Effects of Ammonia and Salinity Stress on Non-Volatile and Volatile Compounds of Ivory Shell (Babylonia areolata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design and Sampling
2.3. FAA Analysis
2.4. 5′-Nucleotide Analysis
2.5. Equivalent Umami Concentration (EUC)
2.6. Organic Acid Analysis
2.7. Inorganic Ion Analysis
2.8. Volatile Compounds Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Comparison of FAA and Flavor 5′-Nucleotide Compositions in Ivory Shells
3.2. Comparison of Organic Acid and Inorganic Ions in Ivory Shells
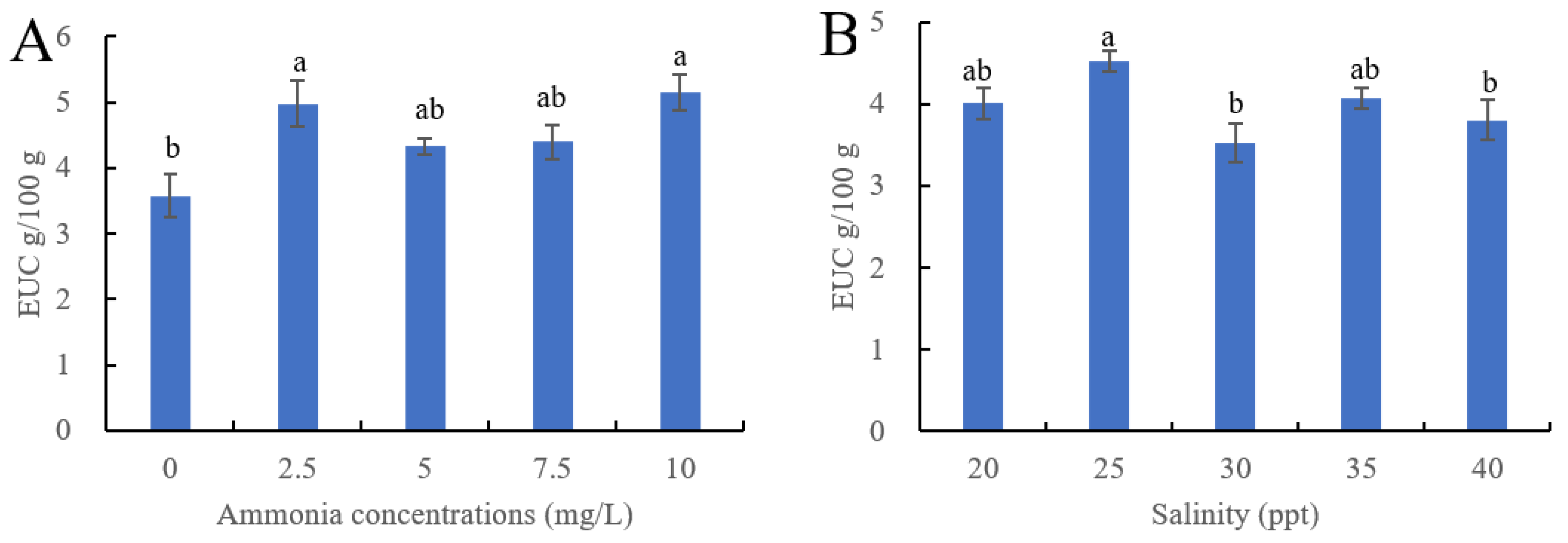
3.3. Effect of Ammonia and Salinity on EUC of Ivory Shells
3.4. Changes in Volatile Components in Ivory Shells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kritsanapuntu, S.; Chaitanawisuti, N.; Santhaweesuk, W.; Natsukari, Y. Growth, production and economic evaluation of earthen ponds for monoculture and polyculture of juveniles spotted babylon (Babylonia areolata) to marketable sizes using large-scale operation. J. Shellfish Res. 2006, 25, 913–918. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Yang, Y.; Yang, Y.; Gu, Z.; Wang, A.; Liu, C. Effects of long-term exposure to ammonia on growth performance, immune response, and body biochemical composition of juvenile ivory shell, Babylonia areolate. Aquaculture 2023, 562, 738857. [Google Scholar] [CrossRef]
- Chaitanawisuti, N.; Kritsanapuntu, S.; Natsukari, Y. Economic analysis of a pilot commercial production for spotted babylon, Babylonia areolata (Link 1807), of marketable sizes using a flow-through culture system in Thailand. Aquac. Res. 2002, 33, 1265–1272. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Yang, Y.; Yang, Y.; Wang, A.; Gu, Z. Effects of salinity on growth performance, physiological response, and body biochemical composition of juvenile ivory shell (Babylonia areolata). Aquaculture 2023, 566, 739193. [Google Scholar] [CrossRef]
- CSY. China Statistical Yearbook; China Statistical Publishing House: Beijing, China, 2022; p. 27. [Google Scholar]
- Lü, W.; Shen, M.; Fu, J.; Li, W.; You, W.; Ke, C. Combined effects of temperature, salinity and rearing density on growth and survival of juvenile ivory shell, babylonia areolata (link 1807) population in Thailand. Aquac. Res. 2017, 48, 1648–1665. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, F.; Huang, J.; Ma, Z.; Jiang, S.; Qiu, L.; Qin, J. Ammonia and salinity tolerance of Penaeus monodon across eight breeding families. Springerplus 2016, 5, 171. [Google Scholar] [CrossRef]
