Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases
Abstract
:1. Introduction
2. Development of NCDs
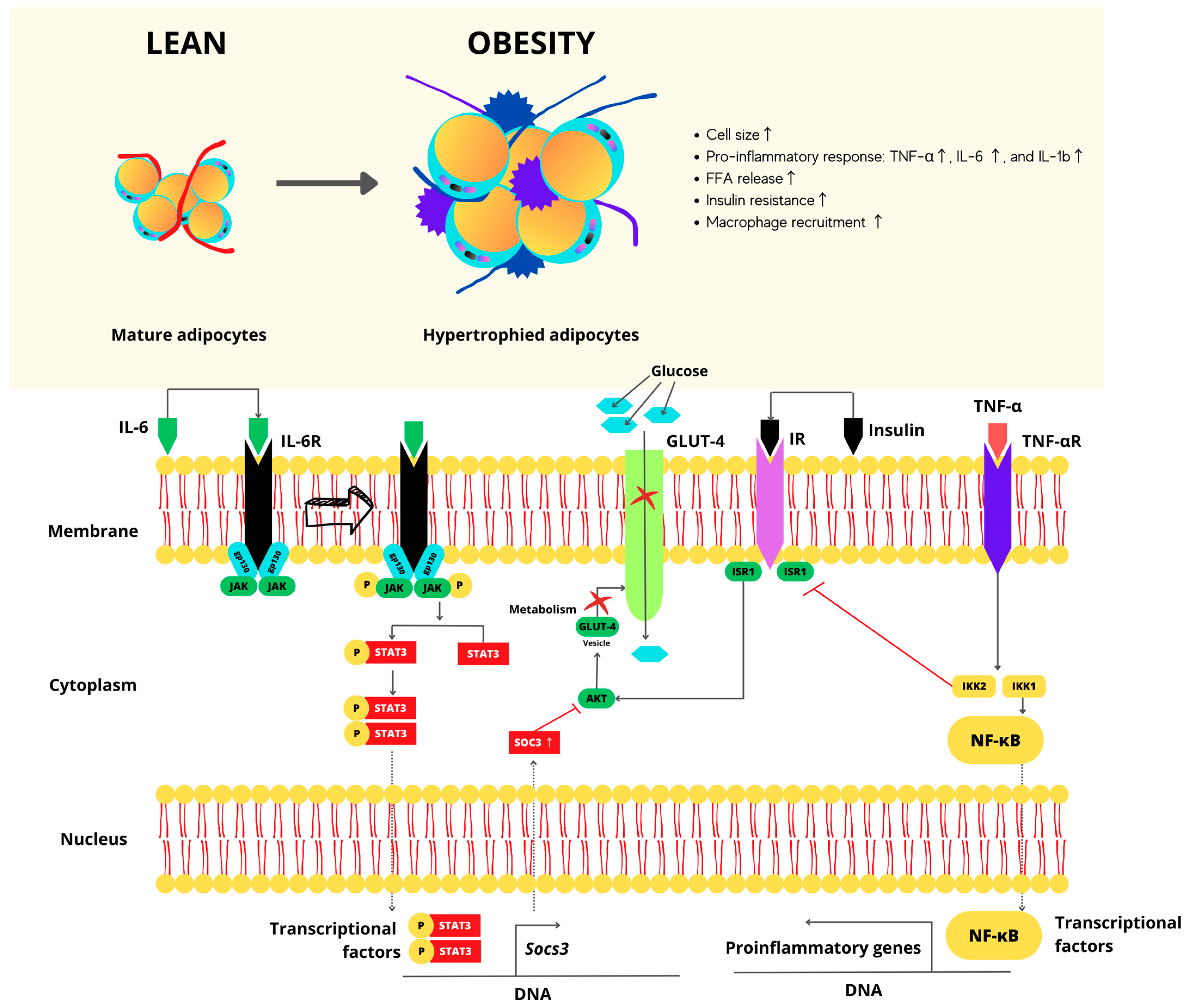
2.1. Molecular Mechanisms in NCDs Illustrated by Overweight and Obese Stages
2.2. Relation of Junk Foods Consumption with NCDs Development
3. Nutraceuticals: Classification and Biological Potential
3.1. Vitamins
3.2. Minerals
3.3. Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R301–R317. [Google Scholar] [CrossRef] [PubMed]
- WHO. Health Systems and Health Security: Developing Conceptual Clarity and a WHO Roadmap for Action; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hosker, D.K.; Elkins, R.M.; Potter, M.P. Promoting Mental Health and Wellness in Youth Through Physical Activity, Nutrition, and Sleep. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 171–193. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, D.; Kroese, F.; Evers, C.; Adriaanse, M.; Gillebaart, M. Healthy diet: Health impact, prevalence, correlates, and interventions. Psychol. Health 2017, 32, 907–941. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Villalobos, J.Á.; Barriguete-Meléndez, J.A.; Lara-Esqueda, A.; Barquera, S.; Rosas-Peralta, M.; Hernández-Ávila, M.; de León-May, M.E.; Admon, L.; Aguilar-Salinas, C.A. Las enfermedades crónicas no transmisibles en México: Sinopsis epidemiológica y prevención integral. Salud Pública México 2008, 50, 419–427. [Google Scholar] [CrossRef]
- Raiten, D.J.; Sakr Ashour, F.A.; Ross, A.C.; Meydani, S.N.; Dawson, H.D.; Stephensen, C.B.; Brabin, B.J.; Suchdev, P.S.; van Ommen, B. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J. Nutr. 2015, 145, 1039S–1108S. [Google Scholar] [CrossRef]
- World Health Organization. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE); World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2006.
- Sahib, N.G.; Anwar, F.; Gilani, A.-H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals—A review. Phytother. Res. 2013, 27, 18. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Pan American Health Organization. Rapid Assessment of Service Delivery for NCDs during the COVID-19 Pandemic in the Americas; Pan American Health Organization: Washington, DC, USA, 2020; p. 8.
