Reducing the Risk of Acrylamide and Other Processing Contaminant Formation in Wheat Products
Abstract
:1. Introduction
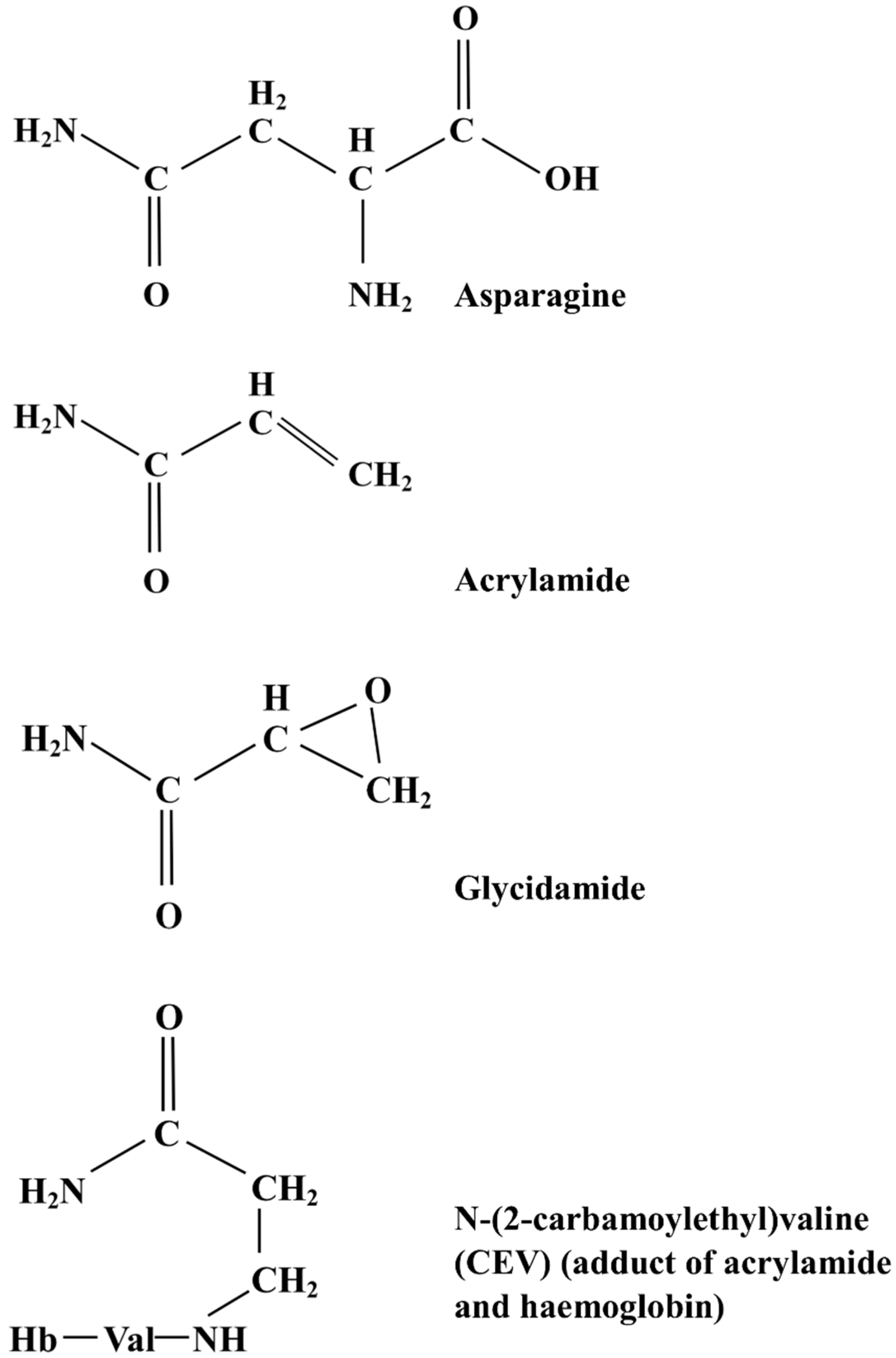
2. Acrylamide
2.1. Acrylamide Toxicity and Dietary Exposure
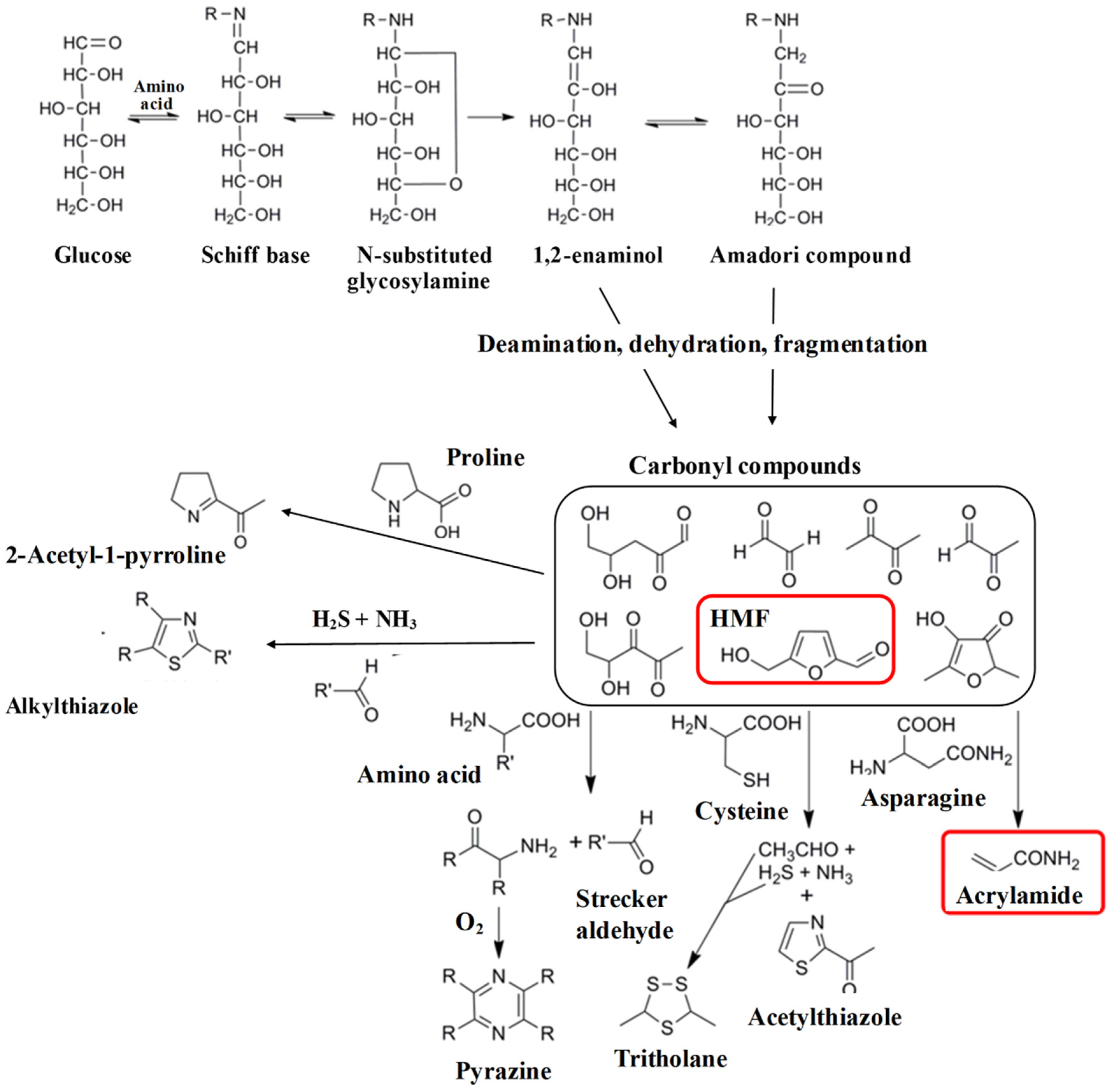
2.2. Formation
2.3. Risk Management Measures (Regulations) and the Challenge of Regulatory Compliance
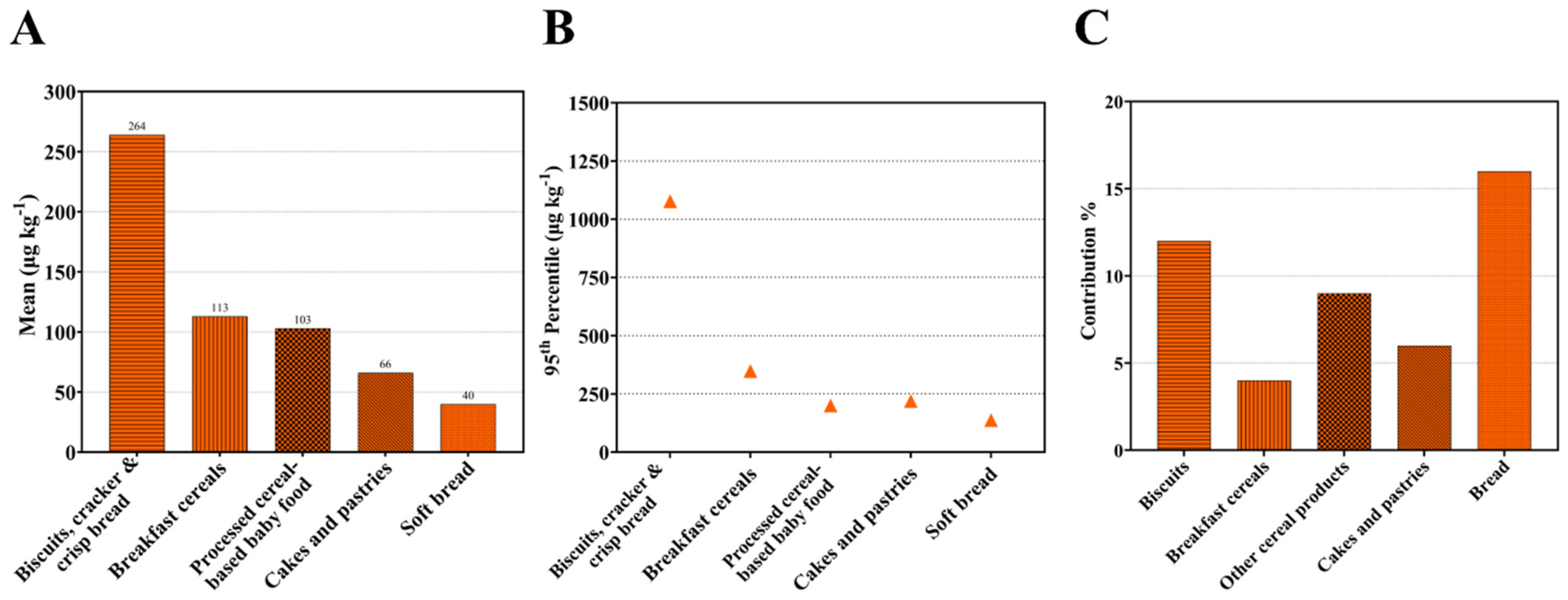
2.4. Acrylamide in Wheat Products and the Contribution of Wheat Products to Dietary Exposure
2.5. Crop Management Measures to Reduce the Acrylamide-Forming Potential of Wheat Grain
2.5.1. Fertilisation
2.5.2. Disease Control
2.6. Genetic Approaches to Reducing the Acrylamide-Forming Potential of Wheat Grain
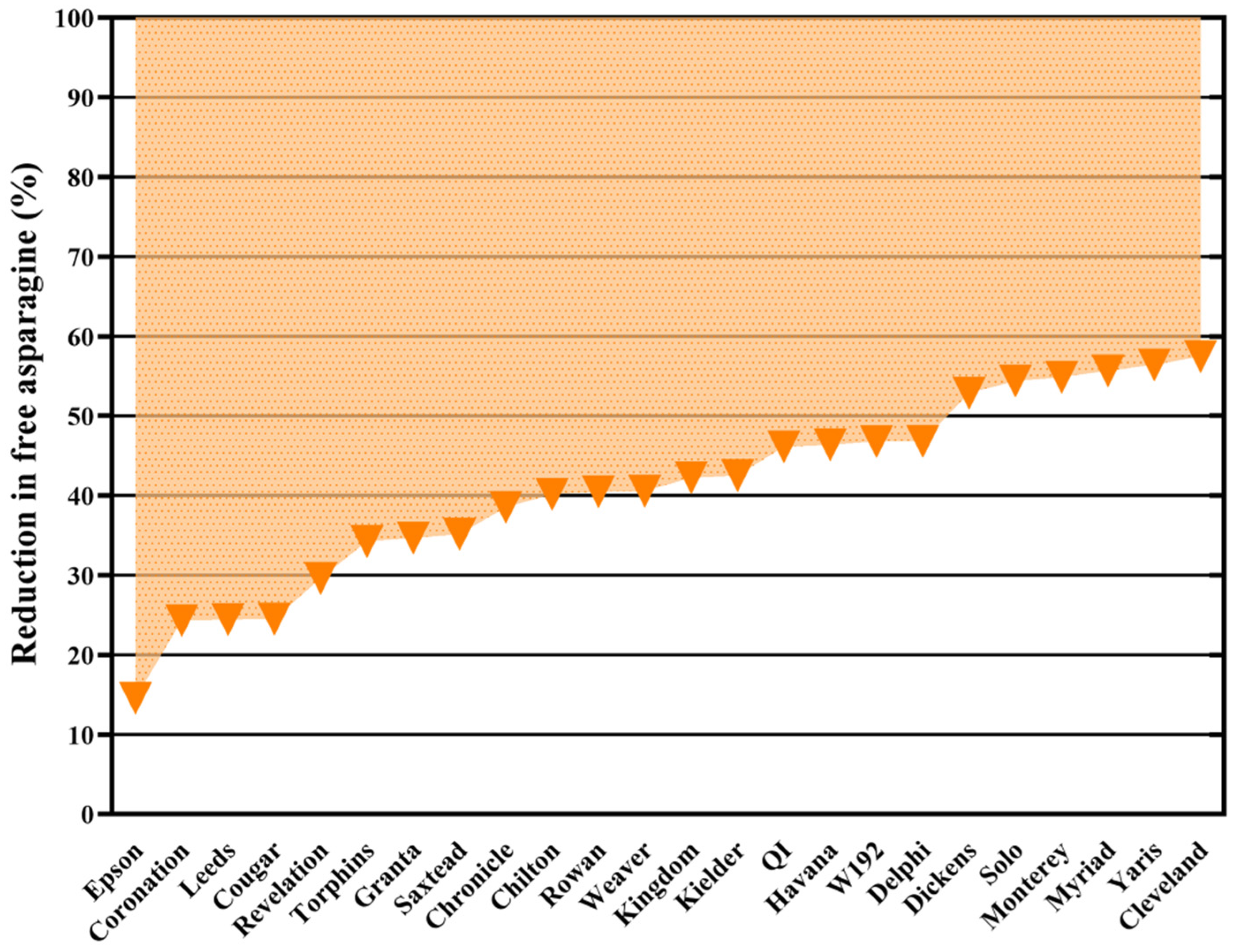
2.6.1. Identifying Quantitative Trait Loci (QTL) That Control Free Asparagine Accumulation in Wheat Grain
2.6.2. The Asparagine Synthetase Gene Family of Wheat and Characterisation of a Natural Genetic Event Associated with Reduced Free Asparagine Concentration in Wheat Grain
2.6.3. The Application of Genome Editing and Chemical Mutagenesis to Reducing Free Asparagine Concentrations in Wheat Grain
2.7. The Use of Imaging Techniques to Predict Free Asparagine Concentrations in Wheat Grain and the Anticipated Development of Free Asparagine Sensors
3. Other Processing Contaminants in Wheat Products
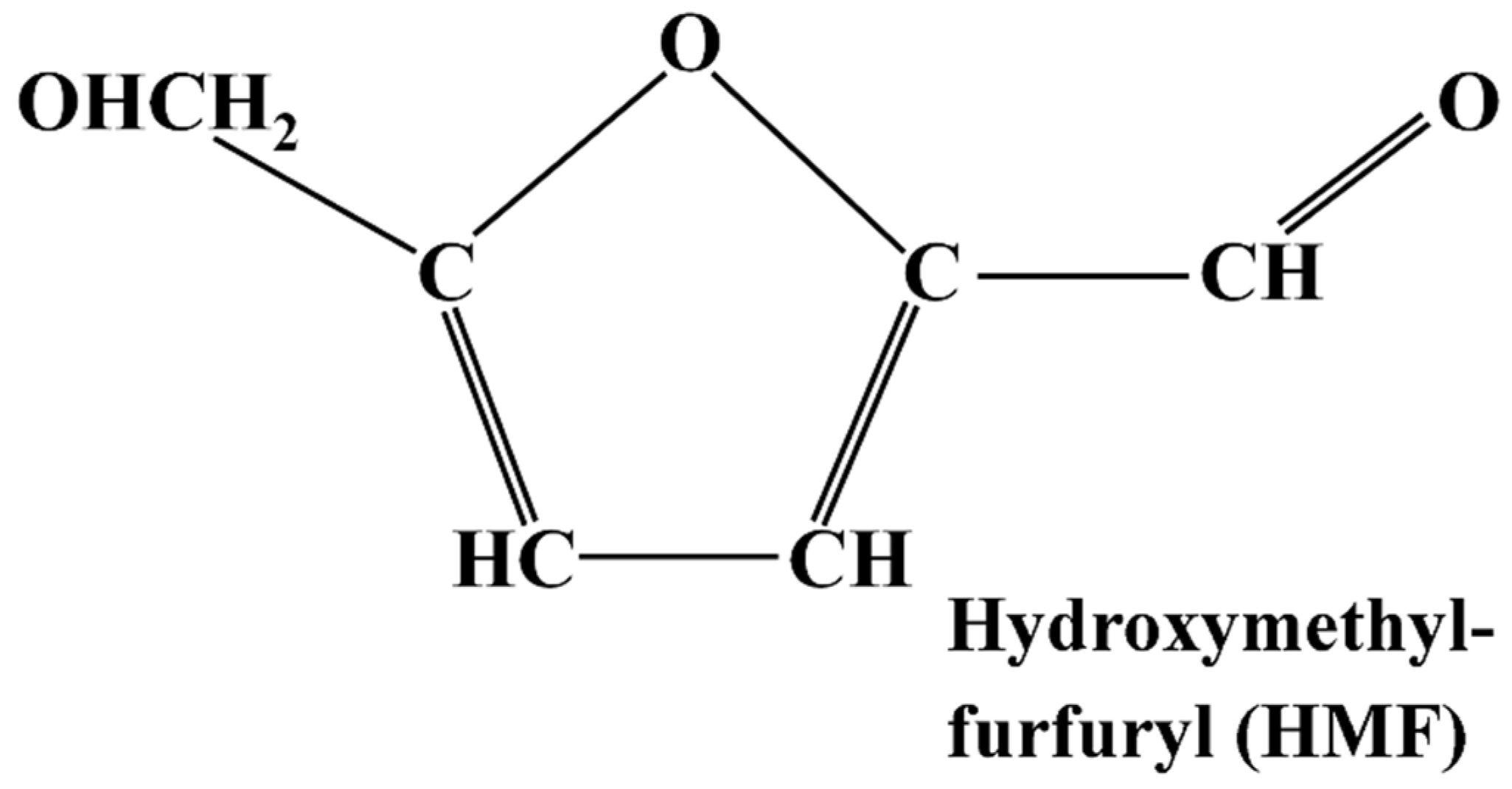
3.1. 5-Hydroxymethylfurfural (HMF)
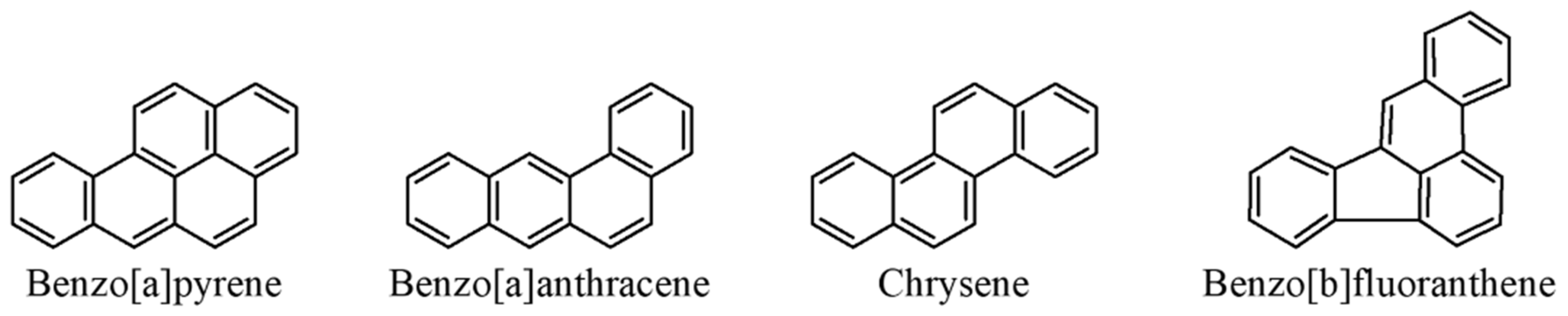
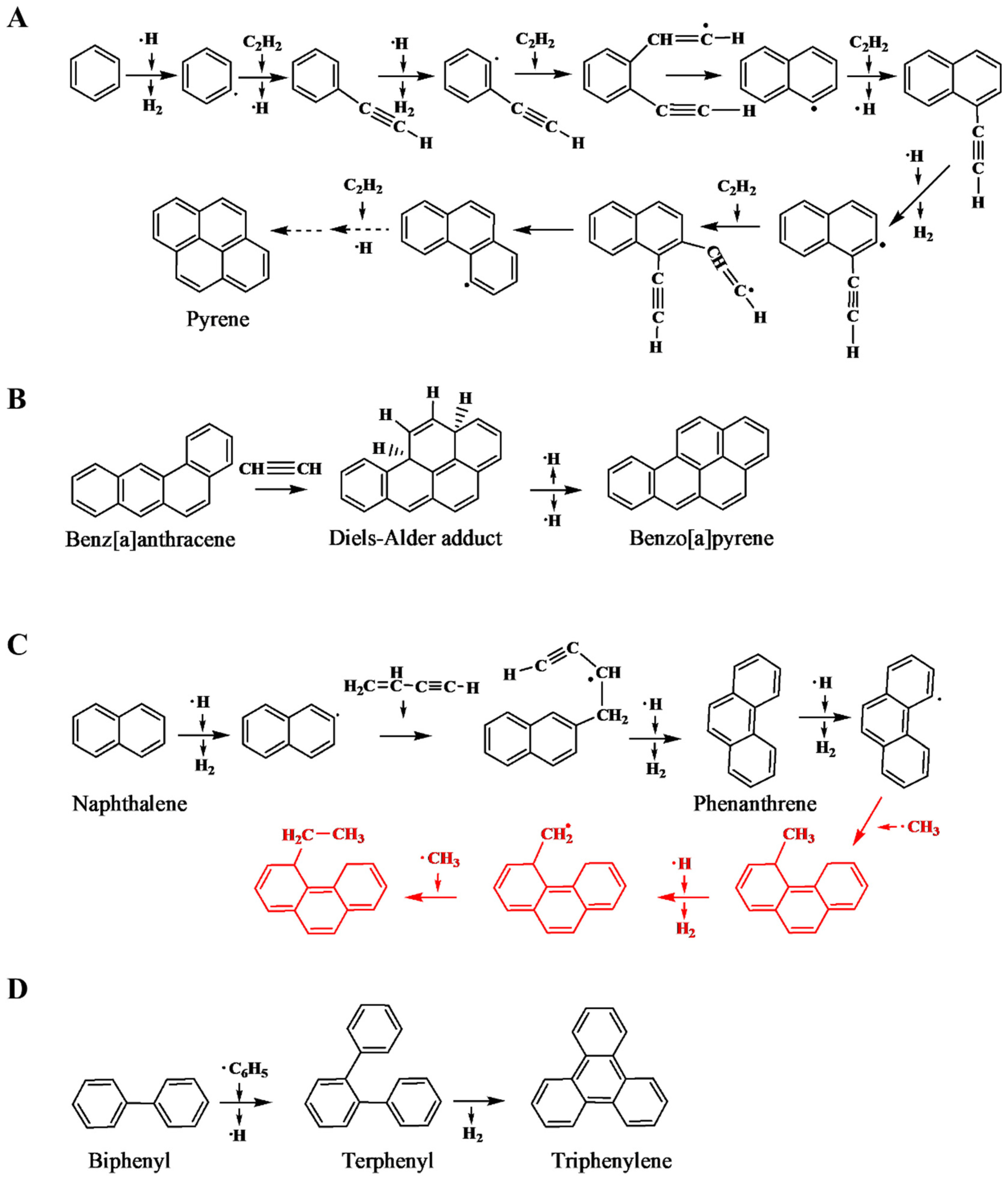
3.2. Polycyclic Aromatic Hydrocarbons (PAHs)