- Long, J.; Cui, Y.; Wang, R.; Chen, Y.; Zhao, N.; Wang, C.; Wang, Z.; Li, Y. Combined effects of high salinity and ammonia-N exposure on the energy metabolism, immune response, oxidative resistance and ammonia metabolism of the Pacific white shrimp Litopenaeus vannamei. Aquac. Rep. 2021, 20, 100648. [Google Scholar] [CrossRef]
- Ran, Z.; Zhang, S.; Zhu, Z.; Ke, A.; Xu, J.; Li, Y.; Liao, K.; Li, S.; Ran, Y.; Yan, X. Effect of salinity on volatiles in the razor clam investigated by head space-solid phase microextraction/gas chromatography-mass spectrometry. Fish. Sci. 2019, 85, 137–146. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, G.; Lin, Z.; Yao, H.; Dong, Y. The razor clam Sinonovacula constricta uses the strategy of conversion of toxic ammonia to glutamine in response to high environmental ammonia exposure. Mol. Biol. Rep. 2020, 47, 9579–9593. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Cong, Y.; Gao, S.; Wu, Z.; Sun, W. Widely targeted metabolomics investigation of the meat taste of Eriocheir sinensis under ammonia exposure. J. Food Compos. Anal. 2023, 121, 105408. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.; Jin, Y.; Deng, Y.; Zhao, Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Shi, H.; Xue, C.; Wang, Q.; Yu, F.; Xue, Y.; Wang, Y.; Li, Z. The flavor profile changes of Pacific oysters (Crassostrea gigas) in response to salinity during depuration. Food Chem. X 2022, 16, 100485. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gu, Z.; Lin, X.; Wang, Y.; Wang, A.; Sun, Y. Effects of high hydrostatic pressure (HHP) and storage temperature on bacterial counts, color change, fatty acids and non-volatile taste active compounds of oysters (Crassostrea ariakensis). Food Chem. 2022, 372, 131247. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, G.; Cao, Z.; Liu, C. Comparison of Biochemical Composition and Non-Volatile Taste Active Compounds of Back and Abdominal Muscles in Three Marine Perciform Fishes, Chromileptes altivelis, Epinephelus akaara and Acanthopagrus schlegelii. Molecules 2022, 27, 4480. [Google Scholar] [CrossRef]
- Chen, L.; Yu, F.; Shi, H.; Wang, Q.; Xue, Y.; Xue, C.; Wang, Y.; Li, Z. Effect of salinity stress on respiratory metabolism, glycolysis, lipolysis, and apoptosis in Pacific oyster (Crassostrea gigas) during depuration stage. J. Sci. Food Agric. 2022, 102, 2003–2011. [Google Scholar] [CrossRef]