- World Health Organization. Guideline for the Pharmacological Treatment of Hypertension in Adults; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2021; pp. 1–48.
- Burton, M.A.; Lillycrop, K.A. Nutritional modulation of the epigenome and its implication for future health. Proc. Nutr. Soc. 2019, 78, 305–312. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2019: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020.
- Robledo-Martínez, R. Las enfermedades no transmisibles en Colombia (Non-communicable diseases in Colombia). Bol. Obs. Salud 2010, 3, 1–9. [Google Scholar]
- World Health Organization. Global Status Report Non Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014.
- Serra-Valdés, M.Á.; Serra-Ruíz, M.; Viera-García, M. Non transmissible chronic diseases: Current magnitude and future trends. Rev. Finlay DOAJ 2018, 8, 140–148. [Google Scholar]
- Salud, S.D. Panorama Epidemiológico de las Enfermedades no Transmisibles en México 2019. 2020. p. 41. Available online: https://www.gob.mx/salud/documentos/panorama-epidemiologico-de-las-enfermedades-no-transmisibles-en-mexico-269304 (accessed on 22 August 2023).
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Gomez, B.; Almeda-Valdes, P.; Tussie-Luna, M.T.; Aguilar-Salinas, C.A. Dyslipidemia in Mexico, a Call for Action. Rev. Investig. Clin. 2018, 70, 211–216. [Google Scholar] [CrossRef]
- Pearce, N.; Ebrahim, S.; McKee, M.; Lamptey, P.; Barreto, M.L.; Mathenson, D.; Walls, H.; Foliaki, S.; Miranda, J.J.; Chimeddamba, O.; et al. Global prevention and control of NCDs: Limitations of the standard approach. J. Public Health Policy 2015, 36, 27. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Plan of Action for the Elimination of Industrially Produced Trans-Fatty Acids 2020–2025; Pan American Health Organization: Washington, DC, USA, 2020.
