Fabrication and Characterization of Apple-Pectin–PVA-Based Nanofibers for Improved Viability of Probiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement of Materials
2.2. Bacterial Culture Activation
2.3. Preparation of Solutions
2.4. Fabrication PEC/PVA Nanofibers
2.5. Encapsulation Efficiency
2.6. Characterization of PEC/PVA Nanofibers
2.6.1. Zeta Potential (ξ)
2.6.2. Thickness
2.6.3. Mechanical Properties of Nanofibers
EAB = (L_max − L_0)/L_0 ∗ 100
2.6.4. Morphological Characterization
2.6.5. Molecular Characterization
2.6.6. Thermogravimetric Analysis
2.7. In Vitro Simulated Gastrointestinal Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Encapsulation Efficiency (EE%)
3.2. Characterization of PEC/PVA Nanofibers
3.2.1. Zeta Potential (ζ)
3.2.2. Moisture Content (%)
3.2.3. Thickness
3.2.4. Mechanical Properties (Tensile Strength and Elongation at Break)
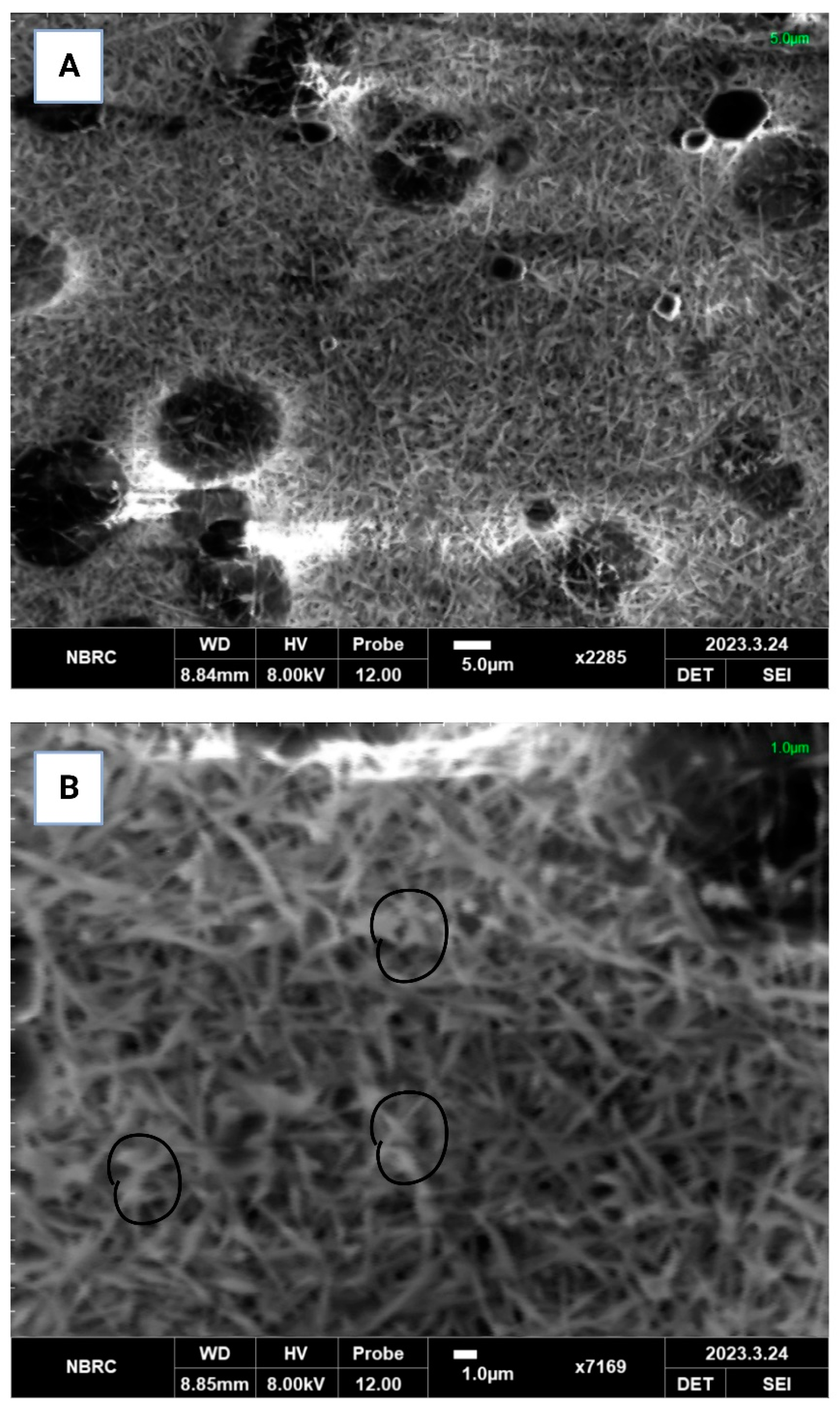
3.2.5. Scanning Electron Microscopy (SEM)
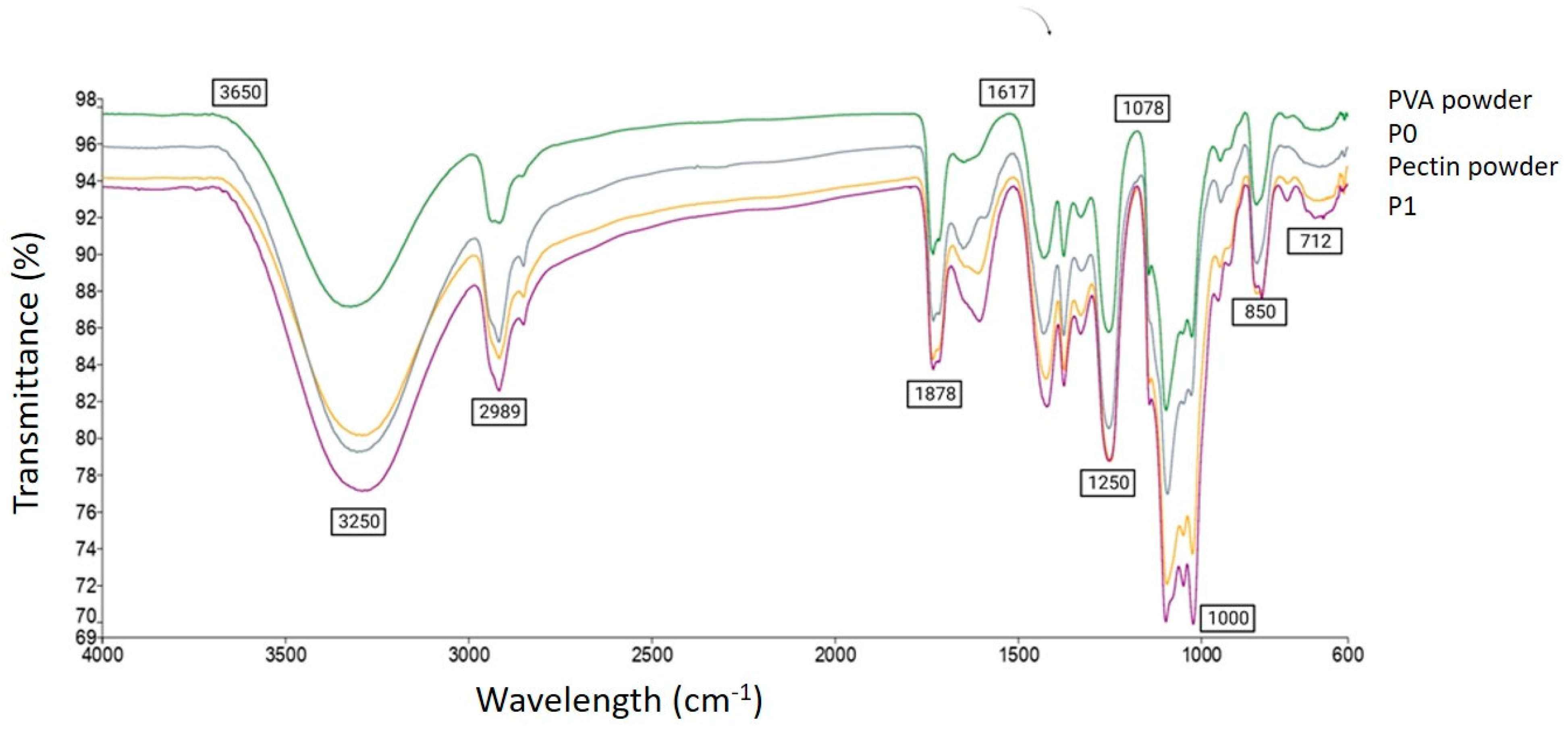
3.2.6. Fourier Transform Infrared Spectrometry (FTIR)
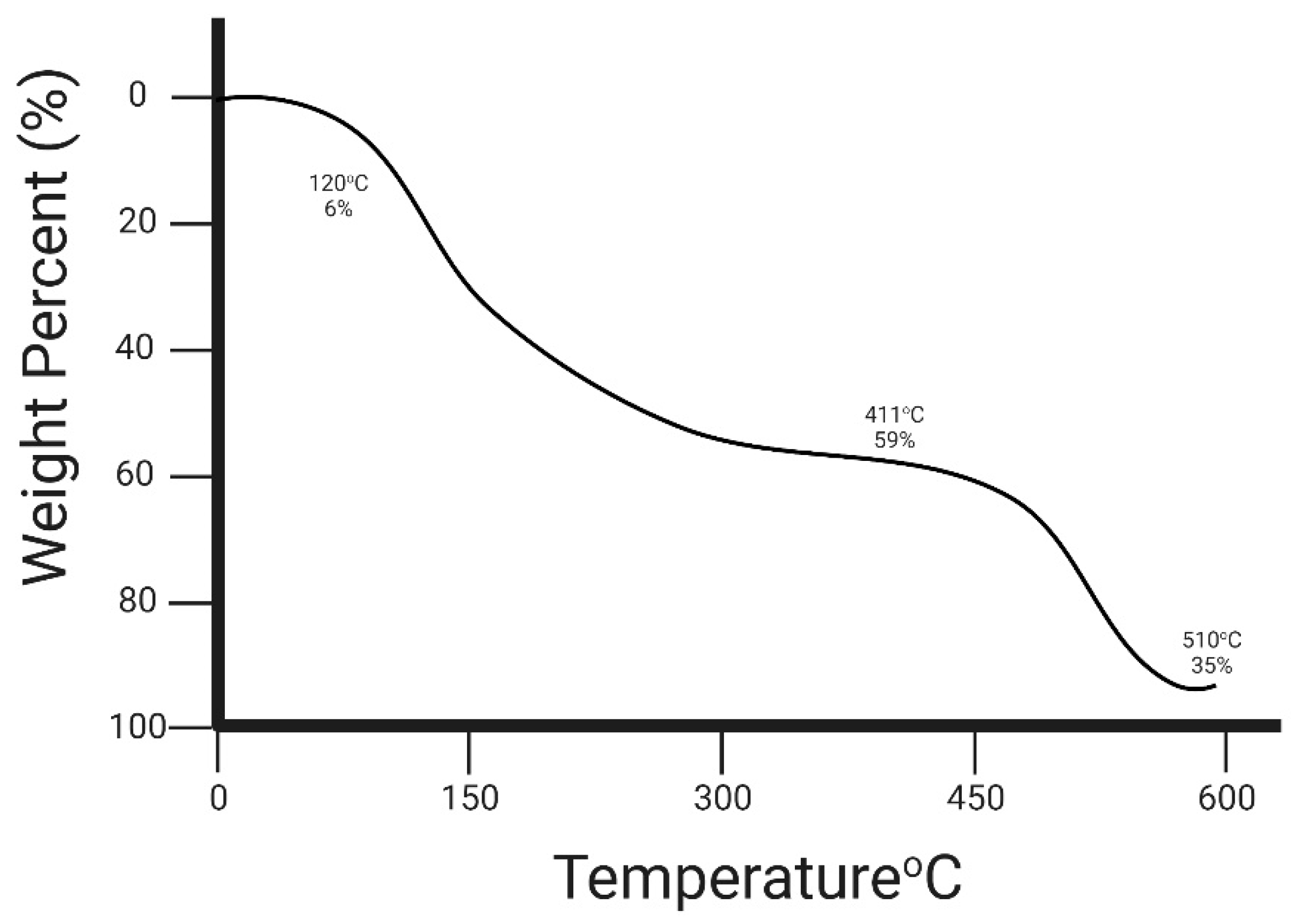
3.2.7. Thermogravimetric Analysis (TGA)
3.3. In Vitro Testing
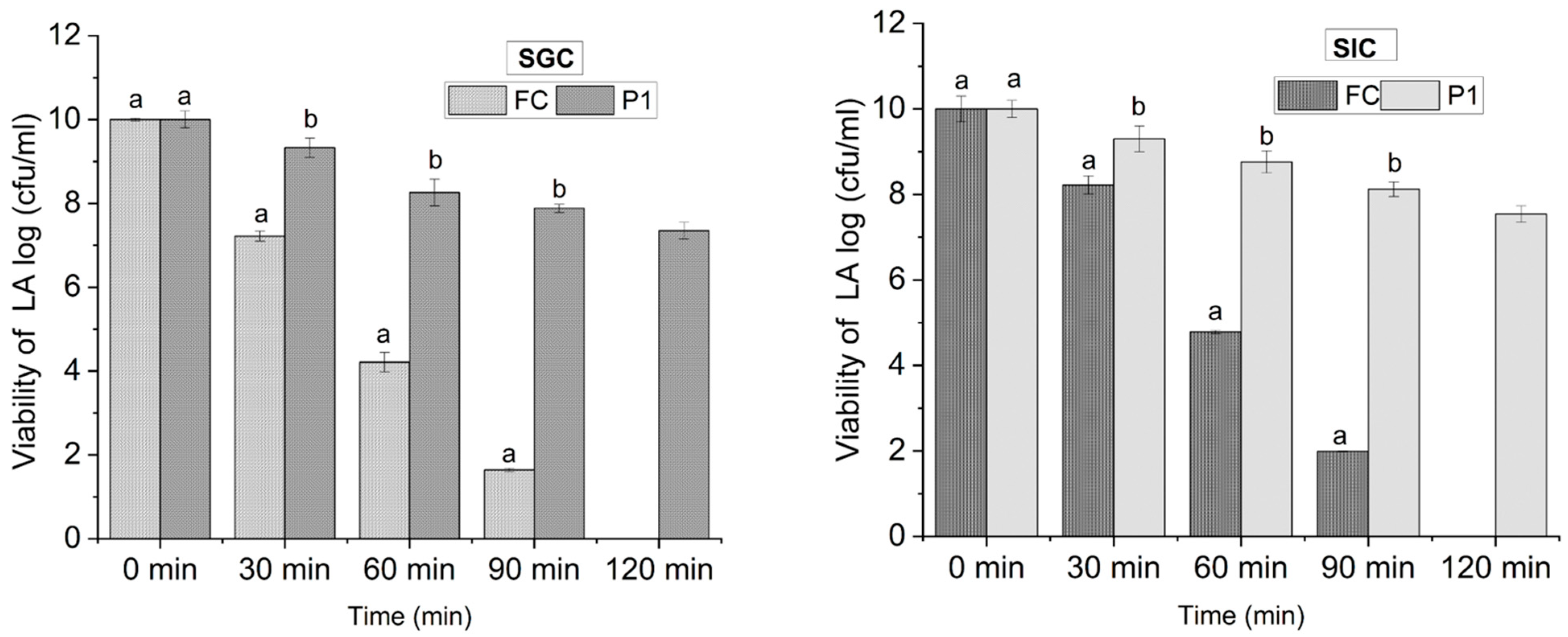
3.3.1. Viability under Simulated Gastric Conditions
3.3.2. Viability under Simulated Intestinal Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lambo, M.T.; Chang, X.; Liu, D. The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef] [PubMed]
- Atraki, R.; Azizkhani, M. Survival of probiotic bacteria nanoencapsulated within biopolymers in a simulated gastrointestinal model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Wang, Q.; Xu, C.; Yu, W.; Yu, H.; Wang, W.; Feng, Z.; Chen, L.; Hou, J.; et al. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chem. 2023, 413, 135680. