Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing
Abstract
:1. Introduction
Food Waste and Circular Bioeconomy
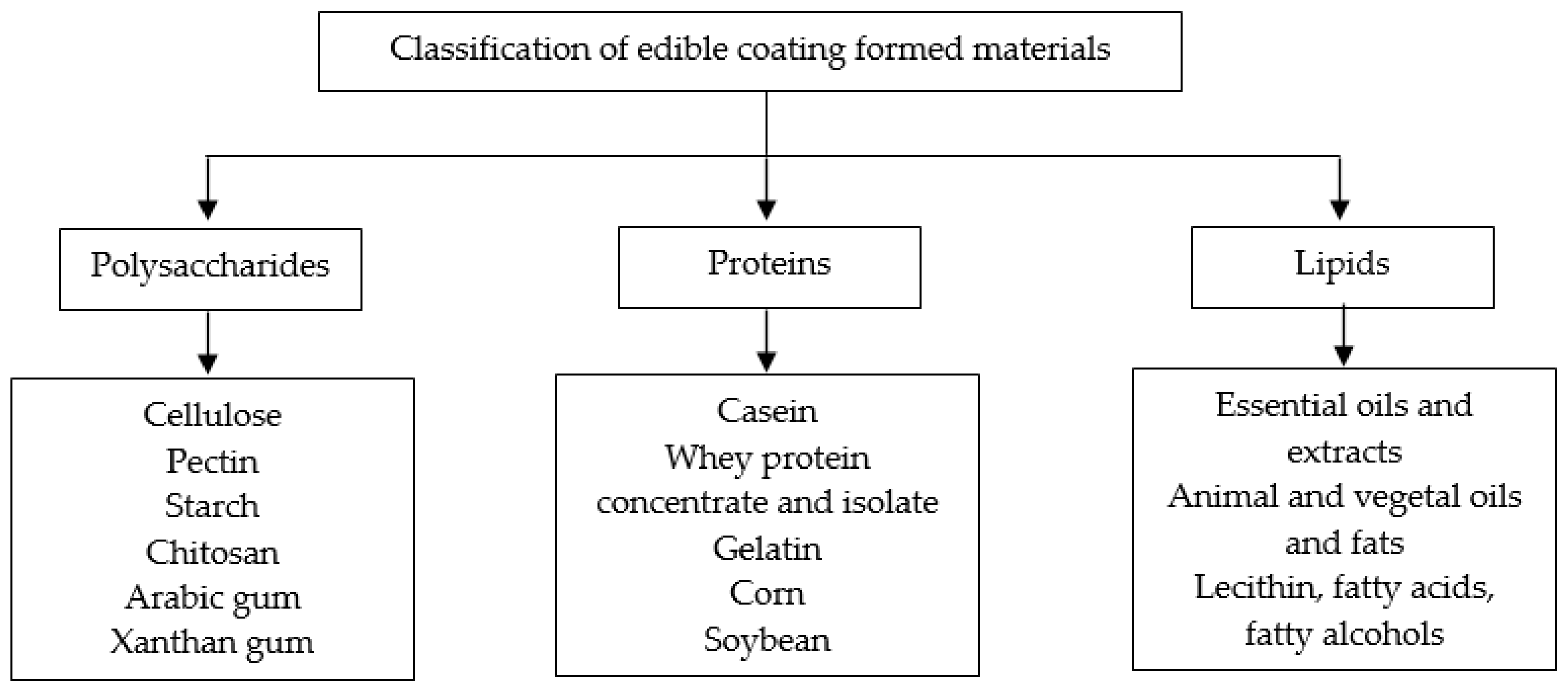
2. Edible Coatings (General Concept)
- Immersion
- Spraying
- Pan coating method
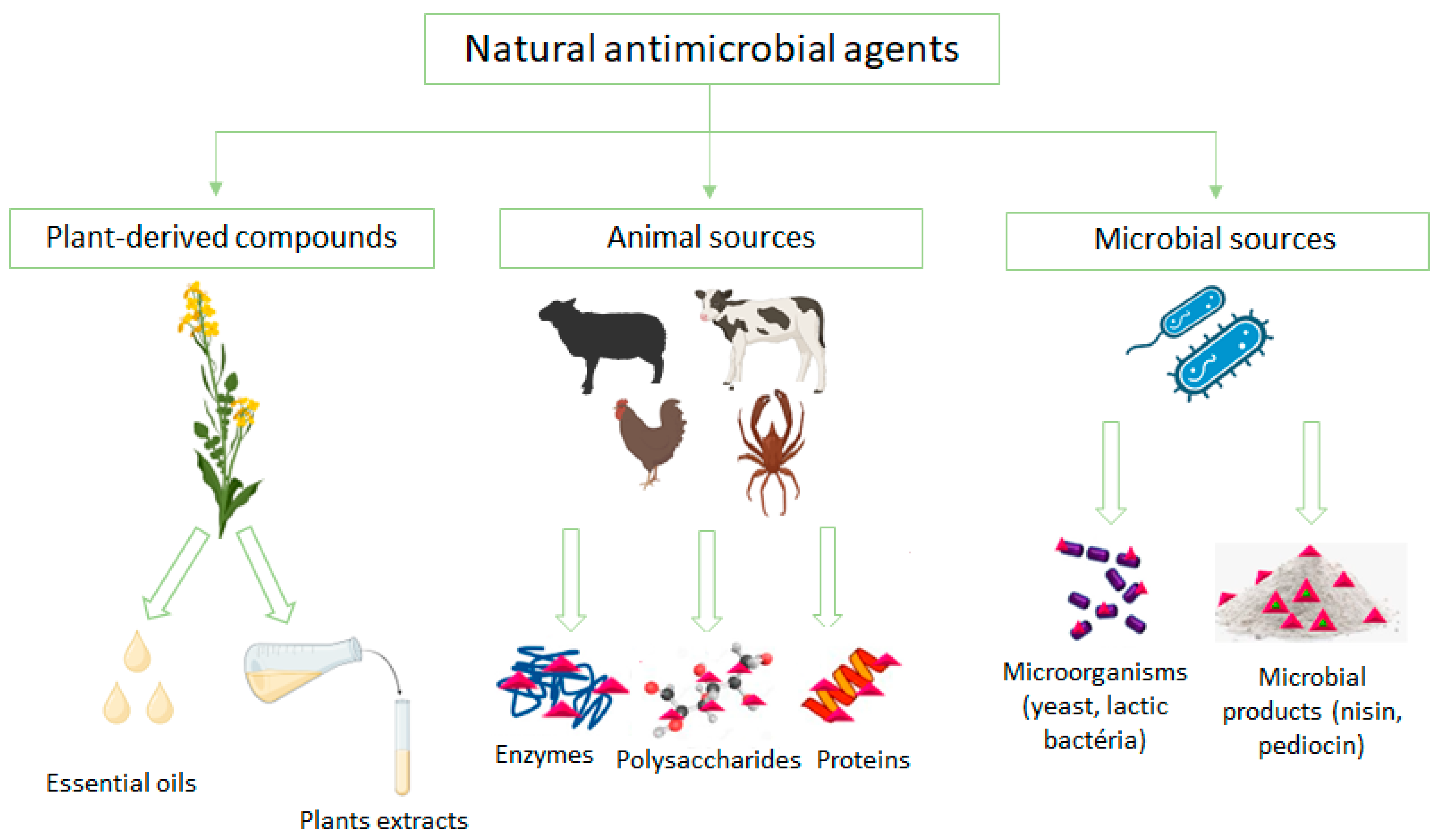
2.1. Antimicrobial Edible Coating
2.2. Characteristics of Antimicrobial Coatings Designed for Food Packaging Use
2.3. Applications of Antimicrobial Edible Coatings: Fruit and Traditional Sausages
2.3.1. Minimally Processed Fruit
2.3.2. Traditional Sausages
2.4. Edible Films and Coatings Obtained from Organic Food Residues–Examples of Waste Valorization and Circular Economy Potential in Food Packaging
3. Recent Developments in Food Packaging with Antimicrobial Properties
Bionanocomposites and Issues Regarding Safety of Heavy and Nanoparticles
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food Preservation Techniques and Nanotechnology for Increased Shelf Life of Fruits, Vegetables, Beverages and Spices: A Review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef]
- Kilcast, D.; Subramaniam, P. Food and Beverage Stability and Shelf Life, 1st ed.; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements- a Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Peralta, H.C. Um Terço Dos Alimentos Produzidos No Mundo é Desperdiçado. Available online: https://www.dn.pt/sociedade/um-terco-dos-alimentos-produzidos-no-mundo-e-desperdicado-14049871.html (accessed on 24 May 2023).
