Production and Characterization of Heme Iron Polypeptide from the Blood of Skipjack Tuna (Katsuwonus pelamis) Using Enzymatic Hydrolysis for Food Supplement Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Tuna Blood Preparation
2.3. Tuna-HIP Production
2.4. Determination of Degree of Hydrolysis of HIP
2.5. Tuna Blood and Tuna-HIP Powder Composition Analysis
2.6. Water Solubility Analysis of HIP
2.7. HIP Molecular Weight Distribution Analysis
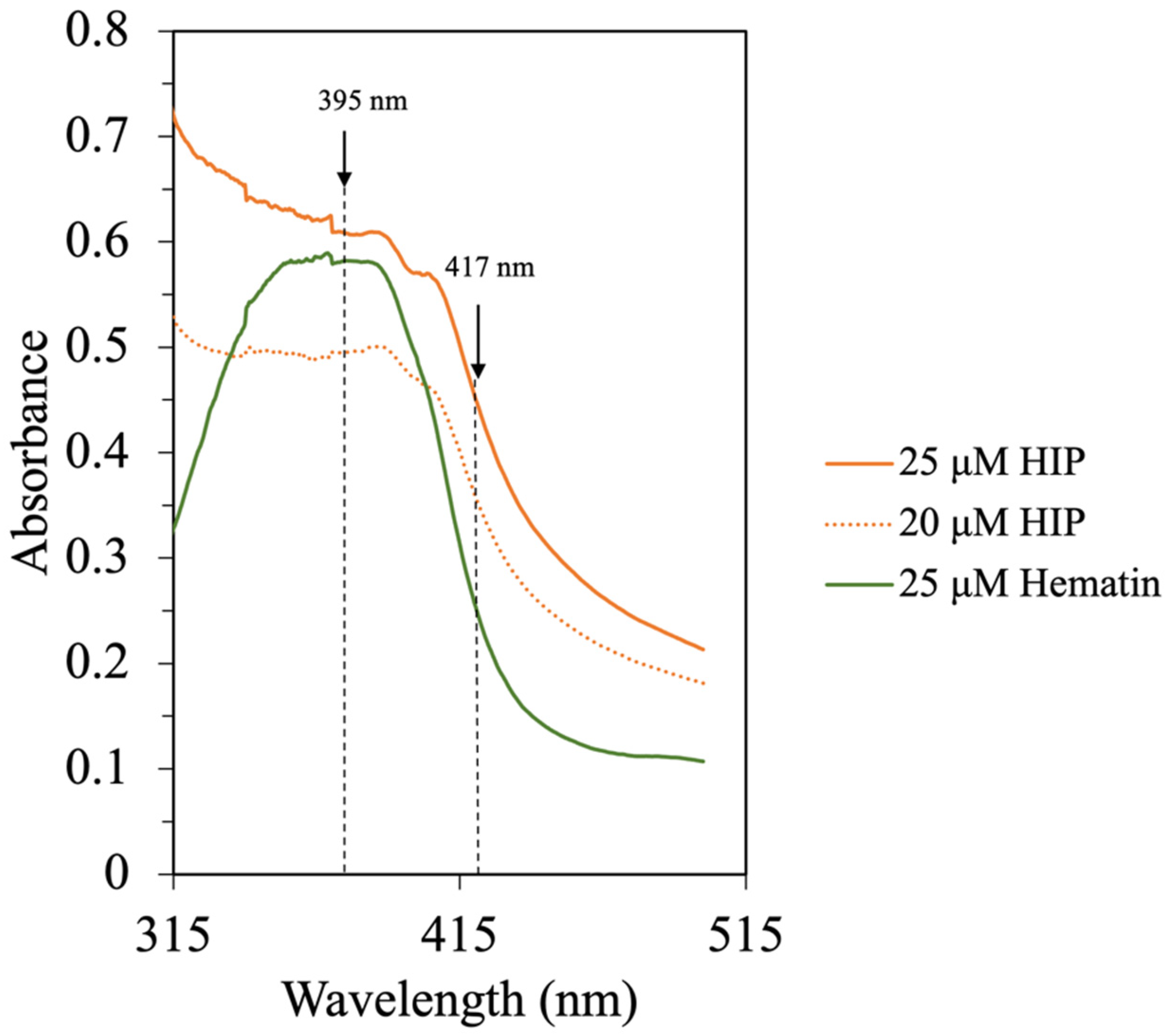
2.8. UV–Visible Difference Spectrometry
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Tuna Batch Variation on Blood Composition and HIP Characteristics
3.1.1. Effect of Tuna Batches on Blood Composition
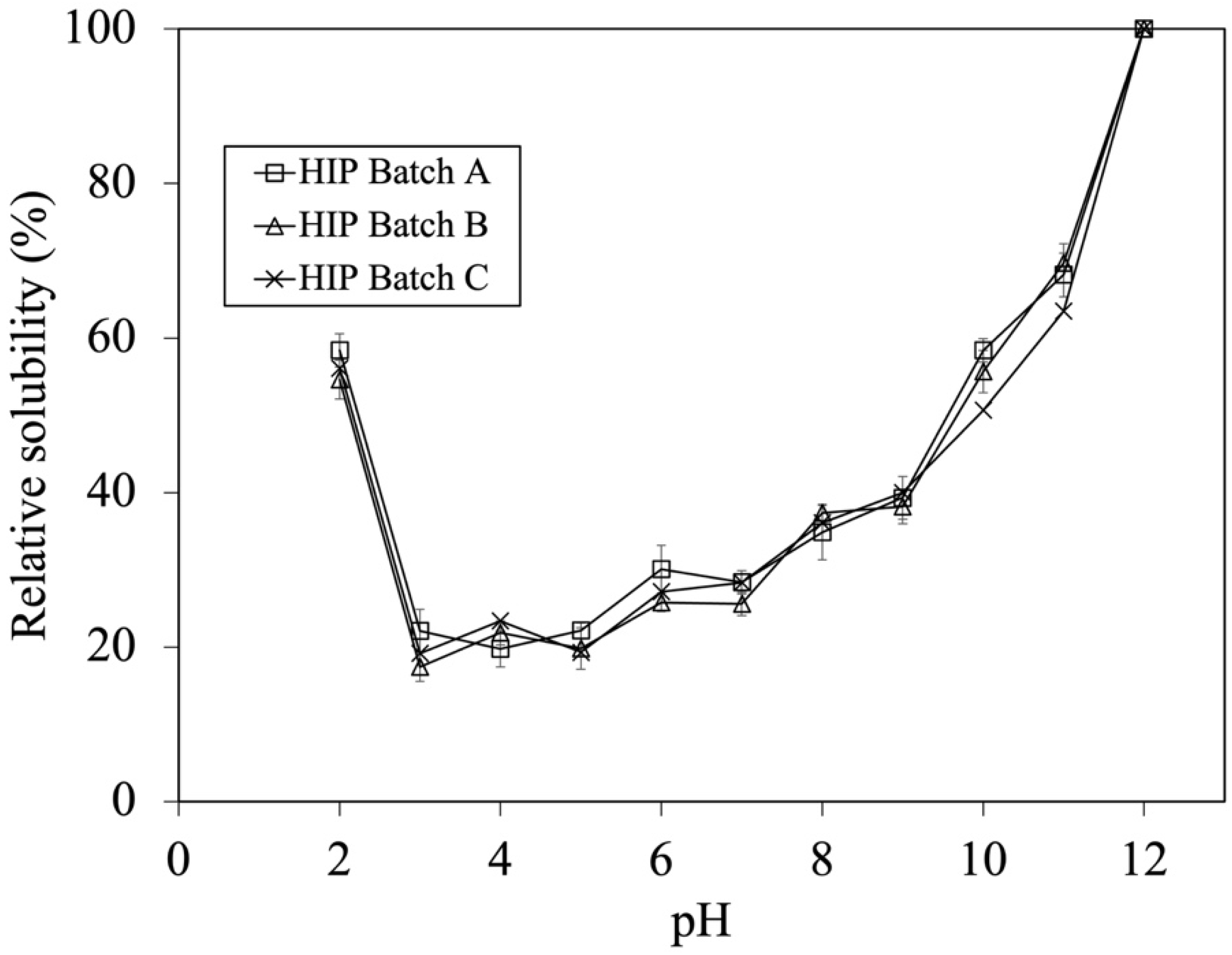
3.1.2. Effect of Tuna Batches on Degree of Hydrolysis, Iron Contents and Solubility of HIP
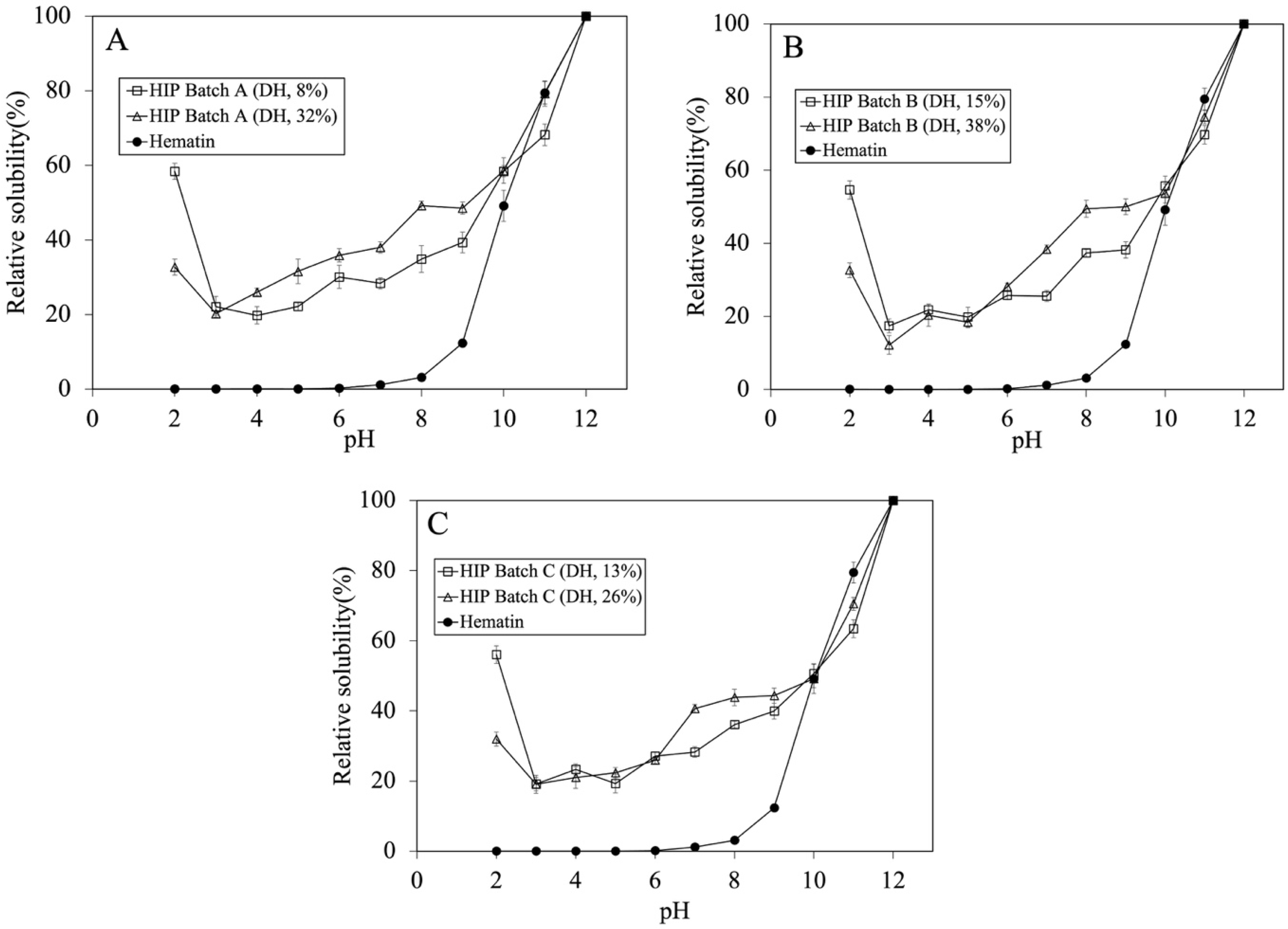