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shewry, P.R.; Hey, S. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Available online: http://www.nabim.org.uk/wheat/wheat-varieties (accessed on 17 August 2023).
- Curtis, T.Y.; Postles, J.; Halford, N.G. Reducing the potential for processing contaminant formation in cereal products. J. Cereal Sci. 2014, 59, 382–392. [Google Scholar] [CrossRef]
- Available online: https://www.efsa.europa.eu/en/topics/topic/chemical-contaminants-food-feed (accessed on 17 August 2023).
- Halford, N.G.; Curtis, T.Y.; Muttucumaru, N.; Postles, J.; Mottram, D.S. Sugars in crop plants. Ann. Appl. Biol. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM Panel). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 60, Some Industrial Chemicals; International Agency for Research on Cancer (IARC): Lyon, France, 1994.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Contaminants. Sixty-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives (Rome, 8–17 February 2005); WHO Technical Reports Series; WHO: Geneva, Switzerland, 2006; Volume 930.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Contaminants in Food; WHO Food Additives Series, Volume 63; FAO JECFA Monographs 2011, Volume 8; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011.
- Zhivagui, M.; Ng, A.W.T.; Ardin, M.; Churchwell, M.I.; Pandey, M.; Renard, C.; Villar, S.; Cahais, V.; Robitaille, A.; Bouaoun, L.; et al. Experimental and pan-cancer genome analyses reveal widespread contribution of acrylamide exposure to carcinogenesis in humans. Genome Res. 2019, 29, 521–531. [Google Scholar] [CrossRef]