- ONU Cerca de 930 Milhões de Toneladas de Comida Vão Parar No Lixo, Alerta FAO. Available online: https://news.un.org/pt/story/2021/09/1764812 (accessed on 24 May 2023).
- Conselho da União Europeia. Um Novo Plano de Ação Para a Economia Circular Para Uma Europa Mais Limpa e Competitiva; COMISSÃO EUROPEIA: Bruxelas, Belgium, 2020. [Google Scholar]
- PHENIX O Desperdício Ainda é Um Tabu Mas a Mudança Está a Caminho! Available online: https://www.wearephenix.com/pt-pt/o-desperdicio-alimentar-em-dados/ (accessed on 24 May 2023).
- Huang, W.; Xu, H.; Xue, Y.; Huang, R.; Deng, H.; Pan, S. Layer-by-Layer Immobilization of Lysozyme–Chitosan–Organic Rectorite Composites on Electrospun Nanofibrous Mats for Pork Preservation. Food Res. Int. 2012, 48, 784–791. [Google Scholar] [CrossRef]
- López-Carballo, G.; Gómez-Estaca, J.; Catalá, R.; Hernández-Muñoz, P.; Gavara, R. Active Antimicrobial Food and Beverage Packaging. In Emerging Food Packaging Technologies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 27–54. [Google Scholar]
- Han, J.H. Emerging Technologies in Food Packaging. Overview. Plast. Film. Food Packag. Mater. Technol. Appl. 2012, 121–126. [Google Scholar] [CrossRef]
- Vinay Pramod Kumar, K.; Suneetha, J.W.; Vinay Pramod Kumar, C.K.; Anila Kumari, B. Active Packaging Systems in Food Packaging for Enhanced Shelf Life. J. Pharmacogn. Phytochem. 2018, 7, 2044–2046. [Google Scholar]
- Jideani, V.A.; Vogt, K. Antimicrobial Packaging for Extending the Shelf Life of Bread—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1313–1324. [Google Scholar] [CrossRef]
- Prasad, P.; Kochhar, A. Active Packaging in Food Industry: A Review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 01–07. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial Food Packaging in Meat Industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on Antimicrobial Packaging for Extending the Shelf Life of Food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Ezeoha, S.L. Production of Biodegradable Plastic Packaging Film from Cassava Starch. IOSR J. Eng. 2013, 3, 14–20. [Google Scholar] [CrossRef]
- APA Bioeconomia. Available online: https://apambiente.pt/apa/bioeconomia (accessed on 24 May 2023).
- Slavutsky, A.M.; Bertuzzi, M.A. Water Barrier Properties of Starch Films Reinforced with Cellulose Nanocrystals Obtained from Sugarcane Bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Isfari, D.; Gemilang Lara, U. Cheese Whey as Potential Resource for Antimicrobial Edible Film and Active Packaging Production. Foods Raw Mater. 2019, 7, 229–239. [Google Scholar] [CrossRef]
- Sánchez Aldana, D.; Andrade-Ochoa, S.; Aguilar, C.N.; Contreras-Esquivel, J.C.; Nevárez-Moorillón, G.V. Antibacterial Activity of Pectic-Based Edible Films Incorporated with Mexican Lime Essential Oil. Food Control 2015, 50, 907–912. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial Cellulose-Lactoferrin as an Antimicrobial Edible Packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Ningtyas, R.; Nugroho, H.; Sabrina, A. Mechanical Properties of Edible Film from Tanduk Banana (Musa corniculata Rumph) Peels for Food Packaging. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 4–9. [Google Scholar] [CrossRef]
- Galus, S.; Kibar, E.A.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings-A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Pérez-Santaescolástica, C.; Munekata, P.E.S.; Feng, X.; Liu, Y.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Active Edible Coatings and Films with Mediterranean Herbs to Improve Food Shelf-Life. Crit. Rev. Food Sci. Nutr. 2022, 62, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Garza, M.Z.; García, S.; del Socorro Flores-González, M.; Arévalo-Niño, K. Edible Active Coatings Based on Pectin, Pullulan, and Chitosan Increase Quality and Shelf Life of Strawberries (Fragaria ananassa). J. Food Sci. 2015, 80, M1823–M1830. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of Oregano Essential Oil and Resveratrol Nanoemulsion Loaded Pectin Edible Coating on the Preservation of Pork Loin in Modified Atmosphere Packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; Alemán, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Development of Active Films of Chitosan Isolated by Mild Extraction with Added Protein Concentrate from Shrimp Waste. Food Hydrocoll. 