Sunflower and Palm Kernel Meal Present Bioaccessible Compounds after Digestion with Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. De-Oiled Flour Preparation
2.2. Chemicals and Reagents
2.3. In Vitro Gastrointestinal Digestion (GID)
2.4. Chemical Characterization and Phenolic Compounds
2.5. Molecular Weight Distribution Profile
2.6. Extract Preparation for Antioxidant Activities
2.7. Oxygen Radical Absorbance Capacity (ORAC)
2.8. DNA Supercoiled Band Protective Capacity
2.9. ABTS and DPPH Radical Scavenging Assays
2.10. Potential Prebiotic Effect
2.11. Statistical Analysis
3. Results
3.1. Chemical Characterization and Total Phenolic Compounds
3.2. Molecular Weight (MW) Distribution by FPLC-SE
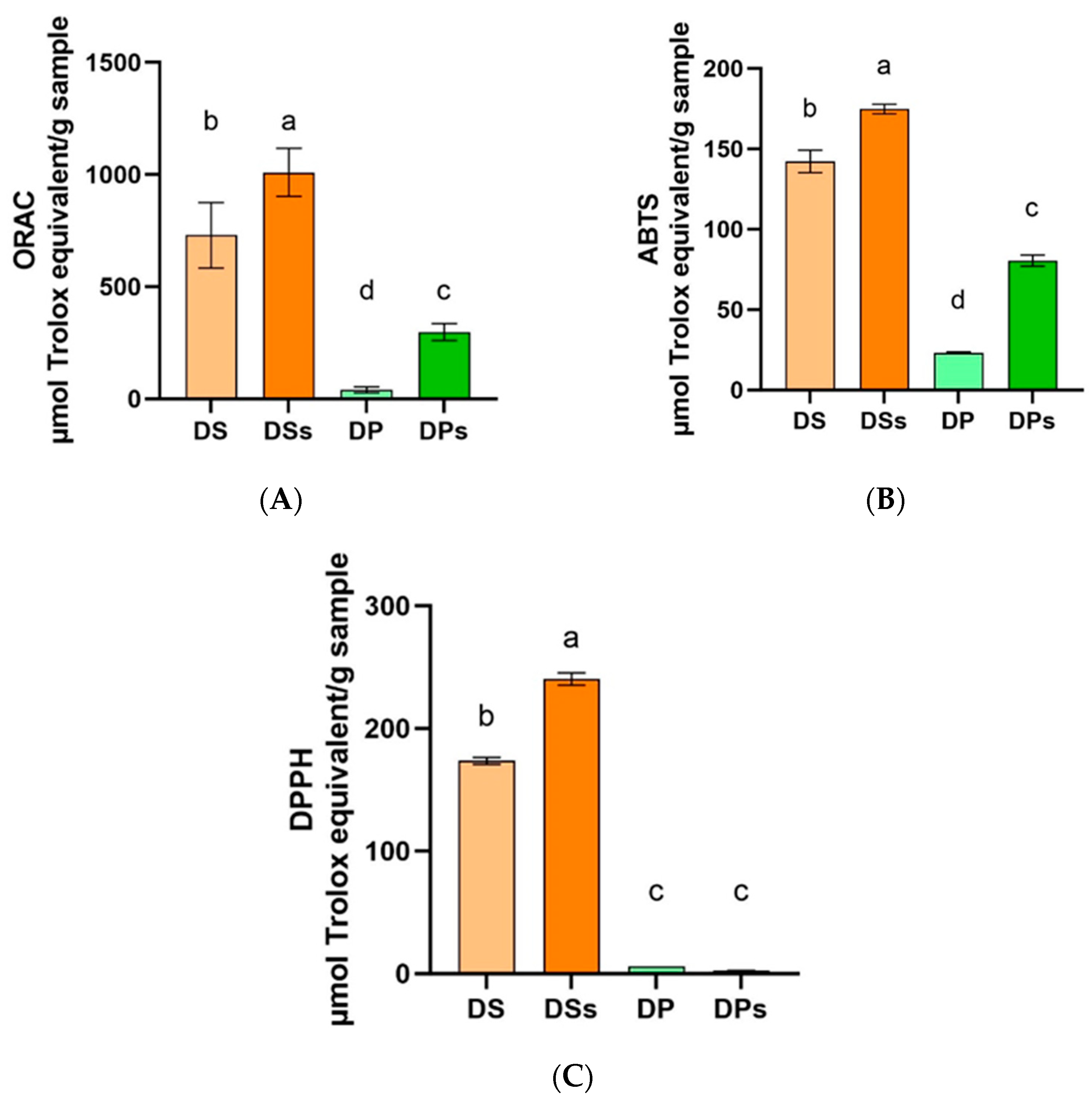
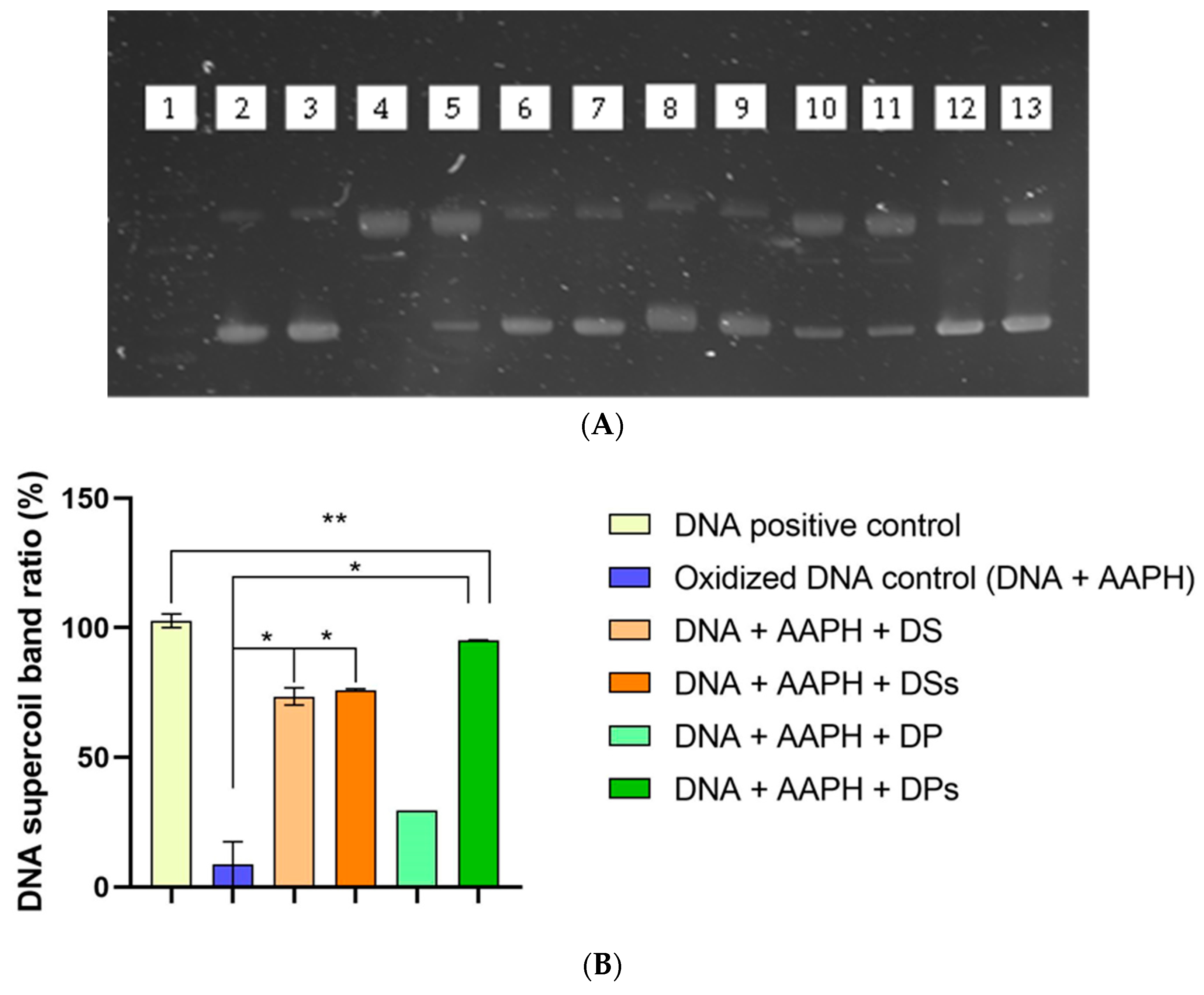