- Bustos, S. Series The Food System and the Challenges of COVID-19: COVID-19 and the Food Phenomena; Food and Agricultural Organization of the United Nations: Rome, Italy, 2020; No. 1. [Google Scholar]
- van Daal, M.T.; Folkerts, G.; Garssen, J.; Braber, S. Pharmacological Modulation of Immune Responses by Nutritional Components. Pharmacol. Rev. 2021, 73, 34. [Google Scholar] [CrossRef] [PubMed]
- FAO; OPS; WEP; UNICEF. Panorama de la Seguridad Alimentaria y Nutrición en Ámerica Latina y el Caribe 2019; Santiago de Chile. 2019, p. 124. Available online: https://www.unicef.org/lac/media/9316/file/PDF (accessed on 22 August 2023).
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Blancas-Flores, G.; Almanza-Pérez, J.C.; López-Roa, R.I.; Alarcón-Aguilar, F.J.; García-Macedo, M.C. La obesidad como un proceso inflamatorio (Obesity as an inflammatory process). In Clinical Interventions in Aging; Hospital Infantil de México: Ciudad de México, Mexico, 2010; pp. 88–97. [Google Scholar]
- González-Chávez, A.; Elizondo-Argueta, S.; Gutiérrez-Reyes, G.; León-Pedroza, J.I. Implicaciones fisiopatológicas entre inflamación crónica y el desarrollo de diabetes y obesidad. Cirugía Cir. 2011, 79, 209–216. [Google Scholar]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Juul, F.; Martinez-Steele, E.; Parekh, N.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018, 120, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Denney, L.; Reidy, K.C.; Eldridge, A.L. Differences in Complementary Feeding of 6 to 23 Month Olds in China, US and Mexico. J. Nutr. Health Food Sci. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- FAO; WHO. Probiotics in food—Health and nutritional properties and guidelines for evaluation. In FAO Food and Nutrition Paper 85; FAO: Rome, Italy, 2006; p. 56. [Google Scholar]
- Kalra, E.K. Nutraceutical—Definition and introduction. AAPS PharmSci. 2003, 5, E25. [Google Scholar] [CrossRef]
- Bigiani, A. Electrophysiology of sodium receptors in taste cells. J. Biomed. Sci. Eng. 2016, 9, 367–383. [Google Scholar] [CrossRef]
- Berdigaliyev, N.; Aljofan, M. An overview of drug discovery and development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Zhang, R.; Monsma, F. Binding kinetics and mechanism of action: Toward the discovery and development of better and best in class drugs. Expert. Opin. Drug Discov. 2010, 5, 1023–1029. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting their Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef]
- Gupta, R.C.; Srivastava, A.; Lall, R. Toxicity Potential of Nutraceuticals. Methods Mol. Biol. 2018, 1800, 367–394. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Gvozdjáková, A.; Pella, D.; Kucharská, J.; Otsuka, K.; Singh, R.B. Omega-3-PUFA, Omega-6-PUFA and Mitochondria. In Mitochondrial Medicine: Mitochondrial Metabolism, Diseases, Diagnosis and Therapy; Gvozdjáková, A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 343–356. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E function and requirements in relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef]
- Cardenas, E.; Ghosh, R. Vitamin E: A dark horse at the crossroad of cancer management. Biochem. Pharmacol. 2013, 86, 845–852. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl Med. 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Pinto, A.; Acuna, A.I.; Beltran, F.A.; Torres-Diaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Klimant, E.; Wright, H.; Rubin, D.; Seely, D.; Markman, M. Intravenous vitamin C in the supportive care of cancer patients: A review and rational approach. Curr. Oncol. 2018, 25, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Pastori, D.; Angelico, F. The role of nutraceuticals for the treatment of non-alcoholic fatty liver disease. Br. J. Clin. Pharmacol. 2017, 83, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, W.G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed. Pharmacother. 2020, 129, 110493. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef]