2015, 43, 91–99. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Mohammad Amini, A.; Zahedi, Y. Characterisation of a New Biodegradable Edible Film Based on Sage Seed Gum: Influence of Plasticiser Type and Concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Vipan, B.; Mahajan, C.; Tandon, R.; Kapoor, S.; Sidhu, M.K. Natural Coatings for Shelf-Life Enhancement and Quality Maintenance of Fresh Fruits and Vegetables-A Review. J. Postharvest Technol. 2018, 06, 12–26. [Google Scholar]
- Appendini, P.; Hotchkiss, J.H. Review of Antimicrobial Food Packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Active Packaging Technologies with an Emphasis on Antimicrobial Packaging and Its Applications. J. Food Sci. 2003, 68, 408–420. [Google Scholar] [CrossRef]
- Han, J.H. Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780080455174. [Google Scholar]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Starch-Based Coatings: Effect on Refrigerated Strawberry (Fragaria ananassa) Quality. J. Sci. Food Agric. 1998, 76, 411–420. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; de Luca Sarantópoulos, C.I.G.; Hubinger, M.D. Selection of an Edible Starch Coating for Minimally Processed Strawberry. Food Bioprocess Technol. 2010, 3, 834–842. [Google Scholar] [CrossRef]
- Bierhals, V.S.; Chiumarelli, M.; Hubinger, M.D. Effect of Cassava Starch Coating on Quality and Shelf Life of Fresh-Cut Pineapple (Ananas Comosus L. Merril Cv “Pérola”). J. Food Sci. 2011, 76, 62–72. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Hubinger, M.D. Fresh Cut ‘Tommy Atkins’ Mango Pre-Treated with Citric Acid and Coated with Cassava (Manihot Esculenta Crantz) Starch or Sodium Alginate. Innov. Food Sci. Emerg. Technol. 2011, 12, 381–387. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Wood, B. Use of Edible Coating to Preserve Pecans at Room Temperature. HortScience 2006, 41, 188–192. [Google Scholar] [CrossRef]
- Nisperos-Carriedo, M.; Baldwin, E.; Shaw, P. Development of an Edible Coating for Extending Postharvest Life of Selected Fruits and Vegetables. Proc. Fla. State Hort. Soc. 1991, 104, 122–125. [Google Scholar]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible Coatings with Antibrowning Agents to Maintain Sensory Quality and Antioxidant Properties of Fresh-Cut Pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Han, J.H.; Seo, G.H.; Park, I.M.; Kim, G.N.; Lee, D.S. Physical and Mechanical Properties of Pea Starch Edible Films Containing Beeswax Emulsions. J. Food Sci. 2006, 71, E290–E296. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of Edible Films and Coatings from Alginates and Carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Costa, C.; Conte, A.; Del Nobile, M.A. Effective Preservation Techniques to Prolong the Shelf Life of Ready-to-Eat Oysters. J. Sci. Food Agric. 2014, 94, 2661–2667. [Google Scholar] [CrossRef]
- Raghav, K.; Agarwal, N.; Saini, M. Edible Coating of Fruits and Vegetables: A Review. Edible Coat. Fruits Veg. a Rev. 2016, I, 188–204. [Google Scholar]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Lipid-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 135–168. [Google Scholar]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar]
- Akita, K.; Associates, J.P.; Building, D. Patent Application Publication (10) Pub. No.: US 2008 / 0283851 A1 Patent Application Publication. U.S. Patent 1(19), US 2008/0280 138 A1, 2008. [Google Scholar]