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Masoodi, F.A.; Gani, A.; Nazir, S.; Jhan, F. Pectin recovery from apple pomace: Physico-chemical and functional variation based on methyl-esterification. Int. J. Food Sci. Technol. 2021, 56, 4669–4679. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Sattari, A.; Alemzadeh, I. Plant-Derived Biopolymers in Food Packaging: Current Status and Market Potential. In Biodegradable Polymer-Based Food Packaging; Springer: Berlin/Heidelberg, Germany, 2022; pp. 13–40. [Google Scholar]
- Aider-Kaci, F.A.; Aidarbekova, S.; Aider, M. Impact of electro-activated whey on growth, acid and bile resistance of Lacticaseibacillus rhamnosus GG and Lactobacillus acidophilus ATCC 4356. Heliyon 2023, 9, e13154. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Wang, W.; Liu, Z.; Gu, L.; Qian, S.; Hou, J.; Jiang, Z. Preparation of pectin-based nanofibers encapsulating Lactobacillus rhamnosus 1.0320 by electrospinning. Food Hydrocoll. 2022, 124, 107216. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.-C.; Chen, W.; Huang, R.; Hong, S.; Wu, Q.; Mei, C. Production of lignin-containing cellulose nanofibers using deep eutectic solvents for UV-absorbing polymer reinforcement. Carbohydr. Polym. 2020, 246, 116548. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, D.; Chauhan, R.; Goel, G. Skimmed milk-based encapsulation for enhanced stability and viability of Lactobacillus gastricus BTM 7 under simulated gastrointestinal conditions. Probiotics Antimicrob. Proteins 2019, 11, 850–856. [Google Scholar] [CrossRef]
- Lemenkova, P. Numerical data modelling and classification in marine geology by the SPSS statistics. Int. J. Eng. Technol. IJET 2019, 5, 90–99. [Google Scholar]