- Eisenbrand, G. Revisiting the evidence for genotoxicity of acrylamide (AA), key to risk assessment of dietary AA exposure. Arch. Toxicol. 2020, 94, 2939–2950. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.; et al. Acrylamide formation mechanisms in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Hodge, J.E. Chemistry of the browning reaction in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Mottram, D.S. The Maillard reaction: Source of flavour in thermally processed foods. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 269–284. [Google Scholar]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation endproducts. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Koubaa, M.; Roohinejad, S.; Mungure, T.; Alaa El-Din, B.; Greiner, R.; Mallikarjunanet, K. Effect of emerging processing technologies on Maillard reactions. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 76–82. [Google Scholar]
- Granvogl, M.; Schieberle, P. Thermally generated 3-aminopropionamide as a transient intermediate in the formation of acrylamide. J. Agric. Food Chem. 2006, 54, 5933–5938. [Google Scholar] [CrossRef]
- Parker, J.K.; Balagiannis, D.P.; Higley, J.; Smith, G.; Wedzicha, B.L.; Mottram, D.S. Kinetic model for the formation of acrylamide during the finish-frying of commercial French fries. J. Agric. Food Chem. 2012, 60, 9321–9331. [Google Scholar] [CrossRef]
- Yasuhara, A.; Tanaka, Y.; Hengel, M.; Shibamoto, T. Gas chromatographic investigation of acrylamide formation in browning model systems. J. Agric. Food Chem. 2003, 51, 3999–4003. [Google Scholar] [CrossRef]