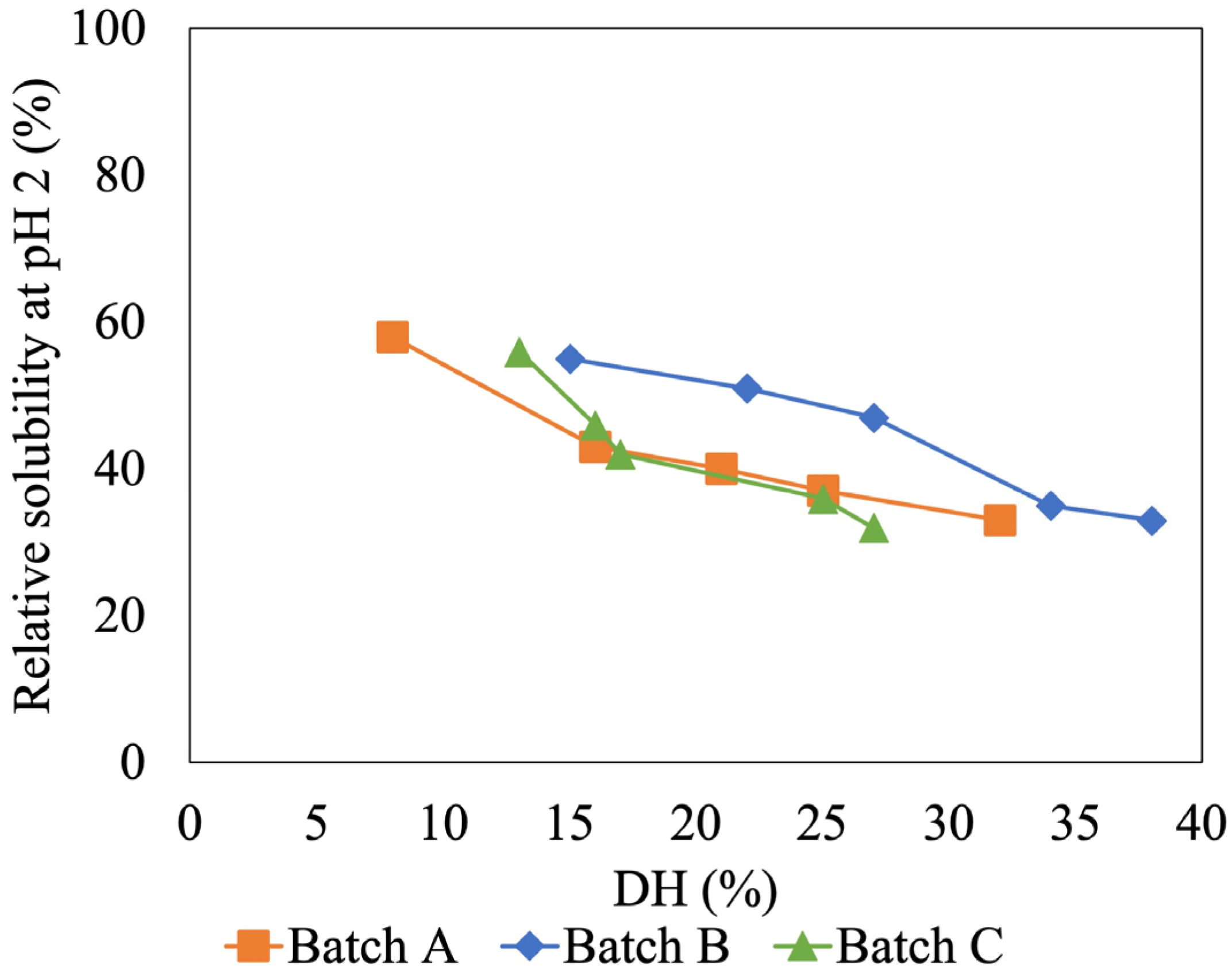
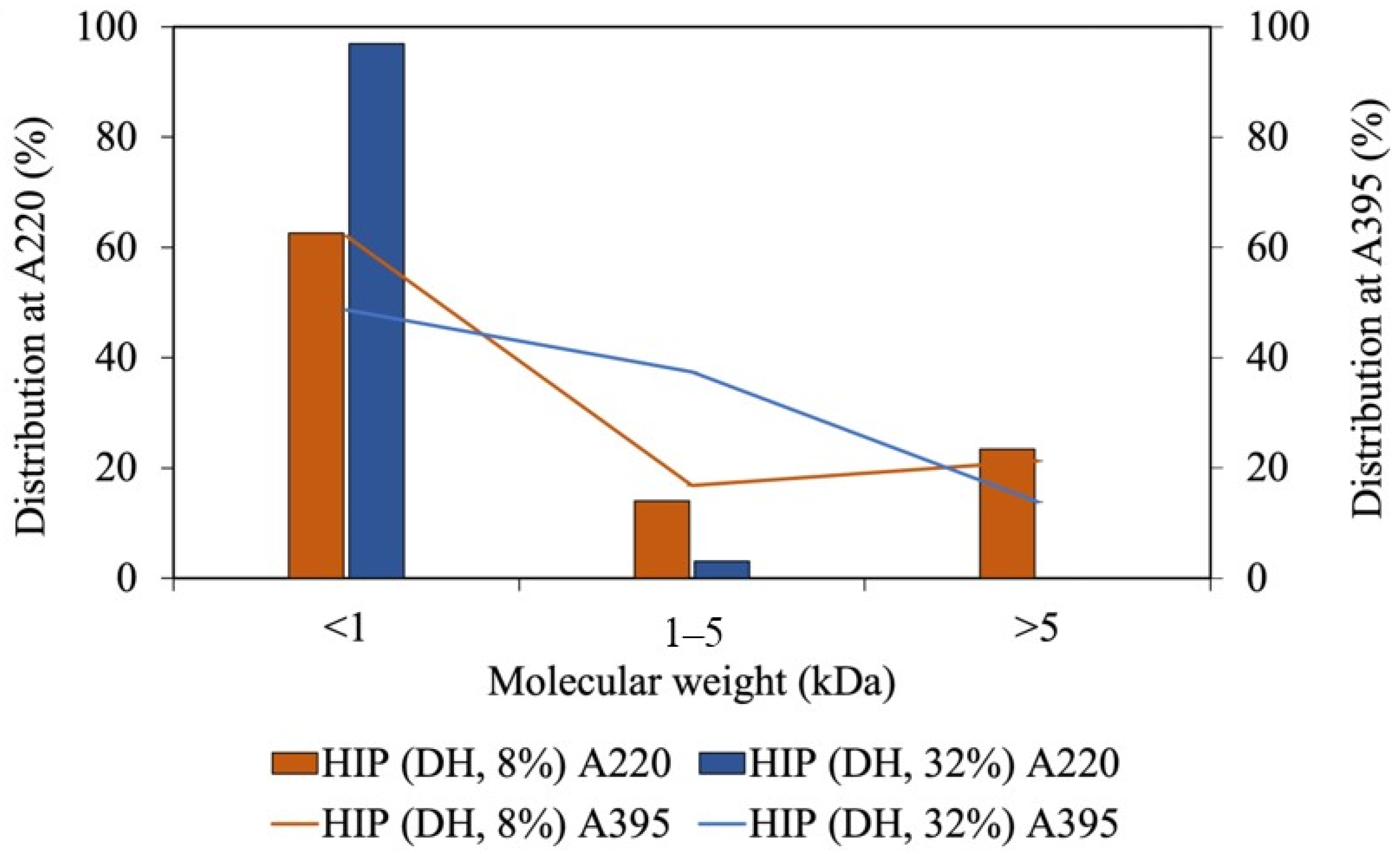
3.2. Effect of Degree of Hydrolysis on Solubility and Molecular Weight of Tuna-HIP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alleyne, M.; Horne, M.K.; Miller, J.L. Individualized Treatment for Iron-deficiency Anemia in Adults. Am. J. Med. 2008, 121, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L. Iron Deficiency Anemia: A Common and Curable Disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Young, I.; Parker, H.M.; Rangan, A.; Prvan, T.; Cook, R.L.; Donges, C.E.; Steinbeck, K.S.; O’dwyer, N.J.; Cheng, H.L.; Franklin, J.L.; et al. Association between Haem and Non-Haem Iron Intake and Serum Ferritin in Healthy Young Women. Nutrients 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Todaka, M.; Oshima, T.; Baba, Y. Production and Characterization of Hydrophilic Heme Iron Preparation from Fish Blood. J. Chem. Eng. Jpn. 2014, 47, 893–899. [Google Scholar] [CrossRef]