- Santiago-Silva, P.; Soares, N.F.F.; Nóbrega, J.E.; Júnior, M.A.W.; Barbosa, K.B.F.; Volp, A.C.P.; Zerdas, E.R.M.A.; Würlitzer, N.J. Antimicrobial Efficiency of Film Incorporated with Pediocin (ALTA® 2351) on Preservation of Sliced Ham. Food Control 2009, 20, 85–89. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeiren, L.; Debevere, J. New Preservation Technologies: Possibilities and Limitations. Int. Dairy J. 2004, 14, 273–285. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of Herbs, Spices and Their Bioactive Compounds in Active Food Packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential Oil Composition, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected from Different Regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Cao, L.; Si, J.Y.; Liu, Y.; Sun, H.; Jin, W.; Li, Z.; Zhao, X.H.; Pan, R. Le Essential Oil Composition, Antimicrobial and Antioxidant Properties of Mosla Chinensis Maxim. Food Chem. 2009, 115, 801–805. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Maksimović, M. Chemical Composition and Bioactivity of Essential Oil from Thymus Species in Balkan Peninsula. Phytochem. Rev. 2015, 14, 335–352. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Shivpuri, A.; Sharma, O.P.; Jhamaria, S.L. Fungitoxic Properties of Plant Extracts against Pathogenic Fungi. J. Mycol. Plant Pathol. 1997, 70, 13–17. [Google Scholar]
- Kapoor, A. Antifungal Activities of Fresh Juice and Aqueous Extracts of Turmeric and Ginger. J. Phytol. Res. 1997, 10, 59–62. [Google Scholar]
- Marino, M.; Bersani, C.; Comi, G. Impedance Measurements to Study the Antimicrobial Activity of Essential Oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Raybaudimassilia, R.; Mosquedamelgar, J.; Martinbelloso, O. Edible Alginate-Based Coating as Carrier of Antimicrobials to Improve Shelf-Life and Safety of Fresh-Cut Melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; De Feo, V.; Altieri, L.; Mancini, E.; De Martino, L.; Luigi Rana, G. An Attempt of Postharvest Orange Fruit Rot Control Using Essential Oils from Mediterranean Plants. J. Med. Food 2010, 13, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Leyva, J.M.; Ortega-Ramírez, L.A.; Carrazco-Lugo, D.K.; Pérez-Carlón, J.J.; Melgarejo-Flores, B.G.; González-Aguilar, G.A.; Miranda, M.R.A. Pectin-Cinnamon Leaf Oil Coatings Add Antioxidant and Antibacterial Properties to Fresh-Cut Peach. Flavour Fragr. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of Antioxidant and Antimicrobial Coating Based on Whey Protein Nanofibrils with TiO2 Nanotubes on the Quality and Shelf Life of Chilled Meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural Antimicrobials as Additives for Edible Food Packaging Applications: A Review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef]
- Min, S.; Krochta, J.M. Inhibition of Penicillium Commune by Edible Whey Protein Films Incorporating Lactoferrin, Lacto-Ferrin Hydrolysate, and Lactoperoxidase Systems. J. Food Sci. 2005, 70, M87–M94. [Google Scholar] [CrossRef]
- Lim, G.-O.; Jang, S.-A.; Song, K. Bin Physical and Antimicrobial Properties of Gelidium Corneum/Nano-Clay Composite Film Containing Grapefruit Seed Extract or Thymol. J. Food Eng. 2010, 98, 415–420. [Google Scholar] [CrossRef]
- Aider, M. Chitosan Application for Active Bio-Based Films Production and Potential in the Food Industry: Review. LWT - Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Effects of Gellan-Based Edible Coating on the Quality of Fresh-Cut Pineapple During Cold Storage. Food Bioprocess Technol. 2014, 7, 2144–2151. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-Chemical Properties of Edible Films Derived from Native and Phosphated Cush-Cush Yam and Cassava Starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Marques, P.T.; Lima, A.M.F.; Bianco, G.; Laurindo, J.B.; Borsali, R.; Le Meins, J.-F.; Soldi, V. Thermal Properties and Stability of Cassava Starch Films Cross-Linked with Tetraethylene Glycol Diacrylate. Polym. Degrad. Stab. 2006, 91, 726–732. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Structural and Mechanical Properties of Edible Films Made from Native and Modified Cush-Cush Yam and Cassava Starch. Food Hydrocoll. 2015, 45, 211–217. [Google Scholar] [CrossRef]
- Schmid, M.; Pröls, S.; Kainz, D.M.; Hammann, F.; Grupa, U. Effect of Thermally Induced Denaturation on Molecular Interaction-Response Relationships of Whey Protein Isolate Based Films and Coatings. Prog. Org. Coatings 2017, 104, 161–172. [Google Scholar] [CrossRef]
- Abigail, O. Volumen de Fruta Fresca Consumida a Nivel Mundial Entre 2018 y 2028. Available online: https://es.statista.com/estadisticas/1308998/consumo-mundial-de-fruta-fresca-a-nivel-mundial/ (accessed on 23 July 2023).