- Claus, A.; Weisz, G.M.; Schieber, A.; Carle, R. Pyrolytic acrylamide formation from purified wheat gluten and gluten-supplemented wheat bread rolls. Mol. Nutr. Food Res. 2006, 50, 87–93. [Google Scholar] [CrossRef]
- Xu, F.; Oruna-Concha, M.J.; Elmore, J.S. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016, 210, 163–171. [Google Scholar] [CrossRef]
- FoodDrinkEurope. Acrylamide Toolbox 2019; FoodDrinkEurope: Brussels, Belgium, 2019. [Google Scholar]
- Boyaci Gunduz, C.P. Formulation and processing strategies to reduce acrylamide in thermally processed cereal-based foods. Int. J. Environ. Res. Public Health 2023, 20, 6272. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 10 January 2011 on Investigations into the Levels of Acrylamide in Food; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- European Commission. Commission Recommendation of 8 November 2013 on Investigations into the Levels of Acrylamide in Food; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- European Commission. Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Mesías, M.; Sáez-Escudero, L.; Morales, F.J.; Delgado-Andrad, C. Reassessment of acrylamide content in breakfast cereals. Evolution of the Spanish market from 2006 to 2018. Food Control 2019, 105, 94–101. [Google Scholar] [CrossRef]
- European Commission. Food Safety: Acrylamide. Available online: https://ec.europa.eu/food/safety/chemical-safety/contaminants/catalogue/acrylamide_en (accessed on 13 July 2023).
- Food and Drug Administration. Guidance for Industry, Acrylamide in Foods; Food and Drug Administration: College Park, MD, USA, 2016.
