutral loss scans. Sci. Rep. 2014, 4, 6581. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M. Characterization of triacylglycerol enantiomers using chiral HPLC/APCI-MS and synthesis of enantiomeric triacylglycerols. Anal. Chem. 2013, 85, 1852–1859. [Google Scholar] [CrossRef]
- Dong, X.Y.; Zhong, J.; Wei, F.; Lv, X.; Wu, L.; Lei, Y.; Liao, B.S.; Quek, S.Y.; Chen, H. Triacylglycerol composition profiling and comparison of high-oleic and normal peanut oils. J. Am. Oil Chem. Soc. 2015, 92, 233–242. [Google Scholar] [CrossRef]
- Liu, S.L.; Dong, X.Y.; Wei, F.; Wang, X.; Lv, X.; Zhong, J.; Wu, L.; Quek, S.Y.; Chen, H. Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content. Ultrason. Sonochem. 2015, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cerrato, A.; Aita, S.E.; Montone, C.M.; Piovesana, S.; Laganà, A.; Cavaliere, C. Degradation of the polar lipid and fatty acid molecular species in extra virgin olive oil during storage based on shotgun lipidomics. J. Chromatogr A. 2021, 1639, 461881. [Google Scholar] [CrossRef]
- Shen, Q.; Dong, W.; Yang, M.; Li, L.; Cheung, H.Y.; Zhang, Z. Lipidomic fingerprint of almonds (Prunus dulcis L. cv Nonpareil) using tio2 nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS and its potential in geographical origin verification. J. Agric. Food Chem. 2013, 61, 7739–7748. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Melo, T.; Barros, M.P.; Domingues, M.R.M.; Domingues, P. Lipidomic profiling of the olive (Olea europaea L.) fruit towards its valorisation as a functional food: In-depth identification of triacylglycerols and polar lipids in Portuguese olives. Molecules 2019, 24, 2555. [Google Scholar] [CrossRef]
- Anagbogu, C.F.; Zhou, J.; Olasupo, F.O.; Nitsa, M.B.; Beckles, D.M. Lipidomic and metabolomic profiles of Coffea canephora L. beans cultivated in Southwestern Nigeria. PLoS ONE 2021, 16, e0234758. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, L.; Zhang, M.; Liu, J.; Marchioni, E. Exploration of natural phosphatidylcholine sources from six beans by UHPLC-Q-HRMS. J. Food Sci. 2020, 85, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Kehelpannala, C.; Rupasinghe, T.; Pasha, A.; Esteban, E.; Hennessy, T.; Bradley, D.; Ebert, B.; Provart, N.J.; Roessner, U. An Arabidopsis lipid map reveals differences between tissues and dynamic changes throughout development. Plant J. 2021, 107, 287–302. [Google Scholar] [CrossRef]
- Bowyer, J.N.; Qin, J.G.; Stone, D.A.J. Protein, lipid and energy requirements of cultured marine fish in cold, temperate and warm water. Rev. Aquac. 2013, 5, 10–32. [Google Scholar] [CrossRef]
- Rocchetti, G.; Vitali, M.; Zappaterra, M.; Righetti, L.; Sirri, R.; Lucini, L.; Dall’Asta, C.; Davoli, R.; Galaverna, G. A molecular insight into the lipid changes of pig Longissimus thoracis muscle following dietary supplementation with functional ingredients. PLoS ONE 2022, 17, e0264953. [Google Scholar] [CrossRef] [PubMed]
- Robson, K.; Birse, N.; Chevallier, O.; Elliott, C. Metabolomic profiling to detect different forms of beef fraud using rapid evaporative ionisation mass spectrometry (REIMS). NPJ Sci. Food. 2022, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Micalizzi, G.; Oteri, M.; Rigano, F.; Sciarrone, D.; Dugo, P.; Mondello, L. Comprehensive lipid profiling in the Mediterranean mussel (Mytilus galloprovincialis) using hyphenated and multidimensional chromatography techniques coupled to mass spectrometry detection. Anal. Bioanal. Chem. 2018, 410, 3297–3313. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Zhang, Y.; Shen, Y.; Su, H.; Jin, J.; Jin, Q.; Wang, X. Characterization of positional distribution of fatty acids and triacylglycerol molecular compositions of marine fish oils rich in omega-3 polyunsaturated fatty acids. Biomed. Res. Int. 2018, 2018, 3529682. [Google Scholar] [CrossRef]
