Arctoscopus japonicus Lipids Enhance Immunity of Mice with Cyclophosphamide-Induced Immunosuppression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of Lipids from A. japonicus Eggs
2.3. Preparation of AJ-PEG
2.4. Establishment of Immunosuppressed Mice Models
2.5. Preparation of Peritoneal Macrophages
2.6. Isolation of Spleen Lymphocytes
2.7. Determination of Peritoneal Macrophage Proliferation and Nitric Oxide (NO) Production
2.8. Measurement of Peritoneal Macrophage Phagocytosis
2.9. Proliferation of Splenic Lymphocytes
2.10. NK Cell Activity of Splenocytes
2.11. Measurement of Immune-Associated Gene Expression
2.12. Measurement of T-Lymphocyte Subsets in the Spleen
2.13. Statistical Analysis
3. Results
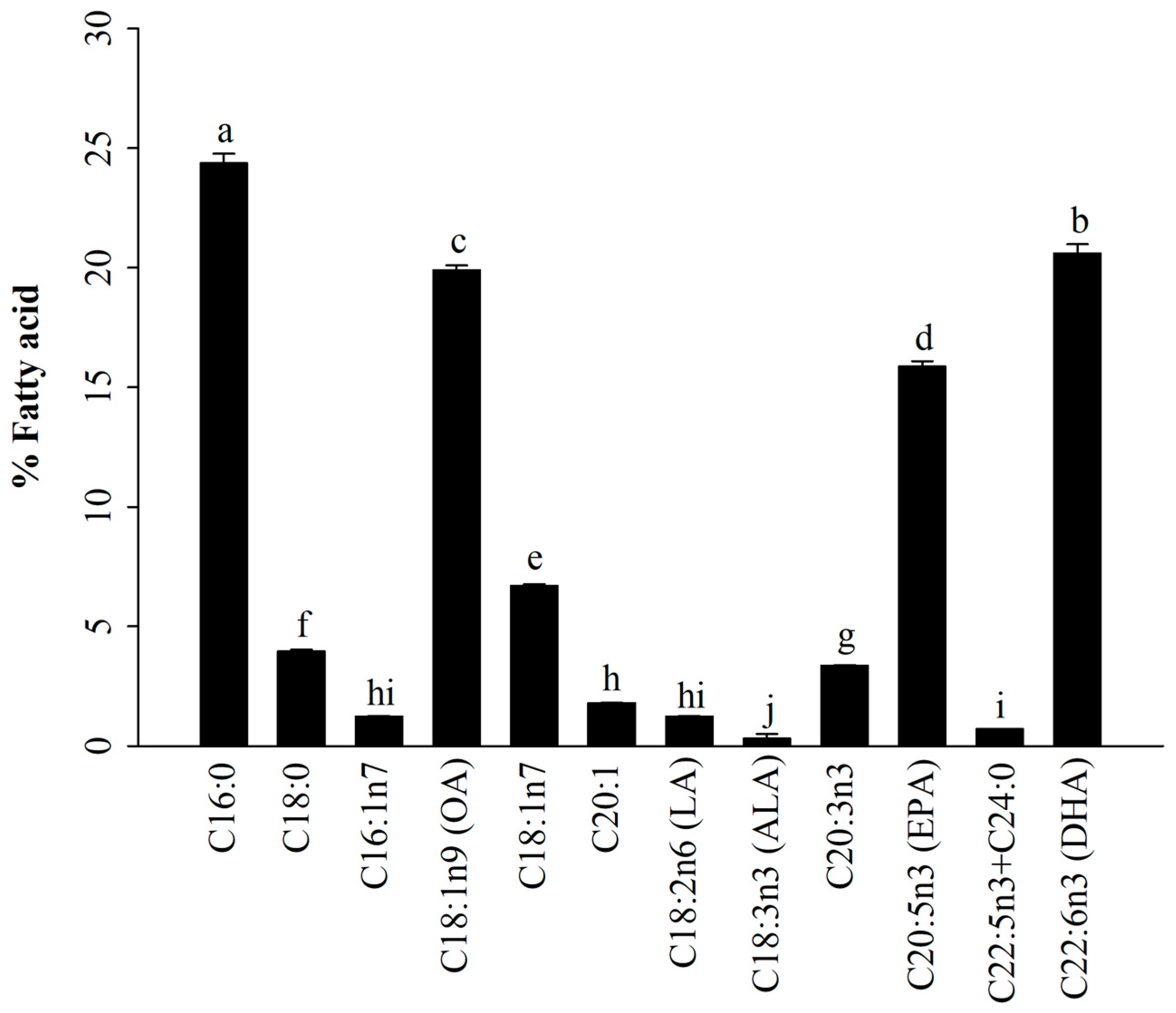
3.1. Fatty Acid Profiles of A. japonicus Lipids Incorporated with PEG6000 (AJ-PEG)
3.2. Effect of AJ-PEG on Spleen Index
3.3. Effect of AJ-PEG on Splenic Lymphocyte Proliferation
3.4. Effect of AJ-PEG on NK Cell Activity of Splenocytes
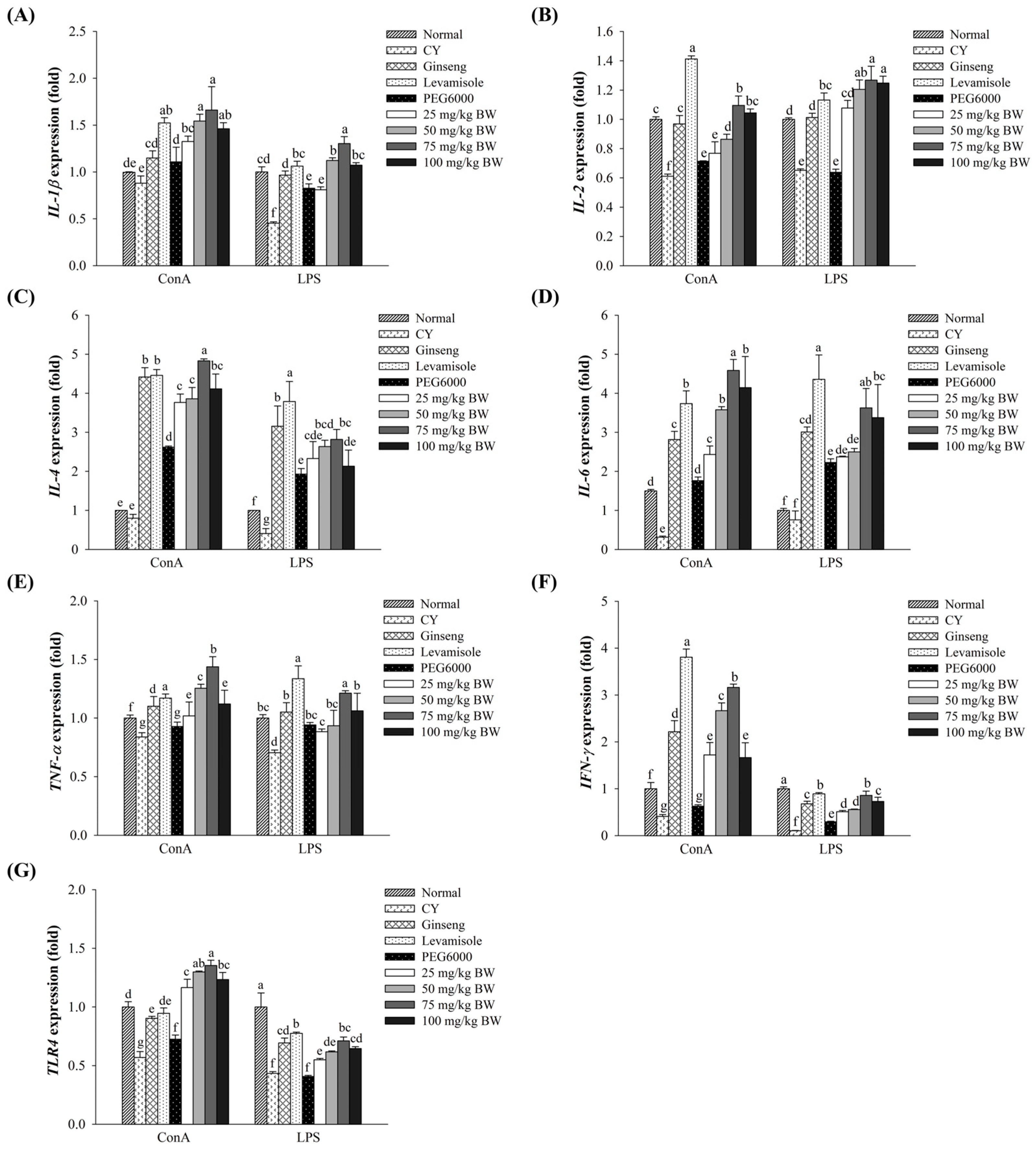
3.5. Effect of AJ-PEG on Gene Expression in Splenic Lymphocytes
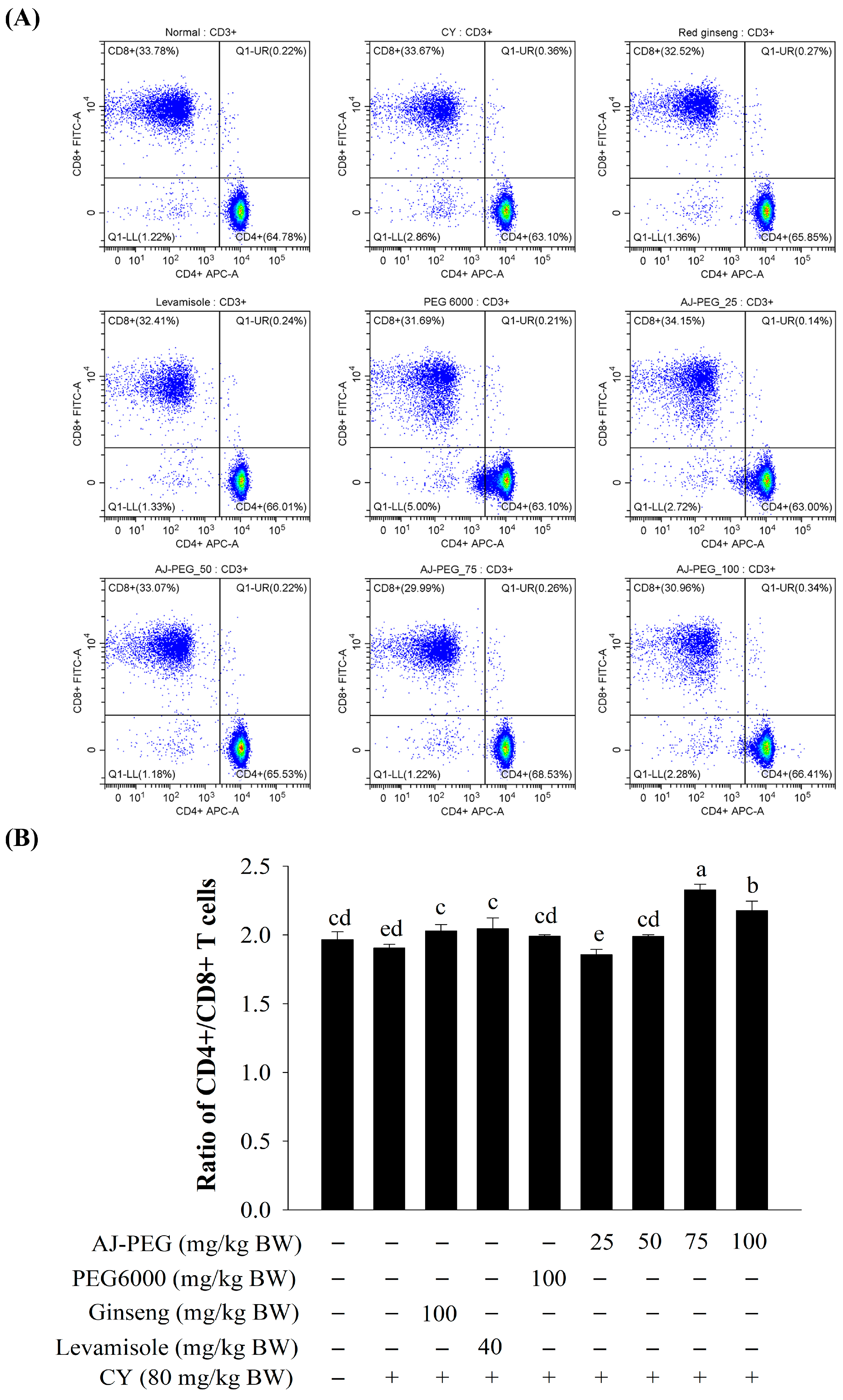
3.6. Effect of AJ-PEG on T Lymphocytes’ Subsets of Splenocytes
3.7. Effect of AJ-PEG on Peritoneal Macrophage Proliferation and NO Production
3.8. Effect of AJ-PEG on Peritoneal Macrophage Phagocytosis
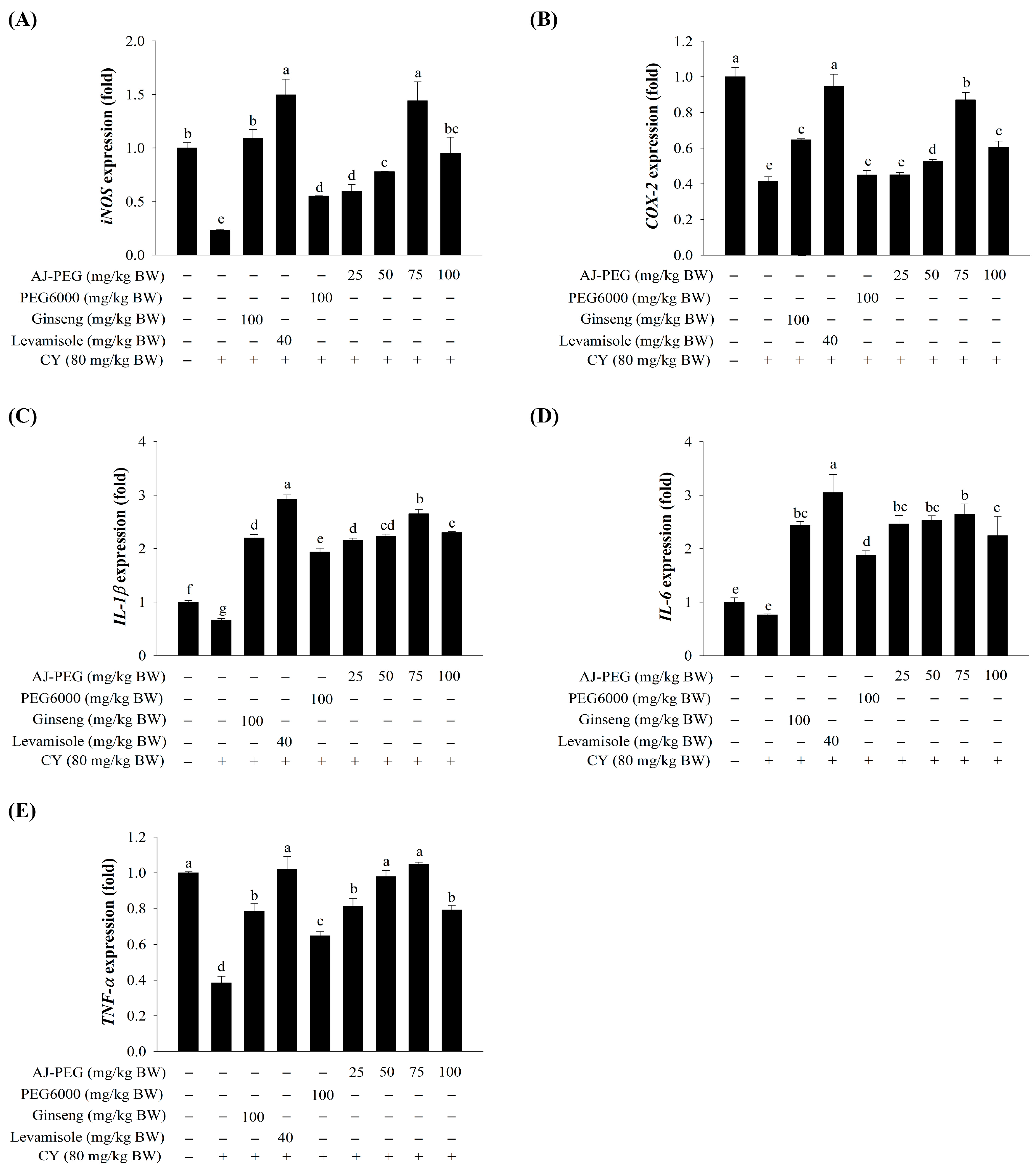
3.9. Effect of AJ-PEG on Gene Expression in Peritoneal Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delves, P.J.; Roitt, I.M. The immune system. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Meng, M.; Feng, C.C.; Wang, X.; Wang, C.L. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-κB pathway. Food Funct. 2019, 10, 4792–4801. [Google Scholar] [CrossRef]