- Homayoonfal, M.; Malekjani, N.; Baeghbali, V.; Ansarifar, E.; Hedayati, S.; Jafari, S.M. Optimization of spray drying process parameters for the food bioactive ingredients. In Critical Reviews in Food Science and Nutrition; Taylor Francis Group: Abingdon, UK, 2022; pp. 1–41. [Google Scholar]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Simonič, M.; Slapničar, Š.; Trček, J.; Matijašić, B.B.; Lorbeg, P.M.; Vesel, A.; Zemljič, L.F.; Fratnik, Z.P. Probiotic Lactobacillus paragasseri K7 nanofiber encapsulation using nozzle-free electrospinning. Appl. Biochem. Biotechnol. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; Rathnayaka, V.; de Silva, R.M.; de Silva, K.N. Colloidal stability of chitin nanofibers in aqueous systems: Effect of pH, ionic strength, temperature & concentration. Carbohydr. Polym. 2020, 235, 116024. [Google Scholar]
- Coelho Braga de Carvalho, A.L.; Ludovici, F.; Goldmann, D.; Silva, A.C.; Liimatainen, H. Silylated thiol-containing cellulose nanofibers as a bio-based flocculation agent for ultrafine mineral particles of chalcopyrite and pyrite. J. Sustain. Metall. 2021, 7, 1506–1522. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Karakaş, C.Y.; Dertli, E.; Yilmaz, M.T. A novel strategy for probiotic bacteria: Ensuring microbial stability of fish fillets using characterized probiotic bacteria-loaded nanofibers. Innov. Food Sci. Emerg. Technol. 2018, 48, 212–218. [Google Scholar] [CrossRef]
- Khoshakhlagh, K.; Mohebbi, M.; Koocheki, A.; Allafchian, A. Encapsulation of D-limonene in Alyssum homolocarpum seed gum nanocapsules by emulsion electrospraying: Morphology characterization and stability assessment. Bioact. Carbohydr. Diet. Fibre 2018, 16, 43–52. [Google Scholar] [CrossRef]