- Han, X.; Gross, R.W. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal. Biochem. 2001, 295, 88–100. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Physical properties and functional roles of lipids in membranes. In New Comprehensive Biochemistry; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 1–41. [Google Scholar]
- Haq, M.; Chun, B.S. Characterization of phospholipids extracted from Atlantic salmon by-product using supercritical CO2 with ethanol as co-solvent. J. Clean. Prod. 2018, 178, 186–195. [Google Scholar] [CrossRef]
- Colombo, S.M.; Foroutani, M.B.; Parrish, C.C. Fats and oils in aquafeed formulations. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Shen, Q.; Song, G.; Wang, H.; Zhang, Y.; Cui, Y.; Xie, H.; Xue, J.; Wang, H. Isolation and lipidomics characterization of fatty acids and phospholipids in shrimp waste through GC/FID and HILIC-QTrap/MS. J. Food Compos. Anal. 2021, 95, 103668. [Google Scholar] [CrossRef]
- Song, G.; Zhang, M.; Zhang, Y.; Wang, H.; Li, S.; Dai, Z.; Shen, Q. In situ method for real-time discriminating salmon and rainbow trout without sample preparation using iknife and rapid evaporative ionization mass spectrometry-based lipidomics. J. Agric. Food Chem. 2019, 67, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Pacetti, D.; Lucci, P.; Frega, N.G. Characterization of phospholipid molecular species in the edible parts of bony fish and shellfish. J. Agric. Food Chem. 2012, 60, 3234–3245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Dong, L.; Chang, J.; Wang, H.; Shen, Q. Lipidomics screening of polyunsaturated phospholipid molecular species in crab (Portunus trituberculatus) muscular tissue: A nontarget approach by HILIC-MS. Eur. J. Lipid Sci. Technol. 2022, 124, 2100097. [Google Scholar] [CrossRef]
- Li, X.; He, Q.; Hou, H.; Zhang, S.; Zhang, X.; Zhang, Y.; Wang, X.; Han, L.; Liu, K. Targeted lipidomics profiling of marine phospholipids from different resources by UPLC-Q-Exactive Orbitrap/MS approach. J. Chromatogr. B 2018, 1096, 107–112. [Google Scholar] [CrossRef]
- Jia, W.; Li, R.; Wu, X.; Liu, S.; Shi, L. UHPLC-Q-Orbitrap HRMS-based quantitative lipidomics reveals the chemical changes of phospholipids during thermal processing methods of Tan sheep meat. Food Chem. 2021, 360, 130153. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Al-Dalali, S.; Zhou, H.; Wang, Z.; Xu, B. Influence of mixture of spices on phospholipid molecules during water-boiled salted duck processing based on shotgun lipidomics. Food Res. Int. 2021, 149, 110651. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Moreira, A.S.P.; Ferreira, P.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. Food grade extraction of Chlorella vulgaris polar lipids: A comparative lipidomic study. Food Chem. 2022, 375, 131685. [Google Scholar] [CrossRef]
- Shi, C.; Guo, H.; Wu, T.; Tao, N.; Wang, X.; Zhong, J. Effect of three types of thermal processing methods on the lipidomics profile of tilapia fillet by UPLC-Q-Extractive Orbitrap mass spectrometry. Food Chem. 2019, 298, 125029. [Google Scholar] [CrossRef]