- Sitzia, J.; Huggins, L. Side effects of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998, 6, 13–21. [Google Scholar]
- Hao, L.X.; Zhao, X.H. Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food Agric. Immunol. 2016, 27, 667–677. [Google Scholar] [CrossRef]
- Han, L.; Meng, M.; Guo, M.; Cheng, D.; Shi, L.; Wang, X.; Wang, C. Immunomodulatory activity of a water-soluble polysaccharide obtained from highland barley on immunosuppressive mice models. Food Funct. 2019, 10, 304–314. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Yu, M.; Wang, Y.; Jiang, T.; Yang, S.; Lv, Z. Glycosaminoglycan from Apostichopus japonicus induces immunomodulatory activity in cyclophosphamide-treated mice and in macrophages. Int. J. Biol. Macromol. 2019, 130, 229–237. [Google Scholar] [CrossRef]
- Zhang, W.N.; Gong, L.L.; Liu, Y.; Zhou, Z.B.; Wan, C.X.; Xu, J.J.; Wu, Q.X.; Chen, L.; Lu, Y.M.; Chen, Y. Immunoenhancement effect of crude polysaccharides of Helvella leucopus on cyclophosphamide-induced immunosuppressive mice. J. Funct. Foods 2020, 69, 103942. [Google Scholar]
- Bai, R.-B.; Zhang, Y.-J.; Fan, J.-M.; Jia, X.-S.; Li, D.; Wang, Y.-P.; Zhou, J.; Yan, Q.; Hu, F.-D. Immune-enhancement effects of oligosaccharides from Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food Funct. 2020, 11, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2018, 13, e0204152. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Inflamm. Allergy Drug Targets. 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, K.; Yang, L.; Liu, R.; Chu, Y.; Qin, X.; Yang, P.; Yu, H. Lipid metabolism in inflammation-related diseases. Analyst 2018, 143, 4526–4536. [Google Scholar] [CrossRef]