- Nitulescu, G.M.; Paunescu, H.; Moschos, S.A.; Petrakis, D.; Nitulescu, G.; Ion, G.N.D.; Spandidos, D.A.; Nikolouzakis, T.K.; Drakoulis, N.; Tsatsakis, A. Comprehensive analysis of drugs to treat SARS-CoV-2 infection: Mechanistic insights into current COVID-19 therapies (Review). Int. J. Mol. Med. 2020, 46, 467–488. [Google Scholar] [CrossRef]
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodstrom-Tullberg, M.; Hjelte, L. Vitamin D treatment modulates immune activation in cystic fibrosis. Clin. Exp. Immunol. 2017, 189, 359–371. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.L.; Sempos, C.T.; Davis, C.D.; Brannon, P.M. Vitamin D: Moving Forward to Address Emerging Science. Nutrients 2017, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Penckofer, S.; Byrn, M.; Adams, W.; Emanuele, M.A.; Mumby, P.; Kouba, J.; Wallis, D.E. Vitamin D Supplementation Improves Mood in Women with Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 8232863. [Google Scholar] [CrossRef]
- Hussien, H.; Abd-Rabou, H.S.; Saad, M.A. The impact of incorporating Lactobacillus acidophilus bacteriocin with inulin and FOS on yogurt quality. Sci. Rep. 2022, 12, 13401. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, A.; Affatato, R.; Centritto, F.; Fratelli, M.; Kurosaki, M.; Barzago, M.M.; Bolis, M.; Terao, M.; Garattini, E.; Paroni, G. All-trans-retinoic Acid Modulates the Plasticity and Inhibits the Motility of Breast Cancer Cells: Role of notch1 and transforming growth factor (TGFbeta). J. Biol. Chem 2015, 290, 17690–17709. [Google Scholar] [CrossRef] [PubMed]
- Szutowicz, A.; Bielarczyk, H.; Jankowska-Kulawy, A.; Ronowska, A.; Pawełczyk, T. Retinoic acid as a therapeutic option in Alzheimer’s disease: A focus on cholinergic restoration. Expert Rev. Neurother. 2015, 15, 239–249. [Google Scholar] [CrossRef]
- Giuli, M.V.; Hanieh, P.N.; Giuliani, E.; Rinaldi, F.; Marianecci, C.; Screpanti, I.; Checquolo, S.; Carafa, M. Current trends in ATRA delivery for cancer theraphy. Pharmaceutics 2020, 12, 707. [Google Scholar] [CrossRef]
- Constantinescu-Aruxandei, D.; Frincu, R.M.; Capra, L.; Oancea, F. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients 2018, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choo, G.S.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, J.Y. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol. Rep. 2019, 42, 1904–1914. [Google Scholar] [CrossRef]
- Lovelace, E.S.; Wagoner, J.; MacDonald, J.; Bammler, T.; Bruckner, J.; Brownell, J.; Beyer, R.P.; Zink, E.M.; Kim, Y.M.; Kyle, J.E.; et al. Silymarin Suppresses Cellular Inflammation By Inducing Reparative Stress Signaling. J. Nat. Prod. 2015, 78, 1990–2000. [Google Scholar] [CrossRef]
- Borah, A.; Paul, R.; Choudhury, S.; Choudhury, A.; Bhuyan, B.; Das Talukdar, A.; Dutta Choudhury, M.; Mohanakumar, K.P. Neuroprotective potential of silymarin against CNS disorders: Insight into the pathways and molecular mechanisms of action. CNS Neurosci. Ther. 2013, 19, 847–853. [Google Scholar] [CrossRef]