- Luo, J.; Monroig, O.; Zhou, Q.; Tocher, D.R.; Yuan, Y.; Zhu, T.; Lu, J.; Song, D.; Jiao, L.; Jin, M. Environmental salinity and dietary lipid nutrition strategy: Effects on flesh quality of the marine euryhaline crab Scylla paramamosain. Food Chem. 2021, 361, 130160. [Google Scholar] [CrossRef]
- Phetsang, H.; Panpipat, W.; Undeland, I.; Panya, A.; Phonsatta, N.; Chaijan, M. Comparative quality and volatilomic characterisation of unwashed mince, surimi, and pH-shift-processed protein isolates from farm-raised hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Food Chem. 2021, 364, 130365. [Google Scholar] [CrossRef]
- Phetsang, H.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.; Chaijan, M. Chemical characteristics and volatile compounds profiles in different muscle part of the farmed hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Int. J. Food Sci. Technol. 2022, 57, 310–322. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Gao, J.; Ma, L.; Huang, X.; Zheng, J.; Dong, X.; Qin, L. Metabolomic approaches to analyze the seasonal variations of amino acid, 5′ -Nucleotide, and lipid profile of clam (Ruditapes philippinarum). Lwt-Food Sci. Technol. 2021, 148, 111709. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 2012, 334–337, 12–23. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Zhang, J.; Sun, Y.; Wang, J. Dietary effects of succinic acid on the growth, digestive enzymes, immune response and resistance to ammonia stress of Litopenaeus vanname. Fish Shellfish Immun. 2018, 78, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Wang, Y.; Yang, Y.; Wang, A.; Gu, Z. Effects of high hydrostatic pressure and storage temperature on fatty acids and non-volatile taste active compounds in red claw crayfish (Cherax quadricarinatus). Molecules 2022, 27, 5098. [Google Scholar] [CrossRef]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 2021, 344, 128632. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, F.; Tang, X.; Shi, Y.; Wang, A.; Gu, Z.; Pan, Z. Comparison of nonvolatile taste active compounds of wild and cultured mud crab Scylla paramamosain. Fish. Sci. 2018, 84, 897–907. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, A.; Xian, J. Variation of free amino acid and carbohydrate concentrations in white shrimp, Litopenaeus vannamei: Effects of continuous cold stress. Aquaculture 2011, 317, 182–186. [Google Scholar] [CrossRef]