- Tang, K.Y.; Jiang, L.; Yeo, J.C.C.; Owh, C.; Ye, E.; Loh, X.J.; Li, Z. Engineering luminescent pectin-based hydrogel for highly efficient multiple sensing. Int. J. Biol. Macromol. 2021, 166, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.K.; Geshlaghi, S.P.; Pirsa, S.; Asdagh, A. Composite film based on potato starch/apple peel pectin/ZrO2 nanoparticles/microencapsulated Zataria multiflora essential oil; investigation of physicochemical properties and use in quail meat packaging. Food Hydrocoll. 2021, 117, 106719. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, C.; Lu, X.; Ng, D.; Truong, Y.B.; Xie, Z. Activated carbon enhanced hydrophobic/hydrophilic dual-layer nanofiber composite membranes for high-performance direct contact membrane distillation. Desalination 2018, 446, 59–69. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Guerra, P.R.; Bahl, M.I.; Torp, A.M.; Hwu, E.T.; Licht, T.R.; Boisen, A. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022, 123, 107112. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Wang, X.; Huang, Y.; Xiao, M.; Hu, Y.; Zhang, J. Antifungal electrospinning nanofiber film incorporated with Zanthoxylum bungeanum essential oil for strawberry and sweet cherry preservation. LWT 2022, 169, 113992. [Google Scholar] [CrossRef]
- Duman, D.; Karadag, A. Inulin added electrospun composite nanofibres by electrospinning for the encapsulation of probiotics: Characterisation and assessment of viability during storage and simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 927–935. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Bai, L.; Deng, J.; Zhou, Q. Biomaterial-based encapsulated probiotics for biomedical applications: Current status and future perspectives. Mater. Des. 2021, 210, 110018. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, C.; Zhou, W.; Luan, Q.; Li, W.; Deng, Q.; Dong, X.; Tang, H.; Huang, F. A pH-responsive gel macrosphere based on sodium alginate and cellulose nanofiber for potential intestinal delivery of probiotics. ACS Sustain. Chem. Eng. 2018, 6, 13924–13931. [Google Scholar] [CrossRef]