- Cejas, J.R.; Almansa, E.; Villamandos, J.E.; Badía, P.; Bolaños, A.; Lorenzo, A. Lipid and fatty acid composition of ovaries from wild fish and ovaries and eggs from captive fish of white sea bream (Diplodus sargus). Aquaculture 2003, 216, 299–313. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between fatty acid profile and anti-inflammatory activity in common Australian seafood by-products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef]
- Holub, D.J.; Holub, B.J. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell Biochem. 2004, 263, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Shan, K.; Chen, H.; Chen, Y.Q. n-3 polyunsaturated fatty acids and their role in cancer chemoprevention. Curr. Pharmacol. Rep. 2015, 1, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C. Omega-3 fatty acids and inflammation: A perspective on the challenges of evaluating efficacy in clinical research. Prostaglandins Other Lipid Mediat 2015, 116–117, 104–111. [Google Scholar] [CrossRef]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Gaugler, T.L.; Fleming, J.A.; Kris-Etherton, P.M. Effects of supplemental long-chain omega-3 fatty acids and erythrocyte membrane fatty acid content on circulating inflammatory markers in a randomized controlled trial of healthy adults. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 161–168. [Google Scholar] [CrossRef]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef]
- Polyviou, T.; MacDougall, K.; Chambers, E.S.; Viardot, A.; Psichas, A.; Jawaid, S.; Harris, H.C.; Edwards, C.A.; Simpson, L.; Murphy, K.G.; et al. Randomised clinical study: Inulin short-chain fatty acid esters for targeted delivery of short-chain fatty acids to the human colon. Aliment. Pharmacol. Ther. 2016, 44, 662–672. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yu, J.; Chen, Y.; Cheng, D.; Wang, X.; Wang, C. Immunomodulatory activity of docosahexenoic acid on RAW264.7 cells activation through GPR120-mediated signaling pathway. J. Agric. Food Chem. 2018, 66, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Bie, N.; Han, L.; Meng, M.; Yan, Z.; Wang, C. The immunomodulatory effect of docosahexaenoic acid (DHA) on the RAW264.7 cells by modification of the membrane structure and function. Food Funct. 2020, 11, 2603–2616. [Google Scholar] [CrossRef]
- Paixão, E.M.d.S.; Oliveira, A.C.d.M.; Pizato, N.; Muniz-Junqueira, M.I.; Magalhães, K.G.; Nakano, E.Y.; Ito, M.K. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: A randomized double-blind controlled trial. Nutr. J. 2017, 16, 71. [Google Scholar] [CrossRef]
- D’Vaz, N.; Amarasekera, M.; Dunstan, J.; Meldrum, S.; Lee-Pullen, T.; Metcalfe, J.; Holt, B.; Serralha, M.; Tulic, M.; Mori, T.; et al. Basic and clinical immunology—3020. Fish oil supplementation in early infancy modulates developing infant immune responses but not clinical allergy. World Allergy Organ. J. 2013, 6, P196. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; You, C.; Chen, B.; Wang, S.; Li, Y. Effects of different dietary ratios of docosahexaenoic to eicosapentaenoic acid (DHA/EPA) on the growth, non-specific immune indices, tissue fatty acid compositions and expression of genes related to LC-PUFA biosynthesis in juvenile golden pompano Trachinotus ovatus. Aquaculture 2019, 505, 488–495. [Google Scholar]
- Cardoso, C.R.; Favoreto, S.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef]
- Yaqoob, P. Monounsaturated fatty acids and immune function. Eur. J. Clin. Nutr. 2002, 56, S9–S13. [Google Scholar] [CrossRef]