- Napolitano, A.; Cerulli, A.; Pizza, C.; Piacente, S. Multi-class polar lipid profiling in fresh and roasted hazelnut (Corylus avellana cultivar “Tonda di Giffoni”) by LC-ESI/LTQOrbitrap/MS/MSn. Food Chem. 2018, 269, 125–135. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, P.; Liu, B.; Meng, X. Effect of traditional Chinese cooking methods on fatty acid profiles of vegetable oils. Food Chem. 2017, 233, 77–84. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, F.; Xu, S.; Wu, B.; Zheng, C.; Lv, X.; Wu, Z.; Chen, H.; Huang, F. Profiling and quantification of lipids in cold-pressed rapeseed oils based on direct infusion electrospray ionization tandem mass spectrometry. Food Chem. 2019, 285, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wei, F.; Xu, S.; Xie, Y.; Lv, X.; Chen, H.; Huang, F. Mass spectrometry-based lipidomics as a powerful platform in foodomics research. Trends Food Sci. Technol. 2021, 107, 358–376. [Google Scholar] [CrossRef]
- Criado-Navarro, I.; Mena-Bravo, A.; Calderón-Santiago, M.; Priego-Capote, F. Determination of glycerophospholipids in vegetable edible oils: Proof of concept to discriminate olive oil categories. Food Chem. 2019, 299, 125136. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.K.; Hollywood, K.A.; Rattray, N.J.; Ward, H.; Trivedi, D.K.; Greenwood, J.; Ellis, D.I.; Goodacre, R. Meat, the metabolites: An integrated metabolite profiling and lipidomics approach for the detection of the adulteration of beef with pork. Analyst 2016, 141, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Long, N.P.; Mo, C.; Dong, Z.; Cui, L.; Kim, G.; Kwon, S.W. Combination of mass spectrometry-based targeted lipidomics and supervised machine learning algorithms in detecting adulterated admixtures of white rice. Food Res. Int. 2017, 100, 814–821. [Google Scholar] [CrossRef]
- Nurseitova, M.A.; Amutova, F.B.; Zhakupbekova, A.A.; Omarova, A.S.; Kondybayev, A.B.; Bayandy, G.A.; Akhmetsadykov, N.N.; Faye, B.; Konuspayeva, G.S. Comparative study of fatty acid and sterol profiles for the investigation of potential milk fat adulteration. J. Dairy Sci. 2019, 102, 7723–7733. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Food authentication: Truffle species classification by non-targeted lipidomics analyses using mass spectrometry assisted by ion mobility separation. Mol. Omics 2022, 18, 616. [Google Scholar] [CrossRef]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhao, L.; Zhang, Y.; Fang, X. Lipidomics reveals the molecular mechanisms underlying the changes in lipid profiles and lipid oxidation in rape bee pollen dried by different methods. Food Res. Int. 2022, 162, 112104. [Google Scholar] [CrossRef]
- Ito, J.; Shimizu, N.; Kobayashi, E.; Hanzawa, Y.; Otoki, Y.; Kato, S.; Hirokawa, T.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. A novel chiral stationary phase LC-MS/MS method to evaluate oxidation mechanisms of edible oils. Sci. Rep. 2017, 7, 10026. [Google Scholar] [CrossRef]
- Grüneis, V.; Fruehwirth, S.; Zehl, M.; Ortner, J.; Schamann, A.; König, J.; Pignitter, M. Simultaneous analysis of epoxidized and hydroperoxidized triacylglycerols in canola oil and margarine by LC-MS. J. Agric. Food Chem. 2019, 67, 10174–10184. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Lee, J.C. LC-MS/MS analysis of lipid oxidation products in blood and tissue samples. Methods Mol. Biol. 2018, 1730, 83–92. [Google Scholar] [PubMed]
- Hu, C.; Wang, M.; Han, X. Shotgun lipidomics in substantiating lipid peroxidation in redox biology: Methods and applications. Redox Biol. 2017, 12, 946–955. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Zhang, D.; Liu, X.; Cao, W.; Chen, Q.; Zheng, O.; Xia, Y. A lipidomic workflow capable of resolving sn- and C=C location isomers of phosphatidylcholines. Chem. Sci. 2019, 10, 10740. [Google Scholar] [CrossRef] [PubMed]
- Kalili, K.M.; de Villiers, A. Systematic optimisation and evaluation of on-line, off-line and stop-flow comprehensive hydrophilic interaction chromatography×reversed phase liquid chromatographic analysis of procyanidins, Part I: Theoretical considerations. J. Chromatogr. A 2013, 1289, 58–68. [Google Scholar] [CrossRef]