- Škrlec, K.; Zupančič, Š.; Mihevc, S.P.; Kocbek, P.; Kristl, J.; Berlec, A. Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. Eur. J. Pharm. Biopharm. 2019, 136, 108–119. [Google Scholar] [CrossRef]
- Zabihollahi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S.; Hamishekar, H. Development and characterization of carboxymethyl cellulose based probiotic nanocomposite film containing cellulose nanofiber and inulin for chicken fillet shelf life extension. Int. J. Biol. Macromol. 2020, 160, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhou, D.; Kang, X. Electrospinning as a novel strategy for the encapsulation of living probiotics in polyvinyl alcohol/silk fibroin. Innov. Food Sci. Emerg. Technol. 2021, 71, 102726. [Google Scholar] [CrossRef]
- Khan, Z.; Minhas, M.U.; Ahmad, M.; Khan, K.U.; Sohail, M.; Khalid, I. Functionalized pectin hydrogels by cross-linking with monomer: Synthesis, characterization, drug release and pectinase degradation studies. Polym. Bull. 2020, 77, 339–356. [Google Scholar] [CrossRef]
- Reguieg, F.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal characterization by DSC and TGA analyses of PVA hydrogels with organic and sodium MMT. Polym. Bull. 2020, 77, 929–948. [Google Scholar] [CrossRef]
- Amin, F.A.Z.; Sabri, S.; Ismail, M.; Chan, K.W.; Ismail, N.; Esa, N.M.; Lila, M.A.M.; Zawawi, N. Probiotic properties of Bacillus strains isolated from stingless bee (Heterotrigona itama) honey collected across Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, W.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. Targeting delivery system for Lactobacillus plantarum based on functionalized electrospun nanofibers. Polymers 2020, 12, 1565. [Google Scholar] [CrossRef]