3.3. Antioxidant Activity by ORAC, ABTS, DPPH, and DNA Supercoiled Band Protective Capacity
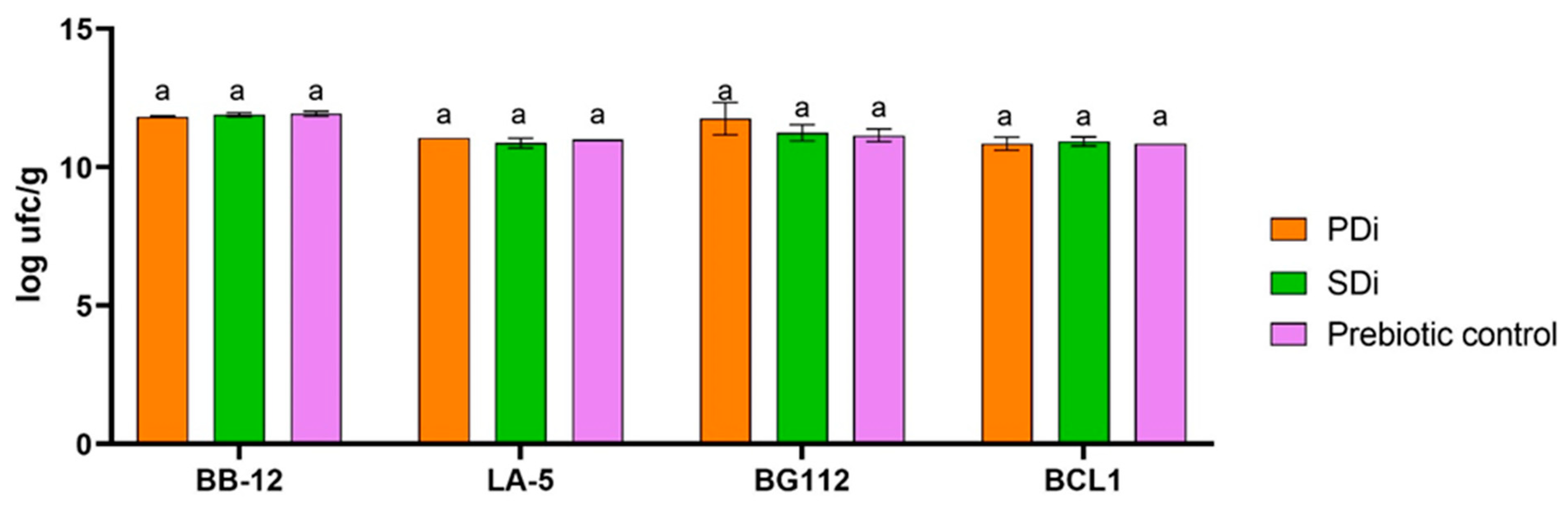
3.4. Potential Prebiotic Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ancuta, P.; Sonia, P. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 21, 7432. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Foreign Agricultural Service: Oilseeds—World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 8 May 2023).