- Song, X.; Lü, W.; Ibrahim, S.; Deng, Y.; Li, Q.; Yue, C. Identification of free amino acids (FAA) that are important as major intracellular osmolytes in the estuarine Hong Kong oyster, Crassostrea hongkongensis. Aquac. Rep. 2023, 28, 101464. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Sun, Z.; Jiang, H.; Qian, Y.; Wang, R.; Li, M. The effects of acute and chronic ammonia exposure on growth, survival, and free amino acid abundance in juvenile Japanese sea perch Lateolabrax japonicus. Aquaculture 2022, 560, 738512. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, L.; Zhao, X.; Cheng, Y. Effects of salinity stress on osmotic pressure, free amino acids, and immune-associated parameters of the juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 2022, 549, 737776. [Google Scholar] [CrossRef]
- Chew, S.F.; Wong, M.Y.; Tam, W.L.; Ip, Y.K. The snakehead Channa asiatica accumulates alanine during aerial exposure, but is incapable of sustaining locomotory activities on land through partial amino acid catabolism. J. Exp. Biol. 2003, 206, 693–704. [Google Scholar] [CrossRef]
- Bi, S.; Chen, L.; Sun, Z.; Wen, Y.; Xue, Q.; Xue, C.; Li, Z.; Sun, C.; Wei, Z.; Liu, H. Investigating infuence of aquaculture seawater with diferent salinities on non-volatile taste-active compounds in Pacifc oyster (Crassostrea gigas). J. Food Meas. Charact. 2021, 15, 2078–2087. [Google Scholar] [CrossRef]
- Zheng, J.; Tao, N.; Gong, J.; Gu, S.; Xu, C. Comparison of non-volatile taste-active compounds between the cooked meats of pre- and post-spawning Yangtze Coilia ectenes. Fish. Sci. 2015, 81, 559–568. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Sarower, M.G.; Hasanuzzaman, A.F.M.; Biswas, B.; Abe, H. Taste producing components in fish and fisheries products: A review. Int. J. Food Ferment. Technol. 2012, 2, 113–121. [Google Scholar]
- Shim, K.; Mok, J.S.; Jeong, Y.; Park, K.; Jang, M. Effect of organic acids on the formation of biogenic amines in fermented anchovy sauce comprising raw anchovy materials with different levels of freshness. J. Food Sci. Technol. 2022, 59, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.K.; Koh, C.B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S.; Kishimura, H.; Tsai, Y.H. Chemical compositions and nutritional value of Asian hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food Chem. 2013, 141, 4138–4145. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Prabu, E.; Felix, S.; Felix, N.; Ahilan, B.; Ruby, P. An overview on significance of fish nutrition in aquaculture industry. Int. J. Fish. Aquat. Stud. 2017, 5, 349–355. [Google Scholar]