- Lebrun, F.; Bazus, A.; Dhulster, P.; Guillochon, D. Solubility of Heme in Heme-Iron Enriched Bovine Hemoglobin Hydrolysates. J. Agric. Food Chem. 1998, 46, 5017–5025. [Google Scholar] [CrossRef]
- Liu, T.-X.; Wang, J.; Zhao, M.-M. In vitro haem solubility of red cell fraction of porcine blood under various treatments. Int. J. Food Sci. Technol. 2010, 45, 719–725. [Google Scholar] [CrossRef]
- He, L.; Yang, F.; Liang, Y.; Zhang, M.; Liu, X.; Zhao, S.; Jin, G. Process optimisation of haemoglobin hydrolysis by complex proteases to produce haem-enriched peptides and its iron uptake property evaluation by Caco-2 cell model. Int. J. Food Sci. Technol. 2020, 55, 3412–3423. [Google Scholar] [CrossRef]
- Vaghefi, N.; Nedjaoum, F.; Guillochon, D.; Bureau, F.; Arhan, P.; Bouglé, D. Influence of the Extent of Hemoglobin Hydrolysis on the Digestive Absorption of Heme Iron. An In Vitro Study. J. Agric. Food Chem. 2002, 50, 4969–4973. [Google Scholar] [CrossRef]
- In, M.J.; Kim, D.C.; Chae, H.J.; Oh, N.S. Effects of degree of hydrolysis and pH on the solubility of heme-iron enriched peptide in hemoglobin hy-drolysate. Biosci. Biotechnol. Biochem. 2003, 67, 365–367. [Google Scholar] [CrossRef]
- Gamarro, E.G.; Orawattanamateekul, W.; Sentina, J.; Gopal, T.S. By-products of tuna processing. GLOBEFISH Res. Programme 2013, 112, 48. [Google Scholar]
- Olson, R.J.; Young, J.W.; Ménard, F.; Potier, M.; Allian, M.; Goñi, N.; Logan, M.; Galván-Magaña, F. Chapter Four–Bioenergetics, Trophic Ecology, and Niche Separation of Tunas. In Advances in Marine Biology; Curry, B.E., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 199–344. [Google Scholar]