- Miao, H.; Chen, L.; Hao, L.; Zhang, X.; Chen, Y.; Ruan, Z.; Liang, H. Stearic acid induces proinflammatory cytokine production partly through activation of lactate-HIF1α pathway in chondrocytes. Sci. Rep. 2015, 5, 13092. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Yang, J.H.; Yoon, S.C.; Chun, Y.Y.; Kim, J.B.; Cha, H.K.; Choi, Y.M. Biomass estimation of sailfin sandfish, Arctoscopus japonicus, in Korean waters. Korean J. Fish. Aquat. Sci. 2009, 42, 487–493. [Google Scholar]
- Shirai, S.M.; Kuranaga, R.; Sugiyama, H.; Higuchi, M. Population structure of the sailfin sandfish, Arctoscopus japonicus (Trichodontidae), in the Sea of Japan. Ichthyol. Res. 2006, 53, 357–368. [Google Scholar] [CrossRef]
- Kang, M.; Fajaryanti, R.; Yoon, S.; Hwang, B. Acoustic characteristic of sailfin sandfish (Arctoscopus japonicus) in Dokdo, Republic of Korea. Ocean Sci. J. 2020, 55, 289–301. [Google Scholar] [CrossRef]
- Jang, H.L.; Shin, S.R.; Yoon, K.Y. Hydrolysis conditions for antioxidant peptides derived from enzymatic hydrolysates of sandfish (Arctoscopus japonicus). Food Sci. Biotechnol. 2017, 26, 1191–1197. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Isolation and characteristics of anti-inflammatory peptides from enzymatic hydrolysates of sandfish (Arctoscopus japonicus) protein. J. Aquat. Food Prod. Technol. 2017, 26, 234–244. [Google Scholar] [CrossRef]
- Jang, H.L.; Young Yoon, K. Optimal conditions of enzymatic hydrolysis for producing anti-inflammatory peptides from sandfish (Arctoscopus japonicus) hydrolysate. Korean J. Food Sci. Technol. 2018, 50, 203–208. [Google Scholar]
- Ishihara, Y.; Watanabe, F. Lipid content and fatty acid composition of Japanese sandfish Arctoscopus japonicus caught offshore of Tottori Prefecture, Japan. Nippon Suisan Gakk. 2013, 79, 229–231. [Google Scholar] [CrossRef]