- Liu, C.H.; Jassey, A.; Hsu, H.Y.; Lin, L.T. Antiviral Activities of Silymarin and Derivatives. Molecules 2019, 24, 1552. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Yang, K.; Chan, C.B. Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity. Acta Pharmacol. Sin. 2018, 39, 893–902. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular Mechanisms and Therapeutic Effects of (-)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; Ponnurangam, S.; Ramamoorthy, P.; Standing, D.; Battafarano, R.J.; Anant, S.; Sharma, P. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS ONE 2012, 7, e30590. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Yosri, N.; El-Mallah, M.F.; Ghonaim, R.; Guo, Z.; Musharraf, S.G.; Du, M.; Khatib, A.; Xiao, J.; Saeed, A.; et al. Screening for natural and derived bio-active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic. Phytomedicine 2021, 85, 153311. [Google Scholar] [CrossRef] [PubMed]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, V.; Chiaramonte, R.; Nizzardo, M.; Cristofaro, B.; Basile, A.; Sherbet, G.V.; Comi, P. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem. Pharmacol. 2007, 74, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Goncalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, K.; Li, G.; Yao, J.; Dai, Q.; Hui, H.; Li, Z.; Guo, Q.; Lu, N. Oroxylin A inhibits hypoxia-induced invasion and migration of MCF-7 cells by suppressing the Notch pathway. Anticancer Drugs 2014, 25, 778–789. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, Y.; Qiao, C.; Ni, T.; Li, Z.; Wang, X.; Guo, Q.; Lu, N.; Wei, L. Oroxylin A promotes PTEN-mediated negative regulation of MDM2 transcription via SIRT3-mediated deacetylation to stabilize p53 and inhibit glycolysis in wt-p53 cancer cells. J. Hematol. Oncol. 2015, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.E.; Song, J.H.; Song, H.H.; Kang, J.W.; Hwang, S.N.; Rhee, K.J.; Shim, A.; Hong, E.H.; Kim, Y.J.; Jeon, S.M.; et al. Antiviral Activity of Oroxylin A against Coxsackievirus B3 Alleviates Virus-Induced Acute Pancreatic Damage in Mice. PLoS ONE 2016, 11, e0155784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zeng, G.; Chen, Y.; Zhang, Q.; Liu, B.; Liu, J.; Chen, H.; Li, M. Oroxylin A accelerates liver regeneration in CCl(4)-induced acute liver injury mice. PLoS ONE 2013, 8, e71612. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Zhang, X.; Tang, J.; Li, Z.; Wang, Q.; Hu, R. Oroxylin A inhibits colitis by inactivating NLRP3 inflammasome. Oncotarget 2017, 8, 58903–58917. [Google Scholar] [CrossRef]
- Lee, A.Y.; Kang, S.; Park, S.J.; Huang, J.; Im, D.S. Anti-Allergic Effect of Oroxylin A from Oroxylum indicum Using in vivo and in vitro Experiments. Biomol. Ther. 2016, 24, 283–290. [Google Scholar] [CrossRef]
- Jeon, S.J.; Bak, H.; Seo, J.; Han, S.M.; Lee, S.H.; Han, S.H.; Kwon, K.J.; Ryu, J.H.; Cheong, J.H.; Ko, K.H.; et al. Oroxylin A Induces BDNF Expression on Cortical Neurons through Adenosine A2A Receptor Stimulation: A Possible Role in Neuroprotection. Biomol. Ther. 2012, 20, 27–35. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kurbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H.B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- Menze, E.T.; Esmat, A.; Tadros, M.G.; Abdel-Naim, A.B.; Khalifa, A.E. Genistein improves 3-NPA-induced memory impairment in ovariectomized rats: Impact of its antioxidant, anti-inflammatory and acetylcholinesterase modulatory properties. PLoS ONE 2015, 10, e0117223. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Arabyan, E.; Hakobyan, A.; Kotsinyan, A.; Karalyan, Z.; Arakelov, V.; Arakelov, G.; Nazaryan, K.; Simonyan, A.; Aroutiounian, R.; Ferreira, F.; et al. Genistein inhibits African swine fever virus replication in vitro by disrupting viral DNA synthesis. Antiviral Res. 2018, 156, 128–137. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013, 4, 200–212. [Google Scholar] [CrossRef]