- Rasul, M.; Jahan, I.; Yuan, C.; Sarkar, M.S.I.; Bapary, M.A.J.; Baten, M.A.; Shah, A.A. Seasonal variation of nutritional constituents in fish of South Asian Countries: A review. Fundam. Appl. Agric. 2021, 6, 193–209. [Google Scholar]
- Chakma, S.; Rahman, A.; Mali, S.K.; Debnath, S.; Hoque, S.; Siddik, M.A. Influence of frozen storage period on the biochemical, nutritional, and microbial quality of Skipjack tuna (Katsuwonus pelamis) collected from the Bay of Bengal coast of Bangladesh. Food Chem. Adv. 2022, 1, 100139. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food Analysis, 4th ed.; Springer: New York, NY, USA; Dordrecht, Germany; Berlin/Heidelberg, Germany; London, UK, 2010. [Google Scholar]
- Pourkhalili, A.; Mirlohi, M.; Rahimi, E. Heme Iron Content in Lamb Meat Is Differentially Altered upon Boiling, Grilling, or Frying as Assessed by Four Distinct Analytical Methods. Sci. World J. 2013, 2013, 374030. [Google Scholar] [CrossRef]
- Barr, I.; Guo, F. Pyridine Hemochromagen Assay for Determining the Concentration of Heme in Purified Protein Solutions. Bio-protocol 2015, 5, e1594. [Google Scholar] [CrossRef]
- Pandey, A.; Joshi, S.; Tekwani, B.; Chauhan, V. A Colorimetric Assay for Heme in Biological Samples Using 96-Well Plates. Anal. Biochem. 1999, 268, 159–161. [Google Scholar] [CrossRef]
- Gu, R.-Z.; Li, C.-Y.; Liu, W.-Y.; Yi, W.-X.; Cai, M.-Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef]
- Kumar, L.A.M.; Padmavati, G. Proximate and Elemental Composition of Stolephorus commersonnii (Lacepede, 1803) from the Coastal Waters of South Andaman. 2017. Available online: https://nopr.niscpr.res.in/handle/123456789/41652 (accessed on 20 June 2023).