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification, and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Sloth, J.J.; Mohammadifar, M.A. Protein extracts from de-oiled sunflower cake: Structural, physico-chemical and functional properties after removal of phenolics. Food Biosci. 2020, 38, 100749. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Pokorny, J. Potential application of oilseeds as source of antioxidants for food lipids—A review. Czech J. Food Sci. 2005, 23, 93–102. [Google Scholar] [CrossRef]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Samperi, R.; Ventura, S.; Chiozzi, R.Z.; Lagana, A. Identification of potential bioactive peptides generated by simulated gastrointestinal digestion of soybean seeds and soy milk proteins. J. Food Compos. Anal. 2015, 44, 205–213. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Filho, J.G.O.; Egea, M.B. Sunflower seed byproduct and its fractions for food application: An attempt to improve the sustainability of the oil process. J. Food Sci. 2021, 86, 1497–1510. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Reid, G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hoseini, S.E. Characterization of Yeast Protein Enzymatic Hydrolysis and Autolysis in Saccharomyces cerevisiae and Kluyveromyces marxianus. J. Food Biosci. Technol. 2015, 5, 19–30. [Google Scholar]
- Cerveró, J.M.; Skovgaard, P.A.; Felby, C.; Sørensen, H.R.; Jørgensen, H. Enzymatic hydrolysis and fermentation of palm kernel press cake for production of bioethanol. Enzym. Microb. Technol. 2010, 46, 177–184. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; Lebeer, S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Brodkorb, A. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2012. [Google Scholar]
- White, J.A.; Hart, R.J.; Fry, J.C. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J. Autom. Chem. Clin. Lab. Autom. 1986, 8, 170–177. [Google Scholar] [CrossRef]
- Spies, J.R. Determination of tryptophan in proteins. Anal. Chem. 1967, 39, 1412–1416. [Google Scholar] [CrossRef]
- WHO Technical Report Series: Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation. 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/43411/WHO_TRS_935_eng.pdf?sequence=1&isAllowed=y (accessed on 10 August 2018).
- Bisinotto, M.S.; da Silva, D.C.; Fino, L.C.; Simabuco, F.M.; Bezerra, R.M.N.; Antunes, A.E.C.; Pacheco, M.T.B. Bioaccessibility of cashew nut kernel flour compounds released after simulated in vitro human gastrointestinal digestion. Food Res. Int. 2021, 139, 109906. [Google Scholar] [CrossRef]
- Heyden, Y.V.; Popovici, S.T.; Schoenmakers, P.J. Evaluation of size-exclusion chromatography and size-exclusion electrochromatography calibration curves. J. Chromatogr. A 2002, 957, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.G.D.E.; Hernández-Ledesma, B.; Amigo, L.; Netto, F.M.; Miralles, B. Identification of peptides released from flaxseed (Linum usitatissimum) protein by Alcalase® hydrolysis: Antioxidant activity. LWT Food Sci. Technol. 2017, 76, 140–146. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z.; Gomes, A.; Fernandes, E.; Lima, J.L.F.D.C.; Bragagnolo, N. In vitro scavenging capacity of annatto seed extracts against reactive oxygen and nitrogen species. Food Chem. 2011, 127, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Pavan, I.C.B.; Yokoo, S.; Granato, D.C.; Meneguello, L.; Carnielli, C.M.; Tavares, M.R.; Simabuco, F.M. Different interactomes for p70-S6K1 and p54-S6K2 revealed by proteomic analysis. Proteomics 2016, 16, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Bakken, H.E. Preventive effect of Nile tilapia hydrolysate against oxidative damage of HepG2 cells and DNA mediated by H2O2 and AAPH. J. Food Sci. Technol. 2015, 52, 6194–6205. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: Use of different assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Garcia-Hernandez, M.H.; Delgado-Portales, R.E.; Corral-Fernandez, N.E.; Cortez-Espinosa, N.; Ruiz-Cabrera, M.A.; Portales-Perez, D.P. In vitro assessment of agave fructans (Agave salmiana) as prebiotics and immune system activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef]
- Ohashi, Y.; Onuma, R.; Naganuma, T.; Ogawa, T.; Naude, R.; Nokihara, K.; Muramoto, K. Antioxidant properties of tripeptides revealed by a comparison of six different assays. Food Sci. Technol. Res. 2015, 21, 695–704. [Google Scholar] [CrossRef]
- Azizi, A.; Kobayashi, I.; Chuuka, J.; Ishigaki, G. Evaluation of corn-soybean inter-cropping systems in southwestern Japan. Trop. Grassl. Forrajes Trop. 2021, 9, 307–314. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Alfieri, F.; Gallo, V.; Miralles, B.; Masi, P.; Romano, A.; Ferranti, P.; Recio, I. Compared digestibility of plant protein isolates by using the INFOGEST digestion protocol. Food Res. Int. 2020, 137, 109708. [Google Scholar] [CrossRef]
- Kaur, R.; Ghoshal, G. Sunflower protein isolates-composition, extraction and functional properties. Adv. Colloid Interface Sci. 2022, 306, 102725. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Ismail, A.; Teruyoshi Yanagita, T.; Esaa, N.M.; Baharuldind, M.T.H. Biochemical characterization of the soluble proteins, protein isolates and hydrolysates from oil palm (Elaeis guineensis) kernel. Food Biosci. 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Dimina, L.; Rémond, D.; Huneau, J.F.; Mariotti, F. Combining plant proteins to achieve amino acid profiles adapted to various nutritional objectives—An exploratory analysis using linear programming. Front. Nutr. 2022, 8, 809685. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Habinshuti, I.; Chen, X.; Yu, J.; Mukeshimana, O.; Duhoranimana, E.; Karangwa, E.; Zhang, X. Antimicrobial, antioxidant, and sensory properties of Maillard reaction products (MRPs) derived from sunflower, soybean, and corn meal hydrolysates. LWT 2019, 101, 694–702. [Google Scholar] [CrossRef]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Bakar, F.A.; Philip, R.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Cheng, Z.; Ren, J.; Li, Y.; Chang, W.; Chen, Z. Study on the multiple mechanisms underlying the reaction between hydroxyl radical and phenolic compounds by qualitative structure and activity relationship. Bioorg. Med. Chem. 2002, 10, 4067–4073. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.; Odinot, E.; Bisotto, A.; Barea, B.; Villeneuve, P.; Sigoillot, J.C.; Record, E.; Faulds, C.B.; Fine, F.; Lesage-Meessen, L. Release of phenolic acids from sunflower and rapeseedmeals using different carboxylic esters hydrolases from Aspergillus niger. Ind. Crops Prod. 2019, 139, 111579. [Google Scholar] [CrossRef]