- Muttucumaru, N.; Halford, N.G.; Elmore, J.S.; Dodson, A.T.; Parry, M.; Shewry, P.R.; Mottram, D.S. The formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat. J. Agric. Food Chem. 2006, 54, 8951–8955. [Google Scholar] [CrossRef]
- Granvogl, M.; Wieser, H.; Koehler, P.; Von Tucher, S.; Schieberle, P. Influence of sulphur fertilization on the amounts of free amino acids in wheat. Correlation with baking properties as well as with 3-aminopropionamide and acrylamide generation during baking. J. Agric. Food Chem. 2007, 55, 4271–4277. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Muttucumaru, N.; Shewry, P.R.; Parry, M.A.; Powers, S.J.; Elmore, J.S.; Mottram, D.S.; Hook, S.; Halford, N.G. Effects of genotype and environment on free amino acid levels in wheat grain: Implications for acrylamide formation during processing. J. Agric. Food Chem. 2009, 57, 1013–1021. [Google Scholar] [CrossRef]
- Oddy, J.; Addy, J.; Mead, A.; Hall, C.; Mackay, C.; Ashfield, T.; McDiarmid, F.; Curtis, T.Y.; Raffan, S.; Wilkinson, M.; et al. Reducing dietary acrylamide exposure from wheat products through crop management and imaging. J. Agric. Food Chem. 2023, 71, 3403–3413. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Raffan, S.; Wan, Y.; King, R.; Gonzalez-Uriarte, A.; Halford, N.G. Contrasting gene expression patterns in grain of high and low asparagine wheat genotypes in response to sulphur supply. BMC Genom. 2019, 20, 628. [Google Scholar] [CrossRef]
- Winkler, U.; Schön, W.J. Amino acid composition of the kernel proteins in barley resulting from nitrogen fertilization at different stages of development. J. Agron. Crop Sci. 1980, 149, 503–512. [Google Scholar]
- Claus, A.; Schreiter, P.; Weber, A.; Graeff, S.; Herrmann, W.; Claupein, W.; Schieber, A.; Carle, R. Influence of agronomic factors and extraction rate on the acrylamide contents in yeast-leavened breads. J. Agric. Food Chem. 2006, 54, 8968–8976. [Google Scholar] [CrossRef]
- Martinek, P.; Klem, K.; Vanova, M.; Bartackova, V.; Vecerkova, L.; Bucher, P.; Hajslova, J. Effects of nitrogen nutrition, fungicide treatment and wheat genotype on free asparagine and reducing sugars content as precursors of acrylamide formation in bread. Plant Soil Environ. 2009, 55, 187–195. [Google Scholar] [CrossRef]
- Curtis, T.; Halford, N.G.; Powers, S.J.; McGrath, S.P.; Zazzeroni, R. Home Grown Cereals Authority Project Report No. 525; Agriculture and Horticulture Development Board: Kenilworth, UK, 2014. [Google Scholar]
- Curtis, T.Y.; Powers, S.J.; Wang, R.; Halford, N.G. Effects of variety, year of cultivation and sulphur supply on the accumulation of free asparagine in the grain of commercial wheat varieties. Food Chem. 2018, 239, 304–313. [Google Scholar] [CrossRef]
- Wilson, T.L.; Guttieri, M.J.; Nelson, N.O.; Fritz, A.; Tilley, M. Nitrogen and sulfur effects on hard winter wheat quality and asparagine concentration. J. Cereal Sci. 2020, 93, 102969. [Google Scholar] [CrossRef]
- Raffan, S.; Oddy, J.; Halford, N.G. The sulphur response in wheat and its implications for acrylamide formation and food safety. Int. J. Mol. Sci. 2020, 21, 3876. [Google Scholar] [CrossRef]
- Zhao, F.J.; Hawkesford, M.J.; McGrath, S.P. Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci. 1999, 30, 1–17. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Bueschl, C.; Schoefbeck, D.; Neumann, N.K.N.; Kluger, B.; Schuster, K.; Krska, R.; Adam, G.; Lemmens, M.; et al. GC-MS based targeted metabolic profiling identifies changes in the wheat metabolome following deoxynivalenol treatment. Metabolomics 2015, 11, 722–738. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Sun, J.; Zhang, A. Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J. Plant Physiol. 2005, 162, 81–89. [Google Scholar] [CrossRef]
- Curtis, T.Y.; Powers, S.J.; Halford, N.G. Effects of fungicide treatment on free amino acid concentration and acrylamide-forming potential in wheat. J. Agric. Food Chem. 2016, 64, 9689–9696. [Google Scholar] [CrossRef]
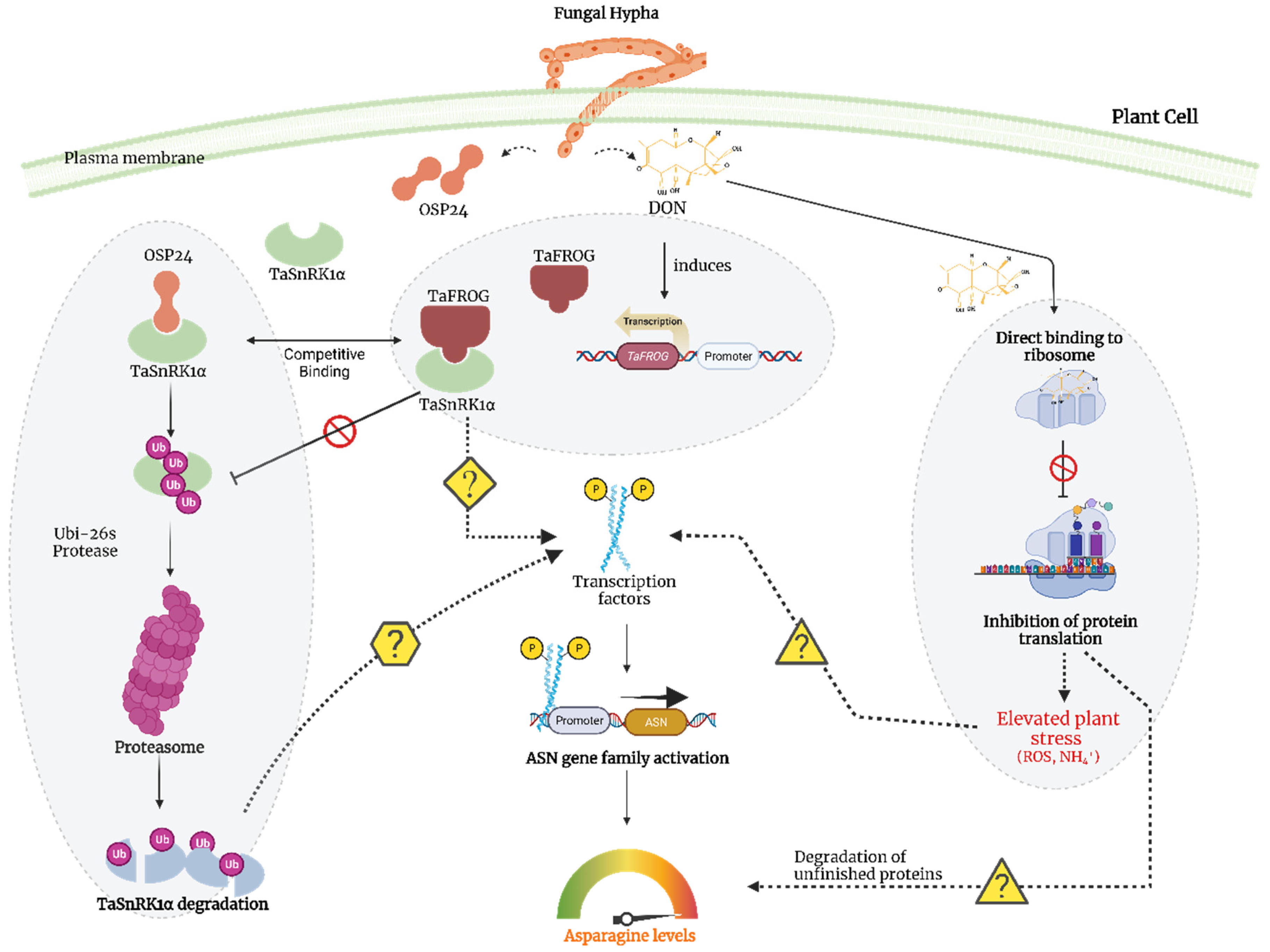
- Perochon, A.; Váry, Z.; Malla, K.B.; Halford, N.G.; Paul, M.J.; Doohan, F.M. The wheat SnRK1α family and its contribution to Fusarium toxin tolerance. Plant Sci. 2019, 288, 110217. [Google Scholar] [CrossRef]