- Deng, W.; Tian, G.; Wang, Z.; Mao, K.; Liu, X.; Ding, Q.; Sang, Y.; Gao, J. Analysis of volatile components changes of Ruditapes philippinarum during boiling by HS-GC-IMS coupled with multivariate analyses. Aquac. Rep. 2022, 25, 101193. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. Lwt-Food Sci. Technol. 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Mamede, R.; Ricardo, F.; Santos, A.; Díaz, S.; Santos, S.A.O.; Bispo, R.; Domingues, M.R.M.; Calado, R. Revealing the illegal harvesting of Manila clams (Ruditapes philippinarum) using fatty acid profiles of the adductor muscle. Food Control 2020, 118, 107368. [Google Scholar] [CrossRef]
- Durnford, E.; Shahidi, F. Flavours of fish meat. In Flavor of Meat, Meat Products, and Seafoods; Shahidi, F., Ed.; Blackie Academic & Professional: New York, NY, USA, 1998; pp. 131–158. [Google Scholar]
- Cha, Y.J.; Cadwallader, K.R.; Baek, H.H. Volatile flavor components in snow crab cooker effluent and effluent concentrate. J. Food Sci. 1993, 58, 525–530. [Google Scholar] [CrossRef]
- Karahadian, C.; Lindsay, R.C. Evaluation of compounds contributing characterizing fishy favors in fish oils. J. Am. Oil. Chem. Soc. 1989, 66, 953–960. [Google Scholar] [CrossRef]
- Gu, S.; Wu, N.; Wang, X.; Zhang, J.; Ji, S. Analysis of key odor compounds in steamed Chinese mitten crab (Eriocheir sinensis). Adv. Mater. Res. 2014, 941–944, 1026–1035. [Google Scholar] [CrossRef]
| Item | Taste Attribute | Ammonia Concentrations (mg/L) | Salinity (ppt) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 7.5 | 10 | 20 | 25 | 30 | 35 | 40 | ||
| Aspartic acid 1 | Umami (+) | 49.77 ± 0.48d | 50.25 ± 0.36cd | 50.80 ± 0.56bc | 51.28 ± 0.41ab | 51.93 ± 0.05a | 51.78 ± 0.18A | 51.66 ± 0.09A | 47.76 ± 0.05B | 47.51 ± 0.06B | 47.22 ± 0.13B |
| Glutamic acid 1 | Umami (+) | 117.25 ± 1.16c | 118.41 ± 0.94c | 119.75 ± 1.35bc | 120.89 ± 0.99ab | 122.45 ± 1.21a | 122.11 ± 0.44A | 121.81 ±0.21A | 112.59 ± 0.11B | 112.01 ± 2.10B | 111.34 ± 0.31B |
| MSG-like FAA | 167.02 ± 1.45c | 168.66 ± 1.24c | 170.55 ± 1.68bc | 172.17 ± 1.33ab | 174.38 ± 1.21a | 173.89 ± 0.60A | 173.47 ± 0.30A | 160.35 ± 0.15B | 159.52 ± 1.87B | 158.56 ± 0.42B | |
| Serine 2 | Sweet (+) | 19.20 ± 0.17c | 19.37 ± 0.13c | 19.57 ± 0.20bc | 19.74 ± 0.15ab | 19.97 ± 0.18a | 19.92 ± 0.07A | 19.87 ± 0.03A | 18.38 ± 0.02B | 18.28 ± 0.04B | 18.17 ± 0.05B |
| Glycine 2 | Sweet (+) | 214.77 ± 2.15c | 216.92 ± 1.94bc | 219.40 ± 2.50abc | 221.53 ± 1.83ab | 224.43 ± 2.25a | 223.79 ± 0.82A | 223.24 ± 0.39A | 206.32 ± 0.20B | 205.26 ± 1.23B | 204.05 ± 0.58B |
| Arginine 2 | Bitter/sweet (+) | 72.03 ± 0.70c | 72.73 ± 0.43c | 73.53 ± 0.80bc | 74.21 ± 0.59 ab | 75.15 ± 0.73a | 74.94 ± 0.27A | 74.77 ± 0.13A | 69.12 ± 0.07B | 68.76 ± 0.07B | 68.35 ± 0.19B |