- Friolli, M.P.S.; Silva, E.K.; Napoli, D.C.S.; Sanches, V.L.; Rostagno, M.A.; Pacheco, M.T.B. High-intensity ultrasound-based process strategies for obtaining edible sunflower (Helianthus annuus L.) flour with low-phenolic and high-protein content. Ultrason. Sonochem. 2023, 97, 106449. [Google Scholar] [CrossRef]
- Tsouko, E.; Alexandri, M.; Fernandes, K.V.; Freire, D.M.G.; Mallouchos, A.; Koutinas, A.A. Extraction of phenolic compounds from palm oil processing residues and their application as antioxidants. Food Technol. Biotechnol. 2019, 57, 29–38. [Google Scholar] [CrossRef]
- Anjum, F.M.; Nadeem, M.; Khan, M.I.; Hussain, S. Nutritional, and therapeutic potential of sunflower seeds: A review. Br. Food J. 2012, 114, 544–552. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Keil, D.; Mehlman, T.; Proper, S.; Shi, X.; Harris, G.K. Essiac tea: Scavenging of reactive oxygen species and effects on DNA damage. J. Ethnopharmacol. 2006, 103, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mu, T.H.; Sun, M.J. Purification, and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Chiaverini, N.; De Ley, M. Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res. 2010, 44, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Bello, B.; Mustafa, S.; Shun, J.; Tengku, T.; Tengku, A.; Tam, Y.J. Evaluation of the effect of soluble polysaccharides of palm kernel cake as a potential prebiotic on the growth of probiotics. 3 Biotech 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Silva, B.V.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals, and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry, and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
| DS | DSs | DSi | DP | DPs | DPi | |
|---|---|---|---|---|---|---|
| Lipids 1 | 0.4 ± 0.0 | nd | nd | 0.4 ± 0.0 | nd | nd |
| Protein 1 | 52.4 ± 0.0 A | 63.7 ± 0.1 A | 36.8 ± 0.2 A | 7.7 ± 0.2 b | 23.7 ± 0.0 a | 7.4 ± 0.0 b |
| Ash 1 | 8.8 ± 0.1 A | 7.2 ± 0.0 B | 6.5 ± 0.0 C | 2.8 ± 0.0 | 23.6 ± 0.1 * | 3.0 ± 0.0 |
| Carbohydrates 1 (*) | 38.5 | 29.1 | 56.7 | 89.1 | 52.7 | 89.7 |
| Fiber 1 | 18.00 ± 0.4 B | nd | 32.6 ± 0.2 A | 85.5 ± 0.1 a | nd | 85.5 ± 0.1 a |
| Total phenolic compounds 2 | 17.7 ± 0.9 B | 22.9 ± 0.9 A | nd | 2.3 ± 0.1 b | 8.3 ± 1.0 a | nd |
| AA (mg/g Protein) | mg/g ref. Protein * | DS | DSs | DP | DPs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Score | Score | Score | Score | |||||||
| Indispensable * | Lys | 45 | 38.83 ± 0.04 A | 0.86 | 36.91 ± 0.02 A | 0.82 | 28.56 ± 0.01 b | 0.63 | 36.07 ± 0.21 a | 0.80 |
| Trp | 6 | nd | 0.00 | 6.37 ± 0.00 | 1.06 | nd | 0.00 | 6.74 ± 0.18 | 1.12 | |
| Phe + Tyr | 38 | 74.36 ± 0.08 A | 1.96 | 74.22 ± 0.09 A | 1.95 | 80.51 ± 0.08 a | 2.12 | 64.83 ± 0.33 b | 1.71 | |
| Met + Cys | 22 | 38.19 ± 0.02 A | 1.74 | 29.61 ± 0.07 B | 1.35 | 19.87 ± 0.01 b | 0.90 | 31.80 ± 0.33 a | 1.41 | |