- Guiochon, G.; Marchetti, N.; Mriziq, K.; Shalliker, R.A. Implementations of two-dimensional liquid chromatography. J. Chromatogr. A 2008, 1189, 109–168. [Google Scholar] [CrossRef]
- Zhou, H.; Xiang, H.; Cai, J.; Wang, Y.; Zhang, M.; Han, Y.; Zhang, Y. Comparison of a point-of-care testing with enzyme-multiplied immunoassay technique and liquid chromatography combined with tandem mass spectrometry methods for therapeutic drug monitoring of mycophenolic acid: A preliminary study. Ther. Drug Monit. 2021, 43, 630–636. [Google Scholar] [CrossRef]

| Sample | Lipid Extraction | Lipidomics Approach | Main Finding | Reference |
|---|---|---|---|---|
| Triacylglycerols in Plant-Based Foods | ||||
| Soy milk | Folch and Bligh method | Ultra-performance liquid chromatography (UPLC)–Q-Exactive Orbitrap mass spectrometry (MS) | 14 lipid molecules were selected as the markers for detecting milk fraud | [64] |
| Soybean oil | Bligh and Dyer method | Shotgun MS-based lipidomics | Identification and quantification of 93 TAG molecules, including 18:2–18:2–18:2 (LLL), 18:2–18:2–18:3 (LLLn), 18:1–18:2–18:2 (OLL), 16:0–18:2–18:2 (OLLn), 16:0–18:1–18:2 (POL), 18:1–18:1–18:2 (OOL), and 18:0–18:2–18:2 (SLL)) | [65] |
| Hazelnut oil | Chloroform/methanol (2:1, v/v) | Chiral HPLC–atmospheric pressure chemical ionization (APCI)–MS coupled with two cellulose tris-(3,5-dimethylphenylcarbamate) columns in a series | Unsaturated fatty acids localized at sn-2 position of TAG structures in hazelnut oil | [66] |
| High-oleic and normal peanut oils | - | APCI-MS | Differences in TAG species between high-oleic and normal peanut oils (e.g., 18:1–18:1–18:1, 16:0–18:1–18:2, and 18:1–16:0–18:1) | [67] |
| Structured lipids | - | APCI-MS/MS | The level of 1,3-di-oleoyl-2-palmitoylglycerol was monitored in the synthesis of structured lipids | [68] |
| Phospholipids in plant-based foods | ||||
| Extra-virgin olive oil | n-Hexane and ethanol/water (80:20, v/v). | Exactive hybrid quadrupole–Orbitrap MS | Identification of 19 phospholipids | [69] |
| Almond | Bligh and Dyer method | Matrix-assisted laser desorption ionization–time-of-flight MS (MALDI-TOF/MS) | Identification of more than 60 phospholipid molecules Proposed that the ratio of m/z 833.6 to 835.6 and m/z 821.6 could be an efficient marker to distinguish almonds from different geographical origins | [70] |
| Olive (Olea europaea L.) | Bligh and Dyer method | High-performance liquid chromatography (HPLC)–electrospray ionization (ESI)-MS/MS | A total of 107 polar lipids belonging to 11 lipid classes, including phospholipids, glyceroglycolipids, glycosphingolipids, and betaine lipids in O. europaea | [71] |
| Coffea canephora L. beans | Methyl-tert-butyl ether (MTBE) | UPLC-MS | PE (34:2) is the predominant phospholipid species in C. canephora beans | [72] |
| Beans (soybean, red kidney bean, red bean, white kidney bean, chickpea, and black beans) | Chloroform/methanol (2:1, v/v) | UHPLC-Q-Exactive Orbitrap/MS | 49 PC species were identified, 16:0_18:1_PC was the major PC species in chickpea | [73] |
| Arabidopsis thaliana | Butanol/methanol (1:1) with 10 mM ammonium formate | ESI/APCI-QqTOF- mass spectrometer | The alteration of lipid species in the model plant A. thaliana at several different developmental stages was monitored, and more than 200 lipid species were identified. | [74] |
| Sample | Lipid Extraction | Lipidomics Approach | Main Finding | Reference |
|---|---|---|---|---|
| Triacylglycerols from Animal-Based Foods | ||||
| Pork | Dichloromethane/methanol (50:50, v/v) | UHPLC-QTOF | 8:0_8:0_34:6 is the major TAG molecule in pork 32 TAG species were altered by changes in diet in pigs | [76] |