- Wu, C.C.; Fang, C.Y.; Cheng, Y.J.; Hsu, H.Y.; Chou, S.P.; Huang, S.Y.; Tsai, C.H.; Chen, J.Y. Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 2017, 24, 2. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Munoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Colucci, R.; Pellegrini, C.; Tirotta, E.; Benvenuti, L.; Segnani, C.; Ippolito, C.; Duranti, E.; Virdis, A.; et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS ONE 2018, 13, e0195502. [Google Scholar] [CrossRef] [PubMed]
- Escande, C.; Nin, V.; Price, N.L.; Capellini, V.; Gomes, A.P.; Barbosa, M.T.; O’Neil, L.; White, T.A.; Sinclair, D.A.; Chini, E.N. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: Implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 2013, 62, 1084–1093. [Google Scholar] [CrossRef]
- Dosedel, M.; Jirkovsky, E.; Macakova, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remiao, F.; Novakova, L.; Mladenka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Staufenbiel, I.; Weinspach, K.; Förster, G.; Geurtsen, W.; Günay, H. Periodontal conditions in vegetarians: A clinical study. Eur J. Clin. Nutr. 2013, 67, 5. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Abdelhamid, L.; Luo, X.M. Retinoic Acid, Leaky Gut, and Autoimmune Diseases. Nutrients 2018, 10, 1016. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium(-)Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef]
 ), Minerals (
), Minerals ( ), and Plants (
), and Plants ( ) lie to dietary source.
) lie to dietary source.
 ), Minerals (
), Minerals ( ), and Plants (
), and Plants ( ) lie to dietary source.
) lie to dietary source.
| Nutraceutical | Biological Activity | Source | Potential Therapeutics |
|---|---|---|---|
| Vitamin E (α-tocopherol) | Lipophilic antioxidant, protection of lipoproteins, PUFA, cellular and intra-cellular membranes from damage [50]. Neuroprotector has anti-inflammatory and hypocholesterolemic properties [51]. | Avocado, banana, tomato, plums, broccoli, spinach, grains, nuts, seeds, and vegetable oils [50,52,53]. | Alzheimer’s disease [51,52], cancer [53], liver disorders [54]. |
| Vitamin C | Acting as an antioxidant, it plays a role in detoxification processes. It participates as an enzymatic cofactor and modulates synaptic activity and neuronal metabolism, among other functions [55,56]. | Tapioca, beet, oranges, other fruits, cabbage, tomato, and corn [57,58]. | Neurodegenerative diseases [56], cancer [58], pain [55], liver diseases [59], infections [57], SARS-CoV-2 infection [60,61,62]. |
| Vitamin D * | Influences the immune response by modulating several immune pathways, inhibiting T cell proliferation as well as interleukin (IL)-17 and interferon (IFN)-γ [62,63]. | Juices, cereals, UV-exposed mushrooms, meat, poultry, and fish [64]. * Exposure to natural sunlight. | Cystic fibrosis [63], colorectal cancer [65], Diabetes mellitus type 2 [66], cardiovascular diseases [67]. |
| Retinoic acid | Inhibition of the NOTCH1 pathway [68]. A potent cell differentiation factor [69]. | Leafy greens, carrots, cantaloupe, and liver [70]. | Breast cancer with HER2-positive [68], neurodegenerative diseases [69]. |
| Selenium | Intake at the recommended level assures the balanced expression of bioactive selenoproteins, which act as oxidoreductases, redox signal regulators, or thyroid hormone activation [71]. | Vegetables, meat, and fish [72]. | Cardiovascular diseases, cancer, and immune disorders [71], SARS-CoV-2 infection and another infection [62,72]. |
| Zinc | Regulates the innate and adaptive immune responses and has several antioxidant effects [73]. | Legumes, fortified cereals, whole grains, red meat, and some shellfish [73]. | Several infections, neurodegenerative diseases [73], cancer [74], diabetes mellitus [75], cardiovascular diseases [76]. |