- Nussbaumer, T.; Warth, B.; Sharma, S.; Ametz, C.; Bueschl, C.; Parich, A.; Pfeifer, M.; Siegwart, G.; Steiner, B.; Lemmens, M.; et al. Joint transcriptomic and metabolomic analyses reveal changes in the primary metabolism and imbalances in the subgenome orchestration in the bread wheat molecular response to Fusarium graminearum. G3 2015, 5, 2579–2592. [Google Scholar] [CrossRef]
- Perochon, A.; Jianguang, J.; Kahla, A.; Arunachalam, C.; Scofield, S.R.; Bowden, S.; Wallington, E.; Doohan, F.M. TaFROG Encodes a Pooideae orphan protein that interacts with SnRK1 and enhances resistance to the mycotoxigenic fungus Fusarium graminearum. Plant Physiol. 2015, 169, 2895–2906. [Google Scholar] [CrossRef]
- Jiang, C.; Hei, R.; Yang, Y.; Zhang, S.; Wang, Q.; Wang, W.; Zhang, Q.; Yan, M.; Zhu, G.; Huang, P.; et al. An orphan protein of Fusarium graminearum modulates host immunity by mediating proteasomal degradation of TaSnRK1α. Nat. Commun. 2020, 11, 4382. [Google Scholar] [CrossRef]
- Corol, D.I.; Ravel, C.; Rakszegi, M.; Charmet, G.; Bedo, Z.; Beale, M.H.; Shewry, P.R.; Ward, J.L. 1H-NMR screening for the high-throughput determination of genotype and environmental effects on the content of asparagine in wheat grain. Plant Biotechnol. J. 2016, 14, 128–139. [Google Scholar] [CrossRef]
- Tafuri, A.; Zuccaro, M.; Ravaglia, S.; Pirona, R.; Masci, S.; Sestili, F.; Lafiandra, D.; Ceriotti, A.; Baldoni, E. Exploring variability of free asparagine content in the grain of bread wheat (Triticum aestivum L.) varieties cultivated in Italy to reduce acrylamide-forming potential. Plants 2023, 12, 1349. [Google Scholar] [CrossRef]
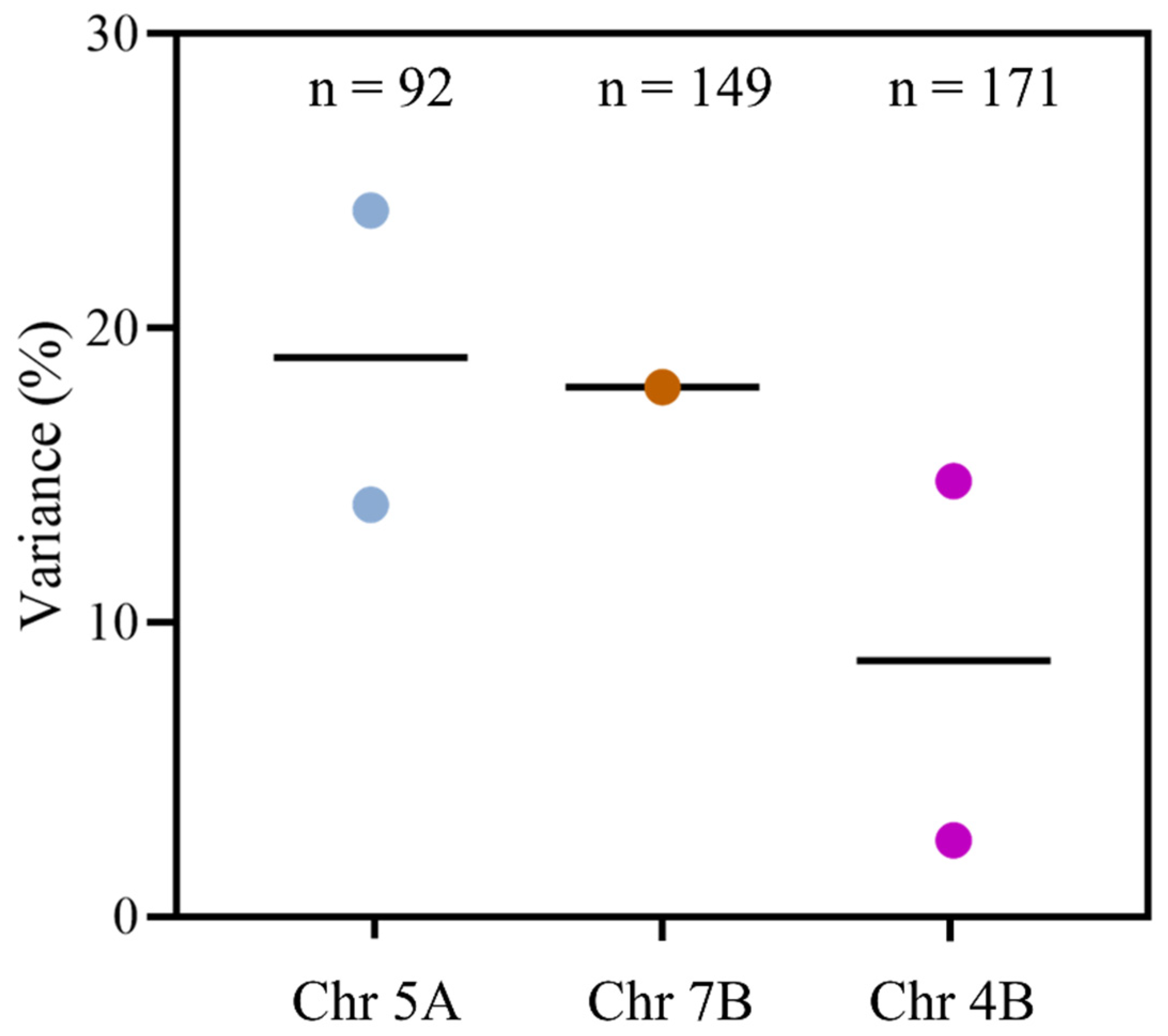
- Emebiri, L.C. Genetic variation and possible SNP markers for breeding wheat with low-grain asparagine, the major precursor for acrylamide formation in heat-processed products. J. Sci. Food Agric. 2014, 94, 1422–1429. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, H.; Chen, J.; Shi, T.; Zhang, C.; Sun, D.; He, Z.; Hao, Y.; Chen, W. Genome-wide association studies of free amino acid levels by six multi-locus models in bread wheat. Front. Plant Sci. 2018, 9, 1196. [Google Scholar] [CrossRef]
- Rapp, M.; Schwadorf, K.; Leiser, W.L.; Würschum, T.; Longin, C.F. Assessing the variation and genetic architecture of asparagine content in wheat: What can plant breeding contribute to a reduction in the acrylamide precursor? Theor. Appl. Genet. 2018, 131, 2427–2437. [Google Scholar] [CrossRef]
- Oddy, J.; Chhetry, M.; Awal, R.; Addy, J.; Wilkinson, M.; Smith, D.; King, R.; Hall, C.; Testa, R.; Murray, E.; et al. Genetic control of grain amino acid composition in a UK soft wheat mapping population. Plant Genome 2023, 16, e20335. [Google Scholar] [CrossRef]
- Xu, H.; Curtis, T.Y.; Powers, S.J.; Raffan, S.; Gao, R.; Huang, J.; Heiner, M.; Gilbert, D.; Halford, N.G. Genomic, biochemical and modelling analyses of asparagine synthetases from wheat. Front. Plant Sci. 2018, 8, 2237. [Google Scholar] [CrossRef]
- Raffan, S.; Halford, N.G. Phylogenetic analysis of cereal asparagine synthetase genes. Ann. Appl. Biol. 2021, 178, 6–22. [Google Scholar] [CrossRef]
- Gao, R.; Curtis, T.Y.; Powers, S.J.; Xu, H.; Huang, J.; Halford, N.G. Food safety: Structure and expression of the asparagine synthetase gene family of wheat. J. Cereal Sci. 2016, 68, 122–131. [Google Scholar] [CrossRef]
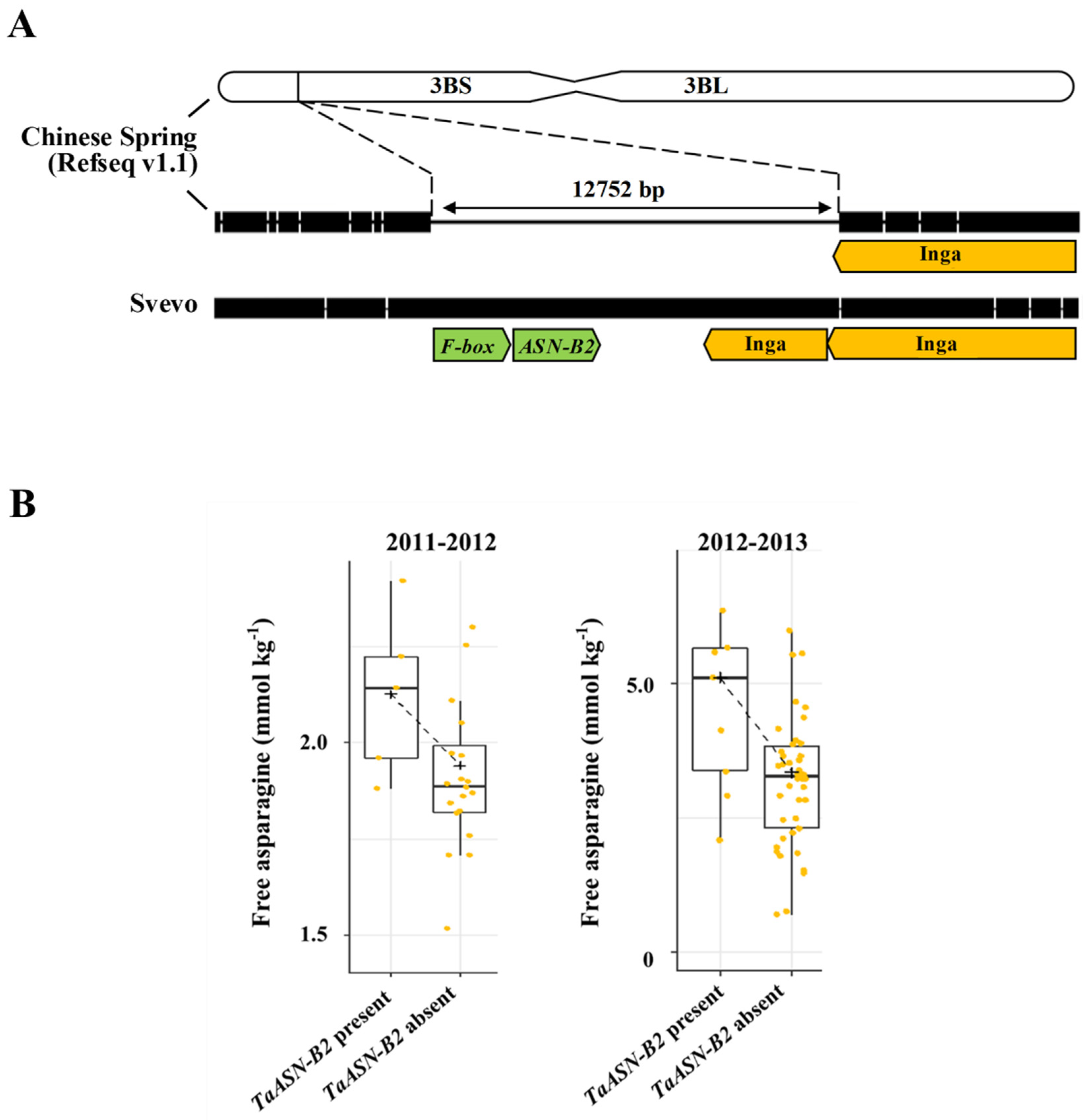
- Oddy, J.; Alárcon-Reverte, R.; Wilkinson, M.; Ravet, K.; Raffan, S.; Minter, A.; Mead, A.; Elmore, J.S.; Moreira de Almeida, I.; Cryer, N.C.; et al. Reduced free asparagine in wheat grain resulting from a natural deletion of TaASN-B2: Investigating and exploiting diversity in the asparagine synthetase gene family to improve wheat quality. BMC Plant Biol. 2021, 21, 302. [Google Scholar] [CrossRef]
- Raffan, S.; Sparks, C.; Huttly, A.; Hyde, L.; Martignago, D.; Mead, A.; Hanley, S.J.; Wilkinson, P.A.; Barker, G.; Edwards, K.J.; et al. Wheat with greatly reduced accumulation of free asparagine in the grain, produced by CRISPR/Cas9 editing of asparagine synthetase gene TaASN2. Plant Biotechnol. J. 2021, 19, 1602–1613. [Google Scholar] [CrossRef]