- Rod-In, W.; Monmai, C.; Lee, S.M.; Jung, S.K.; You, S.; Park, W.J. Anti-Inflammatory effects of lipids extracted from Arctoscopus japonicus eggs on LPS-stimulated RAW264.7 cells. Mar. Drugs 2019, 17, 580. [Google Scholar] [CrossRef]
- Rod-In, W.; Monmai, C.; Shin, I.-S.; You, S.; Park, W.J. Neutral lipids, glycolipids, and phospholipids, isolated from sandfish (Arctoscopus japonicus) eggs, exhibit anti-inflammatory activity in LPS-stimulated RAW264.7 cells through NF-κB and MAPKs pathways. Mar. Drugs 2020, 18, 480. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Lin, S.; Liu, H.; Qiang, Y.; Chen, H.; Jiang, Z.; Zhang, K.; Duan, X.; Xu, Y. Preparation, characterization and in vitro and in vivo evaluation of a solid dispersion of Naringin. Drug Dev. Ind. Pharm. 2018, 44, 1725–1732. [Google Scholar] [CrossRef]
- Ray, A.; Dittel, B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010, 35, 1488. [Google Scholar]
- Weeks, B.A.; Keisler, A.S.; Myrvik, Q.N.; Warinner, J.E. Differential uptake of neutral red by macrophages from three species of estuarine fish. Dev. Comp. Immunol. 1987, 11, 117–124. [Google Scholar] [CrossRef]
- Peng, S.; Yue, Y.; Gao, Q.; Shi, Z.; Yin, F.; Wang, J. Influence of dietary n-3 LC-PUFA on growth, nutritional composition and immune function in marine fish Sebastiscus marmoratus. Chin. J. Oceanol. Limnol. 2014, 32, 1000–1008. [Google Scholar] [CrossRef]
- Ji Eun, K.; Chaiwat, M.; Weerawan, R.-i.; Jang, A.-y.; Sang-Guan, Y.; Sang-min, L.; Woo Jung, P. Immune enhancement effects of Codium fragile anionic macromolecules combined with red ginseng extract in immune-suppressed mice. J. Microbiol. Biotechnol. 2019, 29, 1361–1368. [Google Scholar]