- Maepakdee, S.; Rattanasuwan, P.; Krasaechon, N. Enrichment of Protein and Iron in Snack Products Using Tuna Blood Powder. J. KMUTNB 2014, 24, 168–177. [Google Scholar]
- Sorapukdee, S.; Narunatsopanon, S. Comparative Study on Compositions and Functional Properties of Porcine, Chicken and Duck Blood. Korean J. Food Sci. Anim. Resour. 2017, 37, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Turhan, S.; Ustun, N.S.; Bank, I. Effect of freeze–thaw cycles on total and heme iron contents of bonito (Sarda sarda) and bluefish (Pomatomus saltator) fillets. J. Food Compos. Anal. 2006, 19, 384–387. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Richards, M.P. The effect of Fenton’s reactants and aldehydes on the changes of myoglobin from Eastern little tuna (Euthynnus affinis) dark muscle. Eur. Food Res. Technol. 2010, 232, 221–230. [Google Scholar] [CrossRef]
- Rutherfurd, S.M. Methodology for Determining Degree of Hydrolysis of Proteins in Hydrolysates: A Review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef]
- Collins, J.F.; Anderson, G.J. Chapter 71—Molecular Mechanisms of Intestinal Iron Transport. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L.R., Ghishan, F.K., Wood, J.D., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1921–1947. [Google Scholar]
- Conrad, M.E.; Cortell, S.; Williams, H.L.; Foy, A.L. Polymerization and intraluminal factors in the absorption of hemoglobin-iron. J. Lab. Clin. Med. 1966, 68, 659–668. [Google Scholar]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal iron absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef]
- In, M.-J.; Chae, H.J.; Oh, N.-S. Process development for heme-enriched peptide by enzymatic hydrolysis of hemoglobin. Bioresour. Technol. 2002, 84, 63–68. [Google Scholar] [CrossRef]
- Park, K.-H.; Kim, H.-S.; Han, M.-K.; Kim, U.-H. Heme iron polypeptide polymer with high iron content as an ideal iron supplement. J. Food Biochem. 2010, 34, 896–904. [Google Scholar] [CrossRef]

| Tuna Batch | Fishing Trip Date | Ocean Area | Number of Tuna Fish |
|---|---|---|---|
| Batch A | Jan.–Feb. 2022 | Western Central Pacific (FAO Major Fishing Area 71) | 40 |
| Batch B | Feb.–Mar. 2022 | Western Central Pacific (FAO Major Fishing Area 71) | 40 |
| Batch C | Jan.–Feb. 2022 | Western Central Pacific (FAO Major Fishing Area 71) | 40 |
| Composition (Dry Basis) | Batch A | Batch B | Batch C |
|---|---|---|---|
| Protein (g/100 g) | 58.78 ± 0.56 a | 63.51 ± 1.65 b | 65.99 ± 0.41 c |
| Iron (mg/100 g) ns | 70.54 ± 3.22 | 70.27 ± 1.79 | 69.97 ± 2.21 |
| Total iron (%) ns | 0.07% | 0.07% | 0.07% |
| Heme iron ** (mg/100 g) ns | 54.19 ± 0.38 | 54.76 ± 0.24 | 52.60 ± 0.55 |