| Threonine 2 | Sweet (+) | 9.63 ± 0.09c | 9.71 ± 0.11c | 9.81 ± 0.10bc | 9.90 ± 0.07ab | 10.02 ± 0.09a | 9.99 ± 0.03A | 9.97 ± 0.01A | 9.22 ± 0.01B | 9.17 ± 0.03B | 9.11 ± 0.03B |
| Alanine 2 | Sweet (+) | 83.66 ± 0.84d | 84.49 ± 0.39cd | 85.44 ± 0.96bc | 86.26 ± 0.71 ab | 87.39 ± 0.87a | 87.14 ± 0.32A | 86.92 ± 0.13A | 80.34 ± 0.08B | 79.93 ± 0.05B | 79.45 ± 0.22B |
| Proline 2 | Bitter/sweet (+) | 49.44 ± 0.50c | 49.93 ± 0.43bc | 50.51 ± 0.58abc | 51.00 ± 0.42ab | 51.66 ± 0.52a | 51.52 ± 0.19A | 51.39 ± 0.02A | 47.50 ± 0.05B | 47.25 ± 0.03B | 46.97 ± 0.13B |
| Sweet FAA | 448.73 ± 3.99c | 453.15 ± 3.35bc | 458.26 ± 5.04abc | 462.64 ± 3.07ab | 468.62 ± 4.06a | 467.30 ± 1.68A | 466.16 ± 0.70A | 430.88 ± 0.24B | 428.65 ± 1.35B | 426.10 ± 1.08B | |
| Histidine 3 | Bitter (−) | 9.70 ± 0.07c | 9.77 ± 0.08bc | 9.85 ± 0.08abc | 9.92 ± 0.06ab | 10.02 ± 0.08a | 10.00 ± 0.03A | 9.98 ± 0.01A | 9.24 ± 0.01B | 9.19 ± 0.04B | 9.13 ± 0.03B |
| Tyrosine 3 | Bitter (−) | 8.71 ± 0.04b | 8.76 ± 0.10b | 8.81 ± 0.05ab | 8.85 ± 0.04 ab | 8.91 ± 0.05a | 8.90 ± 0.02A | 8.89 ± 0.15A | 8.25 ± 0.01B | 8.19 ± 0.11B | 8.14 ± 0.03B |
| Valine 3 | Sweet/bitter (−) | 10.25 ± 0.06bc | 10.30 ± 0.07bc | 10.37 ± 0.07abc | 10.43 ± 0.05 ab | 10.51 ± 0.02a | 10.49 ± 0.02A | 10.48 ± 0.09A | 9.71 ± 0.02B | 9.65 ± 0.07B | 9.59 ± 0.03B |
| Methionine 3 | Bitter/sweet/sulfurous (−) | 4.35 ± 0.02b | 4.37 ± 0.03b | 4.40 ± 0.03ab | 4.42 ± 0.02 ab | 4.45 ± 0.04a | 4.45 ± 0.01A | 4.44 ± 0.00A | 4.12 ± 0.01B | 4.09 ± 0.03B | 4.06 ± 0.01B |
| Cystine 3 | Bitter/sweet/sulfurous (−) | 4.91 ± 0.02a | 4.93 ± 0.03a | 4.95 ± 0.02a | 4.96 ± 0.01a | 4.98 ± 0.02a | 4.98 ± 0.01B | 4.98 ± 0.00B | 4.98 ± 0.01B | 4.99 ± 0.04B | 5.00 ± 0.01A |
| Isoleucine 3 | Bitter (−) | 6.21 ± 0.04b | 6.25 ± 0.06b | 6.30 ± 0.05ab | 6.34 ± 0.04 ab | 6.40 ± 0.04a | 6.39 ± 0.02A | 6.38 ± 0.01A | 5.91 ± 0.01B | 5.87 ± 0.04B | 5.83 ± 0.02B |
| Leucine 3 | Bitter (−) | 8.98 ± 0.06c | 9.04 ± 0.11bc | 9.11 ± 0.07abc | 9.17 ± 0.05 ab | 9.26 ± 0.07a | 9.24 ± 0.02A | 9.22 ± 0.01A | 8.54 ± 0.01B | 8.49 ± 0.05B | 8.44 ± 0.03B |
| Phenylalanine 3 | Bitter (−) | 10.93 ± 0.06b | 10.98 ± 0.13ab | 11.05 ± 0.07ab | 11.10 ± 0.05a | 11.18 ± 0.06a | 11.16 ± 0.02A | 11.15 ± 0.01A | 10.34 ± 0.02B | 10.27 ± 0.04B | 10.20 ± 0.04B |
| Tryptophan 3 | Bitter (−) | 10.32 ± 0.04b | 10.37 ± 0.14ab | 10.42 ± 0.05ab | 10.46 ± 0.04a | 10.52 ± 0.05a | 10.50 ± 0.02A | 10.49 ± 0.01A | 9.74 ± 0.02B | 9.68 ± 0.04B | 9.61 ± 0.03B |