- Chen, L.; Qi, Y.; Qi, Z.; Gao, K.; Gong, R.; Shao, Z.; Liu, S.; Li, S.; Sun, Y. A comparative study on the effects of different parts of Panax ginseng on the immune activity of cyclophosphamide-induced immunosuppressed mice. Molecules 2019, 24, 1096. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Maruyama, K.; Suhara, T.; Harashima, H. Factors governing the in vivo tissue uptake of transferrin-coupled polyethylene glycol liposomes in vivo. Int. J. Pharm. 2004, 281, 25–33. [Google Scholar] [CrossRef]
- Kreppel, F.; Kochanek, S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol. Ther. 2008, 16, 16–29. [Google Scholar] [CrossRef]
- Croyle, M.A.; Chirmule, N.; Zhang, Y.; Wilson, J.M. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 2001, 75, 4792–4801. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, F.A. Extrathymic CD4/CD8 double positive T cells. Vet. Immunol. Immunopathol. 1999, 72, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Lim, T.G.; Ahn, S.; Hong, H.D.; Rhee, Y.K.; Kim, K.T.; Lee, E.; Lee, J.H.; Lee, Y.J.; Jung, C.S.; et al. Immune-enhancing effects of a high molecular weight fraction of Cynanchum wilfordii Hemsley in macrophages and immunosuppressed mice. Nutrients 2016, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]


| Group | Dose (mg/kg BW) | Treatment | ||
|---|---|---|---|---|
| Day 1 to 3 (Orally) | Day 4 to 6 (Orally + i.p.) | Day 7 to 10 (Orally) | ||
| Normal | - | Saline | Saline | Saline |
| CY | 80 | Saline | Saline + CY | Saline |
| Ginseng | 100 | Ginseng | Ginseng + CY | Ginseng |
| Levamisole | 40 | Levamisole | Levamisole + CY | Levamisole |
| PEG6000 | 100 | PEG6000 | PEG6000+CY | PEG6000 |
| AJ-PEG | 25 | AJ-PEG | AJ-PEG + CY | AJ-PEG |
| 50 | AJ-PEG | AJ-PEG + CY | AJ-PEG | |
| 75 | AJ-PEG | AJ-PEG + CY | AJ-PEG | |
| 100 | AJ-PEG | AJ-PEG + CY | AJ-PEG | |