| Thr | 23 | 35.93 ± 0.04 B | 1.56 | 37.71 ± 0.03 A | 1.64 | 34.90 ± 0.02 b | 1.52 | 37.85 ± 0.37 a | 1.65 | |
| Leu | 59 | 64.24 ± 0.55 A | 1.09 | 59.32 ± 0.03 B | 1.01 | 74.76 ± 0.01 a | 1.27 | 56.32 ± 0.49 b | 0.95 | |
| Ile | 30 | 45.56 ± 0.48 A | 1.52 | 41.43 ± 0.06 B | 1.38 | 38.92 ± 0.07 a | 1.30 | 38.41 ± 0.33 a | 1.28 | |
| Val | 39 | 58.22 ± 0.05 A | 1.49 | 50.16 ± 0.22 B | 1.29 | 56.86 ± 0.16 b | 1.46 | 51.09 ± 0.33 a | 1.31 | |
| His | 15 | 27.12 ± 0.14 A | 1.81 | 26.16 ± 0.03 B | 1.74 | 15.91 ± 0.01 b | 1.06 | 17.62 ± 0.18 a | 1.17 | |
| Dispensable ** | Asp | 106.66 ± 0.14 B | 111.15 ± 0.07 A | 87.11 ± 0.52 b | 112.79 ± 0.78 a | |||||
| Glu | 220.45 ± 0.28 B | 228.77 ± 0.18 A | 194.86 ± 0.03 b | 198.01 ± 0.02 a | ||||||
| Ser | 46.28 ± 0.15 B | 48.15 ± 0.05 A | 54.50 ± 0.01 b | 55.35 ± 0.49 a | ||||||
| Arg | 90.81 ± 0.23 B | 92.72 ± 0.03 A | 149.74 ± 0.27 a | 121.40 ± 1.09 b | ||||||
| Ala | 44.48 ± 0.12 A | 43.17 ± 0.13 A | 52.71 ± 0.03 a | 45.11 ± 0.40 b | ||||||
| Pro | 38.32 ± 0.12 B | 45.27 ± 0.12 A | 46.16 ± 0.08 b | 47.79 ± 0.52 a | ||||||
| Gly | 57.94 ± 0.28 B | 68.88 ± 0.16 A | 64.63 ± 0.04 b | 79.51 ± 0.71 a | ||||||
| AA distribution | Hydrophobic | 32.70% | 31.55% | 33.49% | 29.55% | |||||
| Hydrophilic | 48.39% | 49.57% | 47.62% | 48.59% | ||||||
| Neutral | 18.91% | 18.88% | 18.89% | 21.86% | ||||||
| Molecular Weight Distribution (Area %) | DS | DSs | DP | DPs |
|---|---|---|---|---|
| >7 kDa | 48.3 ± 4.3 A | 16.02 ± 2.4 B | 10.28 ± 0.4 b | 57.68 ± 4.5 a |
| 5–7 kDa | 1.5 ± 0.7 A | 3.88 ± 1.8 A | 2.88 ± 0.2 a | 2.73 ± 1.0 a |
| 3–5 kDa | 2.7 ± 1.2 A | 6.99 ± 2.2 A | 5.36 ± 0.3 a | 4.52 ± 1.2 a |
| 1–3 kDa | 6.3 ± 3.6 A | 6.42 ± 1.9 A | 26.64 ± 0.7 a | 4.12 ± 1.2 b |
| 0.1–1 kDa | 41.2 ± 3.0 B | 66.7 ± 4.9 A | 54.83 ± 1.0 a | 30.95 ± 3.3 b |
| Total Area (mAU*min) | 392.1 ± 6.5 B | 1094.5 ± 6.5 A | 99.93 ± 1.3 b | 2010.48 ± 6.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisinotto, M.S.; da Silva Napoli, D.C.; Simabuco, F.M.; Bezerra, R.M.N.; Antunes, A.E.C.; Galland, F.; Pacheco, M.T.B. Sunflower and Palm Kernel Meal Present Bioaccessible Compounds after Digestion with Antioxidant Activity. Foods 2023, 12, 3283. https://doi.org/10.3390/foods12173283
Bisinotto MS, da Silva Napoli DC, Simabuco FM, Bezerra RMN, Antunes AEC, Galland F, Pacheco MTB. Sunflower and Palm Kernel Meal Present Bioaccessible Compounds after Digestion with Antioxidant Activity. Foods. 2023; 12(17):3283. https://doi.org/10.3390/foods12173283
Chicago/Turabian StyleBisinotto, Mariana Sisconeto, Daniele Cristina da Silva Napoli, Fernando Moreira Simabuco, Rosângela Maria Neves Bezerra, Adriane Elisabete Costa Antunes, Fabiana Galland, and Maria Teresa Bertoldo Pacheco. 2023. "Sunflower and Palm Kernel Meal Present Bioaccessible Compounds after Digestion with Antioxidant Activity" Foods 12, no. 17: 3283. https://doi.org/10.3390/foods12173283
APA StyleBisinotto, M. S., da Silva Napoli, D. C., Simabuco, F. M., Bezerra, R. M. N., Antunes, A. E. C., Galland, F., & Pacheco, M. T. B. (2023). Sunflower and Palm Kernel Meal Present Bioaccessible Compounds after Digestion with Antioxidant Activity. Foods, 12(17), 3283. https://doi.org/10.3390/foods12173283