| Beef | - | Rapid evaporative ionization MS (REIMS)-based analysis | REIMS-based analysis showed a high accuracy in detecting meat frauds | [77] |
| Salmon muscle | Chloroform: methanol (2:1) | Shotgun-based lipidomics using ESI-MS/MS | Identification of 98 TAG molecules in salmon muscle tissue and their quantitative analysis using multiple neutral-loss scans | [32] |
| Bigeye tuna, bighead carp, and Atlantic salmon heads | Modified Folch method | Q Exactive MS system coupled with UHPLC | Identification of 87–146 TAG species in the tested marine sources | [38] |
| Mediterranean mussel (Mytilus galloprovincialis) | Bligh and Dyer method | ESI-MS/MS | Identification of 34 TAG molecules using product ion scanning mode | [78] |
| Anchovy and tuna oils | - | HPLC-APCI/MS | Identification and quantification of 23 TAG molecules Identification of DHA- and EPA-containing TAGs (e.g., 16:0_18:1_20:5, 18:1_22:6_22:6, and 16:0_18:0_22:6) | [79] |
| Phospholipids from animal-based foods | ||||
| Shrimp waste | Bligh and Dyer and Folch MTBE | GC-FID (HILIC)-QTrap-MS | Identification of 14 PCs, 14 PEs, 9 PIs, and 9 PSs | [84] |
| Salmon muscle | Folch method | Shotgun lipidomics using Triple Quadrupole Mass Spectrometer | Identification of 12 PCs, 14 Pes, 5 PSs, and 2 PIs Finding major species, including 16:0–22:6 PC, 18:1–20:5 PE, 18:2–20:4 PE, and 22:5–22:6 PS | [2] |
| Atlantic salmon king salmon rainbow trout | - | Quadrupole TOF-MS | Determination of predominant phospholipids, such as 18:0_20:5_PE 18:1_20:5_PE, 18:0_20:5_PE, 18:0_22:6_PE, and 18:0_22:6_ PI | [85] |
| Sea fish, freshwater fish, and shellfish | Bligh and Dyer method | HPLC-ESI-MS/ MS | Identification of 18 PCs, 24 PEs, 15 PSs, and 8 PIs | [86] |
| Crabs | Bligh and Dyer method | Hydrophilic interaction chromatography-MS (HILIC-MS) | Identification of 21 PCs, 9 PEs, 2 PSs, and 6 PIs, as well as EPA- and DHA-containing PLs, including 18:0_20:5_PC, 18:1_20:5_PE 16:0_22:6_PE, and 18:0_20:5_PI | [89] |
| Shrimp head, codfish roe, and squid gonad | Bligh and Dyer method | UPLC-Q-Exactive Orbitrap/MS | Identification of 310 phospholipid molecules with 34 different fatty acyl chain combinations | [87] |
| Sheep meat | 100% isopropanol alcohol | UPLC-QOrbitrap HRMS | Finding a total of 90 lipids belonging to six lipid subclasses, such as sphingomyelin, ceramide, lysophosphatidylcholine, phosphatidylcholine, phosphatidylethanolamines, and triacylglycerol | [90] |
| Duck | Methanol: Chloroform (2:1) | ESI-MS/MS | Identification and quantification of a total of 118 phospholipid species in duck meat | [88] |
| Chlorella vulgaris | Ethanol dichloromethane:methanol (2:1, v/v) | HILIC–ESI–MS | More than 30 phospholipids were identified in the lipid extract of C. vulgaris Using ultrasonication in lipid extraction increased the yields of polar lipids from C. vulgaris | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, J.; Kang, J.; Kim, H.; Moon, C. A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods. Foods 2023, 12, 3177. https://doi.org/10.3390/foods12173177
Yeo J, Kang J, Kim H, Moon C. A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods. Foods. 2023; 12(17):3177. https://doi.org/10.3390/foods12173177
Chicago/Turabian StyleYeo, JuDong, JaeYoon Kang, HyeonJin Kim, and Chaeeun Moon. 2023. "A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods" Foods 12, no. 17: 3177. https://doi.org/10.3390/foods12173177
APA StyleYeo, J., Kang, J., Kim, H., & Moon, C. (2023). A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods. Foods, 12(17), 3177. https://doi.org/10.3390/foods12173177