- Raffan, S.; Oddy, J.; Meade, A.; Barker, G.; Curtis, T.; Usher, S.; Burt, C.; Halford, N.G. Field assessment of genome edited, low asparagine wheat: Europe’s first CRISPR wheat field trial. Plant Biotechnol. J. 2023, 21, 1097–1099. [Google Scholar] [CrossRef]
- Rakszegi, M.; Kisgyörgy, B.N.; Tearall, K.; Shewry, P.R.; Láng, L.; Phillips, A.; Bedő, Z. Diversity of agronomic and morphological traits in a mutant population of bread wheat studied in the Healthgrain program. Euphytica 2010, 174, 409–421. [Google Scholar] [CrossRef]
- Alarcón-Reverte, R.; Xie, Y.; Stromberger, J.; Cotter, J.D.; Mason, R.E.; Pearce, S. Induced mutations in ASPARAGINE SYNTHETASE-A2 reduce free asparagine concentration in the wheat grain. Crop Sci. 2022, 62, 1484–1496. [Google Scholar] [CrossRef]
- Burnett, A.C.; Anderson, J.; Davidson, K.J.; Ely, K.S.; Lamour, J.; Li, Q.; Morrison, B.D.; Yang, D.; Rogers, A.; Serbin, S.P. A best-practice guide to predicting plant traits from leaf-level hyperspectral data using partial least squares regression. J. Exp. Bot. 2021, 72, 6175–6189. [Google Scholar] [CrossRef]
- Helou, C.; Gadonna-Widehem, P.; Robert, N.; Branlard, G.; Thebault, J.; Librere, S.; Jacquot, S.; Mardon, J.; Piquet-Pissaloux, A.; Chapron, S.; et al. The impact of raw materials and baking conditions on Maillard reaction products, thiamine, folate, phytic acid and minerals in white bread. Food Funct. 2016, 7, 2498–2507. [Google Scholar] [CrossRef]
- Leiva, G.E.; Naranjo, G.B.; Malec, L.S. A study of different indicators of Maillard reaction with whey proteins and different carbohydrates under adverse storage conditions. Food Chem. 2017, 215, 410–416. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Thakur, J.S. HMF as a quality indicator in Garcinia indica fruit juice concentrate. Curr. Res. Nutr. Food Sci. 2018, 6, 227–233. [Google Scholar] [CrossRef]
- Li, W.; Qu, X.N.; Han, Y.; Zheng, S.W.; Wang, J.; Wang, Y.P. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int. J. Mol. Sci. 2015, 16, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Husøy, T.; Haugen, M.; Murkovic, M.; Jöbstl, D.; Stølen, L.H.; Bjellaas, T.; Rønningborg, C.; Glatt, H.; Alexander, J. Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure. Food Chem. Toxicol. 2008, 46, 3697–3702. [Google Scholar] [CrossRef] [PubMed]
- Durling, L.J.K.; Busk, L.; Hellman, B.E. Evaluation of the DNA damaging effect of the heat-induced food toxicant 5-hydroxymethylfurfural (HMF) in various cell lines with different activities of sulfotransferases. Food Chem. Toxicol. 2009, 47, 880–884. [Google Scholar] [CrossRef]
- Murkovic, M.; Pichler, N. Analysis of 5-hydroxymethylfurfural in coffee, dried fruits and urine. Mol. Nutr. Food Res. 2006, 50, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2021, 40, 545–561. [Google Scholar] [CrossRef]
- Lee, H.S.; Nagy, S. Relative reactivities of sugars in the formation of 5-hydroxymethyl furfural in sugar-catalyst model systems. J. Food Process. Preserv. 1990, 14, 171–178. [Google Scholar] [CrossRef]
- Mesías, M.; Sáez-Escudero, L.; Morales, F.J.; Delgado-Andrade, C. Occurrence of furosine and hydroxymethylfurfural in breakfast cereals. Evolution of the Spanish market from 2006 to 2018. Foods 2019, 8, 158. [Google Scholar] [CrossRef]
- Petisca, C.; Henriques, A.R.; Pérez-Palacios, T.; Pinho, O.; Ferreira, I.M.P.L.V.O. Assessment of hydroxymethylfurfural and furfural in commercial bakery products. J. Food Compos. Anal. 2014, 33, 20–25. [Google Scholar] [CrossRef]
- Ortu, E.; Caboni, P. Levels of 5-hydroxymethylfurfural, furfural, 2-furoic acid in sapa syrup, Marsala wine and bakery products. Int. J. Food Prop. 2017, 20 (Suppl. 3), S2543–S2551. [Google Scholar] [CrossRef]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V.; Niaz, M.S.; Ramesh, A. Polycyclic aromatic hydrocarbons and digestive tract cancers: A perspective. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2011, 29, 324–357. [Google Scholar] [CrossRef]
- Hessel, S.; Lampen, A.; Seidel, A. Polycyclic aromatic hydrocarbons in food—Efflux of the conjugated biomarker 1-hydroxypyrene is mediated by Breast Cancer Resistance Protein (ABCG2) in human intestinal Caco-2 cells. Food Chem. Toxicol. 2013, 62, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Crowell, S.R.; Hanson-Drury, S.; Williams, D.E.; Corley, R.A. In vitro metabolism of benzo[a]pyrene and dibenzo[def,p]chrysene in rodent and human hepatic microsomes. Toxicol. Lett. 2014, 228, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zelinkova, Z.; Wenzl, T. The Occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM Panel). Polycyclic aromatic hydrocarbons in food. EFSA J. 2008, 724, 1–114. [Google Scholar]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef]