- Lopes, L.A.A.; Carvalho, R.d.S.F.; Magalhães, N.S.S.; Madruga, M.S.; Athayde, A.J.A.A.; Portela, I.A.; Barão, C.E.; Pimentel, T.C.; Magnani, M.; Stamford, T.C.M. Microencapsulation of Lactobacillus acidophilus La-05 and incorporation in vegan milks: Physicochemical characteristics and survival during storage, exposure to stress conditions, and simulated gastrointestinal digestion. Food Res. Int. 2020, 135, 109295. [Google Scholar] [CrossRef]
| Parameter | P0 (*) | P1 (*) |
|---|---|---|
| Zeta potential (mV) | −7.55 ± 0.08 a | −10.22 ±0.01 a |
| Moisture content (%) | 14.10 ± 0.06 a | 14.27 ± 0.05 a |
| Encapsulation efficiency (%) | --- | 82.90% |
| Mechanical properties | ||
| Thickness (mm) | 0.128 ± 0.05 a | 0.137 ± 0.01 b |
| Tensile strength (MPa) | 13.35 ± 0.04 a | 19.45 ± 0.01 b |
| Elongation at break (%) | 22.05 ± 0.05 a | 36.98 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, A.; Irshad, S.; Walayat, N.; Khan, M.R.; Iqbal, M.W.; Luo, X. Fabrication and Characterization of Apple-Pectin–PVA-Based Nanofibers for Improved Viability of Probiotics. Foods 2023, 12, 3194. https://doi.org/10.3390/foods12173194
Nawaz A, Irshad S, Walayat N, Khan MR, Iqbal MW, Luo X. Fabrication and Characterization of Apple-Pectin–PVA-Based Nanofibers for Improved Viability of Probiotics. Foods. 2023; 12(17):3194. https://doi.org/10.3390/foods12173194
Chicago/Turabian StyleNawaz, Asad, Sana Irshad, Noman Walayat, Mohammad Rizwan Khan, Muhammad Waheed Iqbal, and Xiaofang Luo. 2023. "Fabrication and Characterization of Apple-Pectin–PVA-Based Nanofibers for Improved Viability of Probiotics" Foods 12, no. 17: 3194. https://doi.org/10.3390/foods12173194
APA StyleNawaz, A., Irshad, S., Walayat, N., Khan, M. R., Iqbal, M. W., & Luo, X. (2023). Fabrication and Characterization of Apple-Pectin–PVA-Based Nanofibers for Improved Viability of Probiotics. Foods, 12(17), 3194. https://doi.org/10.3390/foods12173194