| Lysine 3 | Sweet/bitter (−) | 15.40 ± 0.11c | 15.50 ± 0.09bc | 15.63 ± 0.13abc | 15.73 ± 0.09ab | 15.88 ± 0.11a | 15.85 ± 0.04A | 15.82 ± 0.02A | 14.65 ± 0.02B | 14.57 ± 0.05B | 14.47 ± 0.04B |
| Bitter FAA | 89.76 ± 0.49c | 90.27 ± 0.80bc | 90.89 ± 0.97abc | 91.38 ± 0.45ab | 92.11 ± 0.51a | 91.96 ± 0.21A | 91.83 ± 0.30A | 85.48 ± 0.14B | 84.99 ± 0.48B | 84.47 ± 0.26B | |
| Asparagine 4 | Flat/tasteless | 10.79 ± 0.07b | 10.86 ± 0.16b | 10.94 ± 0.08ab | 11.00 ± 0.06ab | 11.10 ± 0.07a | 11.08 ± 0.03A | 11.06 ± 0.01A | 10.25 ± 0.02A | 10.19 ± 0.04AB | 10.12 ± 0.03B |
| Glutamine 4 | Flat/tasteless | 53.94 ± 0.53d | 54.47 ± 0.34cd | 55.08 ± 0.61bc | 55.60 ± 0.45ab | 56.31 ± 0.55a | 56.15 ± 0.20A | 56.02 ± 0.10A | 51.78 ± 0.05B | 51.51 ± 0.13B | 51.21 ± 0.15B |
| Tasteless FAA | 64.73 ± 0.52d | 65.33 ± 0.94cd | 66.02 ± 0.66bc | 66.60 ± 0.48ab | 67.41 ± 0.61a | 67.23 ± 0.22A | 67.08 ± 0.11A | 62.03 ± 0.06B | 61.70 ± 0.16B | 61.33 ± 0.18B | |
| Total FAA | 770.23 ± 7.19c | 777.41 ± 6.61bc | 785.71 ± 8.53abc | 792.80 ± 6.11ab | 802.52 ± 7.52a | 800.37 ± 2.74A | 798.53 ± 1.32A | 738.75 ± 0.78B | 734.86 ± 3.76B | 730.46 ± 2.05B | |
| Item | Ammonia Concentrations (mg/L) | Salinity (ppt) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 7.5 | 10 | 20 | 25 | 30 | 35 | 40 | |
| CMP | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| UMP | 3.75 ± 0.57a | 3.54 ± 0.38ab | 2.21 ± 0.05b | 2.12 ± 0.07b | 4.83 ± 0.96a | 1.53 ± 0.19AB | 1.53 ± 0.26AB | 2.12 ± 0.09A | 1.49 ± 0.20B | 1.66 ± 0.65AB |
| GMP | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| IMP | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| AMP | 129.90 ± 16.37c | 180.96 ± 12.11a | 155.01 ± 0.13b | 155.93 ± 1.37b | 180.83 ± 5.34a | 140.41 ± 12.46AB | 159.66 ± 8.53A | 133.87 ± 8.15B | 148.14 ± 17.03AB | 146.30 ± 12.72AB |
| Total | 133.64 ± 17.94c | 184.50 ± 11.73a | 157.22 ± 0.07b | 158.05 ± 1.30b | 186.66 ± 6.29a | 141.95 ± 12.66AB | 161.19 ± 8.80A | 135.99 ± 8.06B | 149.63 ± 16.83AB | 147.96 ± 12.07AB |
| Item | Ammonia Concentrations (mg/L) | Salinity (ppt) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 7.5 | 10 | 20 | 25 | 30 | 35 | 40 | |
| Lactic acid | 2.56 ± 0.29b | 2.33 ± 0.01b | 2.27 ± 0.31b | 3.74 ± 0.48a | 2.08 ± 0.26b | 2.39 ± 0.16A | 2.31 ± 0.01A | 2.57 ± 0.10A | 2.06 ± 0.50A | 2.21 ± 0.42A |
| Acetic acid | 5.43 ± 0.87a | 4.50 ± 0.19a | 4.63 ± 0.25a | 4.45 ± 0.19a | 5.10 ± 0.42a | 4.28 ± 1.06AB | 4.13 ± 1.05AB | 3.28 ± 0.24BC | 4.69 ± 0.26A | 2.63 ± 0.39C |