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793. [Google Scholar] [CrossRef]
- Ibáñez, R.; Agudo, A.; Berenguer, A.; Jakszyn, P.; Tormo, M.J.; Sanchéz, M.J.; Quirós, J.R.; Pera, G.; Navarro, C.; Martinez, C.; et al. Dietary intake of polycyclic aromatic hydrocarbons in a Spanish population. J. Food Prot. 2005, 68, 2190–2195. [Google Scholar] [CrossRef]
- Martí-Cid, R.; Llobet, J.M.; Castell, V.; Domingo, J.L. Evolution of the dietary exposure to polycyclic aromatic hydrocarbons in Catalonia, Spain. Food Chem. Toxicol. 2008, 46, 3163–3171. [Google Scholar] [CrossRef]
- Orecchio, S.; Papuzza, V. Levels, fingerprint and daily intake of polycyclicaromatic hydrocarbons (PAHs) in bread baked using wood as fuel. J. Hazard. Mater. 2009, 164, 876–883. [Google Scholar] [CrossRef]
- Ahmed, M.T.; Hadi, E.A.; Samahy, S.E.; Youssof, K. The influence of baking fuel on residues of polycyclic aromatic hydrocarbons and heavy metals in bread. J. Hazard. Mater. 2000, 80, 1–8. [Google Scholar] [CrossRef]
- Rey-Salgueiro, L.; García-Falcón, M.S.; Martínez-Carballo, E.; Simal-Gándara, J. Effects of toasting procedures on the levels of polycyclic aromatic hydrocarbons in toasted bread. Food Chem. 2008, 108, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Rascón, A.J.; Azzouz, A.; Ballesteros, E. Assessing polycyclic aromatic hydrocarbons in cereal-based foodstuffs by using a continuous solid-phase extraction system and gas chromatography–mass spectrometry. Food Control 2018, 92, 92–100. [Google Scholar] [CrossRef]
- Ciecierska, M.; Obiedziński, M.W. Polycyclic aromatic hydrocarbons in the bakery chain. Food Chem. 2013, 141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chawda, S.; Tarafdar, A.; Sinha, A.; Mishra, B.K. Profiling and health risk assessment of PAHs content in tandoori and tawa bread from India. Polycycl. Aromat. Compd. 2020, 40, 21–32. [Google Scholar] [CrossRef]
- Tran-Lam, T.T.; Hai Dao, Y.; Kim Thi Nguyen, L.; Kim Ma, H.; Nguyen Tran, H.; Truong Le, G. Simultaneous determination of 18 polycyclic aromatic hydrocarbons in daily foods (Hanoi Metropolitan Area) by gas chromatography–tandem mass spectrometry. Foods 2018, 7, 201. [Google Scholar] [CrossRef]
- Iwegbue, C.M.; Onyonyewoma, U.A.; Bassey, F.I.; Nwajei, G.E.; Martincigh, B.S. Concentrations and health risk of polycyclic aromatic hydrocarbons in some brands of biscuits in the Nigerian market. Hum. Ecol. Risk Assess. 2015, 21, 338–357. [Google Scholar] [CrossRef]


| Food | Indicative Value 2011 | Indicative Value 2013 | Benchmark Level 2017 | Under Discussion | |
|---|---|---|---|---|---|
| Benchmark Level | Maximum Level | ||||
| Soft bread | 150 | 80 | 50 | 50 | 75 |
| Soft bread (other) | 150 | 100 | |||
| Breakfast cereals: bran products, whole grain cereals, gun puffed grain | 400 | 400 | 300 | 300 | 500 |
| Breakfast cereals: wheat and rye-based | 300 | 300 | 300 | 400 | |
| Breakfast cereals: maize, oat, spelt, barley and rice-based | 200 | 150 | |||
| Biscuits | 500 | 500 | 350 | 300 | 500 |
| Crackers | 500 | 500 | 400 | ||
| Crispbread | 500 | 450 | 350 | 300 | 400 |
| Gingerbread | - | 1000 | 800 | ||
| Cereal-based baby foods | 100 | 50 | 40 | ||
| Biscuits and rusks for infants and young children | 250 | 200 | 150 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, N.; Halford, N.G. Reducing the Risk of Acrylamide and Other Processing Contaminant Formation in Wheat Products. Foods 2023, 12, 3264. https://doi.org/10.3390/foods12173264
Kaur N, Halford NG. Reducing the Risk of Acrylamide and Other Processing Contaminant Formation in Wheat Products. Foods. 2023; 12(17):3264. https://doi.org/10.3390/foods12173264
Chicago/Turabian StyleKaur, Navneet, and Nigel G. Halford. 2023. "Reducing the Risk of Acrylamide and Other Processing Contaminant Formation in Wheat Products" Foods 12, no. 17: 3264. https://doi.org/10.3390/foods12173264