| Gene | Accession No. | Sequence of Primer (5′ to 3′) | |
|---|---|---|---|
| Forward Primer | Reverse Primer | ||
| iNOS | BC062378.1 | TTCCAGAATCCCTGGACAAG | TGGTCAAACTCTTGGGGTTC |
| IL-1β | NM_008361.4 | GGGCCTCAAAGGAAAGAATC | TACCAGTTGGGGAACTCTGC |
| IL-2 | NM_008366.3 | CCTGAGCAGGATGGAGAATTACA | TCCAGAACATGCCGCAGAG |
| IL-4 | NM_021283.2 | ACAGGAGAAGGGACGCCAT | GAAGCCCTACAGACGAGCTCA |
| IL-6 | NM_031168.2 | AGTTGCCTTCTTGGGACTGA | CAGAATTGCCATTGCACAAC |
| IFN-γ | NM_008337.3 | CTCAAGTGGCATAGATGT | GAGATAATCTGGCTCTGCAGGATT |
| TNF-α | D84199.2 | ATGAGCACAGAAAGCATGATC | TACAGGCTTGTCACTCGAATT |
| TLR4 | NM_021297.3 | CGCTCTGGCATCATCTTCAT | GTTGCCGTTTCTTGTTCTTCC |
| COX-2 | NM_011198.4 | AGAAGGAAATGGCTGCAGAA | GCTCGGCTTCCAGTATTGAG |
| β-actin | NM_007393.5 | CCACAGCTGAGAGGGAAATC | AAGGAAGGCTGGAAAAGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Rod-in, W.; Jang, A.-y.; Park, W.J. Arctoscopus japonicus Lipids Enhance Immunity of Mice with Cyclophosphamide-Induced Immunosuppression. Foods 2023, 12, 3292. https://doi.org/10.3390/foods12173292
Choi J, Rod-in W, Jang A-y, Park WJ. Arctoscopus japonicus Lipids Enhance Immunity of Mice with Cyclophosphamide-Induced Immunosuppression. Foods. 2023; 12(17):3292. https://doi.org/10.3390/foods12173292
Chicago/Turabian StyleChoi, JeongUn, Weerawan Rod-in, A-yeong Jang, and Woo Jung Park. 2023. "Arctoscopus japonicus Lipids Enhance Immunity of Mice with Cyclophosphamide-Induced Immunosuppression" Foods 12, no. 17: 3292. https://doi.org/10.3390/foods12173292
APA StyleChoi, J., Rod-in, W., Jang, A.-y., & Park, W. J. (2023). Arctoscopus japonicus Lipids Enhance Immunity of Mice with Cyclophosphamide-Induced Immunosuppression. Foods, 12(17), 3292. https://doi.org/10.3390/foods12173292