| Silymarin ** | It reduces inflammation and fibrogenesis, stimulates liver regeneration, exerts membrane-stabilizing and antioxidant activity [59]. Induces growth inhibition and apoptosis by suppressing the MAPK signaling pathway [77]. | Fruits and seeds of the milk thistle plant (Silybum marianum L.) [77]. | Liver diseases [59], human gastric cancer KIM 2019 [77], inflammation [78], neurodegenerative diseases [79], viral infections [80]. |
| Epicatechin | Antioxidant activity reduces inflammation, improves endothelial function and insulin resistance [81]. | Grapes, berries, apples, beans, peas, tea, and cocoa products [82]. | Cardiovascular diseases [81], cancer [83], diabetes mellitus [82]. |
| Curcumin | An antioxidant, this multitargeted agent has been shown to exhibit anti-inflammatory activity by suppressing numerous cells signaling pathways, including NF-κB, STAT3, Nrf2, ROS, and COX-2 [84]. Reduced Notch-1 activation, expression of Jagged-1 and its downstream target Hes-1 [85]. | Rhizome of Curcuma longa [84,86]. | Esophageal cancer [85], inflammatory and neurodegenerative diseases, diabetes mellitus [84], liver diseases [86], SARS-CoV-2 infection [87,88]. |
| Resveratrol | Possesses antioxidant, anti-inflammatory, cardioprotective, and anticancer properties [89]. Induces an increase in the levels of the pro-apoptotic proteins p53, its effector p21waf and Bax [90]. | Peanuts, grapes, pines, berries, red wine and Polygonum cuspidatum [89,91]. | Leukemia [90], cardiovascular diseases, SARS-CoV-2 infection [88], cancer [89], Metabolic syndrome [91], Alzheimer’s [92]. |
| Oroxylin A | Anti-inflammatory and anticancer properties. Reduced Notch-1 activation [93] inhibited glycolysis [94]. | Scutellaria radix and the root of Scutellaria baicalensis [93]. | Breast cancer [93,94], viral infections [95], liver diseases [96], colitis [97], allergy [98], neurodegenerative diseases [99]. |
| Quercetin | Antioxidant, anti-inflammatory, antithrombotic and vasodilatory actions. Decreased plasma concentrations of atherogenic oxidized LDL [100,101]. | Onions, broccoli, apples, berry crops, grapes, tea, and wine [100,102]. | Cardiovascular diseases [100], cancer [101], allergy [102,103], neurodegenerative diseases [103]. |
| Genistein | Antioxidant and anti-inflammatory activity [104]. Acts in several signaling pathways [105]. | Fruit, nuts, vegetables, legumes, and soy products [105]. | Cancer [83,105], viral infections [106], Huntington’s disease [104], diabetes mellitus [107]. |
| Apigenin | Anti-inflammatory and anticancer properties [108]. | Fruits and vegetables [108]. | Cancer [83], viral infections [108], Alzheimer’s [109], obesity [110], metabolic syndrome [111]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza-Juárez, A.; Pérez-Carrillo, E.; Arredondo-Espinoza, E.U.; Islas, J.F.; Benítez-Chao, D.F.; Escamilla-García, E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods 2023, 12, 3262. https://doi.org/10.3390/foods12173262
Garza-Juárez A, Pérez-Carrillo E, Arredondo-Espinoza EU, Islas JF, Benítez-Chao DF, Escamilla-García E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods. 2023; 12(17):3262. https://doi.org/10.3390/foods12173262
Chicago/Turabian StyleGarza-Juárez, Aurora, Esther Pérez-Carrillo, Eder Ubaldo Arredondo-Espinoza, José Francisco Islas, Diego Francisco Benítez-Chao, and Erandi Escamilla-García. 2023. "Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases" Foods 12, no. 17: 3262. https://doi.org/10.3390/foods12173262