| Total Heme iron (%) ns | 75.88 ± 3.24 | 77.99 ± 3.15 | 74.57 ± 3.66 |
| Composition (Dry Basis) | Batch A | Batch B | Batch C | |||
|---|---|---|---|---|---|---|
| Blood | HIP (4 h) | Blood | HIP (4 h) | Blood | HIP (4 h) | |
| Iron (mg/100 g) ns | 70.54 ± 3.22 | 67.93 ± 0.60 | 70.27 ± 1.79 | 70.92 ± 0.61 | 69.97 ± 2.21 | 67.71 ± 2.66 |
| Heme iron (mg/100 g) ns | 54.19 ± 0.38 | 54.25 ± 0.61 | 54.76 ± 0.24 | 55.06 ± 0.38 | 52.60 ± 0.55 | 51.71 ± 0.59 |
| Sources | Enzyme Conditions | %DH | Molecular Weight | Solubility | References |
|---|---|---|---|---|---|
| Skipjack tuna | Alcalase, pH 8.5, 0.5 h | 8–15% | <1 kDa and 1–5 kDa | pH 2, pH > 7 | This study |
| Bovine | Pepsin, pH 4, 24 h | 11% | 4–5 kDa | pH 2–12 except 5.5 | [6] |
| Pepsin, Subtilisin | 15% | N/A | pH2 | [9] | |
| Esparase + Flavourzyme | 19.8% | N/A | pH > 6 | [10,32] | |
| Porcine | Alcalase | N/A | 80 kDa and 250 kDa | Wide range pH (N/A specific pH) | [33] |
| Alcalase + Flavourzyme, pH 7.5 | 8–12% | 1–7.5 kDa | pH 2 | [7] | |
| Neutrase, acid enzyme, alkaline enzyme, and Papain | 6–12% | 3–14.4 kDa | pH > 8 | [8] | |
| Yellowtail fish | Alcalase, pH 10, 24 h | N/A | 2–3.6 kDa | pH < 4, pH > 6 | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tansukkasem, S.; Kaewpathomsri, P.; Jonjaroen, V.; Payongsri, P.; Lertsiri, S.; Niamsiri, N. Production and Characterization of Heme Iron Polypeptide from the Blood of Skipjack Tuna (Katsuwonus pelamis) Using Enzymatic Hydrolysis for Food Supplement Application. Foods 2023, 12, 3249. https://doi.org/10.3390/foods12173249
Tansukkasem S, Kaewpathomsri P, Jonjaroen V, Payongsri P, Lertsiri S, Niamsiri N. Production and Characterization of Heme Iron Polypeptide from the Blood of Skipjack Tuna (Katsuwonus pelamis) Using Enzymatic Hydrolysis for Food Supplement Application. Foods. 2023; 12(17):3249. https://doi.org/10.3390/foods12173249
Chicago/Turabian StyleTansukkasem, Satita, Piriya Kaewpathomsri, Veasarach Jonjaroen, Panwajee Payongsri, Sittiwat Lertsiri, and Nuttawee Niamsiri. 2023. "Production and Characterization of Heme Iron Polypeptide from the Blood of Skipjack Tuna (Katsuwonus pelamis) Using Enzymatic Hydrolysis for Food Supplement Application" Foods 12, no. 17: 3249. https://doi.org/10.3390/foods12173249
APA StyleTansukkasem, S., Kaewpathomsri, P., Jonjaroen, V., Payongsri, P., Lertsiri, S., & Niamsiri, N. (2023). Production and Characterization of Heme Iron Polypeptide from the Blood of Skipjack Tuna (Katsuwonus pelamis) Using Enzymatic Hydrolysis for Food Supplement Application. Foods, 12(17), 3249. https://doi.org/10.3390/foods12173249