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Aguilar-Toalá, J.E.; Liceaga, A.M. Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities. Polysaccharides 2023, 4, 99–115. [Google Scholar] [CrossRef]
- Hugo, C.J.; Hugo, A. Current Trends in Natural Preservatives for Fresh Sausage Products. Trends Food Sci. Technol. 2015, 45, 12–23. [Google Scholar] [CrossRef]
- Islam, R.U.; Khan, M.A.; Islam, S.U. Plant Derivatives as Promising Materials for Processing and Packaging of Meat-Based Products—Focus on Antioxidant and Antimicrobial Effects. J. Food Process. Preserv. 2017, 41, e12862. [Google Scholar] [CrossRef]
- Honikel, K.O. The Use and Control of Nitrate and Nitrite for the Processing of Meat Products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-Evaluation of Sodium Nitrate (E 251) and Potassium Nitrate (E 252) as Food Additives. EFSA J. 2017, 15, 1–123. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, A.R.; Han, J. Antioxidant and Antimicrobial Activities of Leafy Green Vegetable Extracts and Their Applications to Meat Product Preservation. Food Control 2013, 29, 112–120. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural Antimicrobial/Antioxidant Agents in Meat and Poultry Products as Well as Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2016, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and By-Products as Source of Natural Antioxidants in Meat and Meat Products: A Review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, J.; Ramírez-Toro, C.; Bolívar, G.; Ceballos-González, C. Mechanisms of Action of Novel Ingredients Used in Edible Films to Preserve Microbial Quality and Oxidative Stability in Sausages—A Review. Trends Food Sci. Technol. 2019, 89, 100–109. [Google Scholar] [CrossRef]
- Fuller, G.W. New Food Product Development; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429062711. [Google Scholar]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar]
- Chien, P.-J.; Sheu, F.; Yang, F.-H. Effects of Edible Chitosan Coating on Quality and Shelf Life of Sliced Mango Fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Nélio Ranieli Ferreira de, P.; Eduardo Valério de Barros, B.; Rodrigues, L.J.; Carvalho, R.A.; Piccoli, R.H. Qualidade de Produtos Minimamente Processados e Comercializados Em Gôndolas de Supermercados Nas Cidades de Lavras—MG, Brasília—DF e São Paulo—SP. Ciência e Agrotecnol. 2009, 33, 219–227. [Google Scholar] [CrossRef]
- Nascimento, M.S. Efeito de Revestimentos Ativos No Período de Vida Útil Do Chouriço Tradicional Português. Master’s Thesis, Universidade Tecnica de Lisboa, Lisbon, Portugal, 2012; p. 125. [Google Scholar]
- Janjarasskul, T.; Krochta, J.M. Edible Packaging Materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Pérez, M.J.; Quintanar-Guerrero, D.; Mercado-Silva, E.; Real-Sandoval, S.A.; Zambrano-Zaragoza, M.L. The Effects of Tocopherol Nanocapsules/Xanthan Gum Coatings on the Preservation of Fresh-Cut Apples: Evaluation of Phenol Metabolism. Food Bioprocess Technol. 2015, 8, 1791–1799. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the Shelf Life of Fresh-Cut ‘Royal Delicious’ Apple with Edible Coatings and Anti-Browning Agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Drăghici, M.C.; Popescu, P.A.; Popa, V.I.; Bujor, O.-C.; Ion, V.A.; Popa, M.E. Latest Developments in Edible Coatings on Minimally Processed Fruits and Vegetables: A Review. Foods 2021, 10, 2821. [Google Scholar] [CrossRef]
- Nain, N.; Katoch, G.K.; Kaur, S.; Rasane, P. Recent Developments in Edible Coatings for Fresh Fruits and Vegetables. J. Hortic. Res. 2021, 29, 127–140. [Google Scholar] [CrossRef]
- Popescu, P.A.; Palade, L.M.; Nicolae, I.C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, J.; Zhu, Y.; Han, W.; Xuan, H.; Ge, L. The Preservation Effect of Ascorbic Acid and Calcium Chloride Modified Chitosan Coating on Fresh-Cut Apples at Room Temperature. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 502, 102–106. [Google Scholar] [CrossRef]