| Citric acid | 26.07 ± 2.69d | 31.44 ± 0.68c | 35.94 ± 2.90ab | 34.28 ± 0.52bc | 39.00 ± 1.53a | 32.60 ± 1.2A | 29.57 ± 0.25B | 26.54 ± 0.53C | 30.12 ± 0.98B | 28.84 ± 1.14B |
| Malic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Succinic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Item | Ammonia Concentrations (mg/L) | Salinity (ppt) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 7.5 | 10 | 20 | 25 | 30 | 35 | 40 | |
| Ca2+ (mg/g) | 3.00 ± 0.09c | 4.27 ± 0.06a | 3.04 ± 0.06c | 3.41 ± 0.03b | 2.98 ± 0.18c | 2.70 ± 0.11D | 3.66 ± 0.36A | 3.28 ± 0.04BC | 3.46 ± 0.09AB | 3.00 ± 0.07CD |
| Na+ (mg/g) | 4.77 ± 0.08b | 4.81 ± 0.10ab | 4.77 ± 0.04b | 4.92 ± 0.04ab | 5.17 ± 0.35a | 3.97 ± 0.03E | 4.31 ± 0.09D | 5.04 ± 0.12C | 5.58 ± 0.11B | 7.06 ± 0.06A |
| K+ (mg/g) | 3.64 ± 0.02a | 3.58 ± 0.03a | 3.65 ± 0.03a | 3.66 ± 0.01a | 3.59 ± 0.09a | 3.59 ± 0.11C | 3.76 ± 0.04B | 3.69 ± 0.04BC | 3.48 ± 0.04D | 4.02 ± 0.04A |
| Mg2+ (mg/g) | 3.99 ± 0.09a | 3.72 ± 0.04cd | 3.96 ± 0.22ab | 3.81 ± 0.03bc | 3.58 ± 0.06d | 3.67 ± 0.28B | 3.69 ± 0.07B | 3.89 ± 0.09B | 4.05 ± 0.03B | 4.94 ± 0.33A |
| Zn2+ (mg/kg) | 16.88 ± 0.63c | 15.45 ± 0.13d | 27.37 ± 0.40a | 24.01 ± 0.06b | 16.76 ± 0.04c | 16.87 ± 0.37C | 28.19 ± 0.63A | 16.11 ± 0.27CD | 15.93 ± 0.59D | 21.13 ± 0.38B |
| Mn2+ (mg/kg) | 1.34 ± 0.05c | 1.53 ± 0.06ab | 1.50 ± 0.03b | 1.62 ± 0.05a | 1.36 ± 0.09c | 1.15 ± 0.02D | 1.52 ± 0.01A | 1.34 ± 0.00C | 1.40 ± 0.06B | 1.56 ± 0.00A |
| Cl− (mg/g) | 1.25 ± 0.05c | 1.31 ± 0.01b | 1.33 ± 0.00b | 1.30 ± 0.01b | 1.43 ± 0.01a | 1.35 ± 0.03B | 1.28 ± 0.04B | 1.38 ± 0.07B | 1.45 ± 0.06B | 1.72 ± 0.20A |
| PO43− (mg/g) | 2.59 ± 0.24a | 2.64 ± 0.17a | 2.79 ± 0.36a | 2.70 ± 0.00a | 2.96 ± 0.08a | 3.02 ± 0.25B | 3.26 ± 0.12AB | 3.25 ± 0.10AB | 3.11 ± 0.15B | 3.54 ± 0.37A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Sun, Y.; Hong, X.; Yu, F.; Yang, Y.; Wang, A.; Gu, Z. Effects of Ammonia and Salinity Stress on Non-Volatile and Volatile Compounds of Ivory Shell (Babylonia areolata). Foods 2023, 12, 3200. https://doi.org/10.3390/foods12173200
Liu C, Sun Y, Hong X, Yu F, Yang Y, Wang A, Gu Z. Effects of Ammonia and Salinity Stress on Non-Volatile and Volatile Compounds of Ivory Shell (Babylonia areolata). Foods. 2023; 12(17):3200. https://doi.org/10.3390/foods12173200
Chicago/Turabian StyleLiu, Chunsheng, Yunchao Sun, Xin Hong, Feng Yu, Yi Yang, Aimin Wang, and Zhifeng Gu. 2023. "Effects of Ammonia and Salinity Stress on Non-Volatile and Volatile Compounds of Ivory Shell (Babylonia areolata)" Foods 12, no. 17: 3200. https://doi.org/10.3390/foods12173200