- Vilaplana, R.; Guerrero, K.; Guevara, J.; Valencia-Chamorro, S. Chitosan Coatings to Control Soft Mold on Fresh Blackberries (Rubus Glaucus Benth.) during Postharvest Period. Sci. Hortic. 2020, 262, 109049. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Prudencio, S.H.; Mali, S.; Pedrine Colabone Celligoi, M.A. Assessment of a New Edible Film Biodegradable Based on Starch-Nystose to Increase Quality and the Shelf Life of Blackberries. Food Biosci. 2021, 42, 101173. [Google Scholar] [CrossRef]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry Leaf Extracts Incorporated Chitosan Coatings for Preserving Postharvest Quality of Fresh Blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Khorram, F.; Ramezanian, A.; Hosseini, S.M.H. Effect of Different Edible Coatings on Postharvest Quality of ‘Kinnow’ Mandarin. J. Food Meas. Charact. 2017, 11, 1827–1833. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible Coatings Incorporating Pomegranate Peel Extract and Biocontrol Yeast to Reduce Penicillium Digitatum Postharvest Decay of Oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rios, D.A.; Nakamoto, M.M.; Braga, A.R.C.; da Silva, E.M.C. Food Coating Using Vegetable Sources: Importance and Industrial Potential, Gaps of Knowledge, Current Application, and Future Trends. Appl. Food Res. 2022, 2, 100073. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of Antioxidant Chitosan Film with Banana Peels Extract and Its Application as Coating in Maintaining the Storage Quality of Apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent Advances in Edible Coating of Food Products and Its Legislations: A Review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Oyom, W.; Xu, H.; Liu, Z.; Long, H.; Li, Y.; Zhang, Z.; Bi, Y.; Tahergorabi, R.; Prusky, D. Effects of Modified Sweet Potato Starch Edible Coating Incorporated with Cumin Essential Oil on Storage Quality of ‘Early Crisp’. LWT 2022, 153, 112475. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of Postharvest Shelf Life of Strawberries (Fragaria ananassa) Using a Coating of Chitosan-Whey Protein Isolate Conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Mohd, S.; Gull, A.; Ahad, T.; Malik, A.R.; Ahmad, T.; Ahmad, F.; Gani, A. Effect of Gum Arabic, Xanthan and Carrageenan Coatings Containing Antimicrobial Agent on Postharvest Quality of Strawberry: Assessing the Physicochemical, Enzyme Activity and Bioactive Properties. Int. J. Biol. Macromol. 2021, 183, 2100–2108. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of Strawberries with an Antifungal Edible Coating Using Peony Extracts in Chitosan. Food Bioprocess Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent Advances in Polysaccharide-Based Edible Coatings for Preservation of Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.U.; Min, B. Packaging and Storage. In Handbook of Fermented Meat and Poultry; Fidel, T., Ed.; Blackwell: Oxford, UK, 2007; pp. 289–300. ISBN 0813814774. [Google Scholar]
- Spotti, E.; Berni, E. Starter Cultures: Molds. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; Blackwell: Oxford, UK, 2007; pp. 171–176. [Google Scholar]
- Labadie, J. Spoilage Microrganisms: Risks and Control. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; Blackwell: Oxford, UK, 2007; pp. 421–426. [Google Scholar]
- Martín-Sánchez, A.M.; Chaves-López, C.; Sendra, E.; Sayas, E.; Fenández-López, J.; Pérez-Álvarez, J.Á. Lipolysis, Proteolysis and Sensory Characteristics of a Spanish Fermented Dry-Cured Meat Product (Salchichón) with Oregano Essential Oil Used as Surface Mold Inhibitor. Meat Sci. 2011, 89, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.-H.; Eo, J.-H.; Choi, Y.-H. Gelatin Coating on Quality Attributes of Sausage during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2011, 31, 834–842. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active Film from Chitosan Incorporating Green Tea Extract for Shelf Life Extension of Pork Sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Catarino, M.D.; Alves-Silva, J.M.; Fernandes, R.P.; Gonçalves, M.J.; Salgueiro, L.R.; Henriques, M.F.; Cardoso, S.M. Development and Performance of Whey Protein Active Coatings with Origanum Virens Essential Oils in the Quality and Shelf Life Improvement of Processed Meat Products. Food Control 2017, 80, 273–280. [Google Scholar] [CrossRef]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Basak, G.; Awasthi, M.G. Materiality of Edible Film Packaging in Muscle Foods: A Worthwhile Conception. J. Packag. Technol. Res. 2020, 4, 117–132. [Google Scholar] [CrossRef]
- Naufalin, R.; Wicaksono, R.; Arsil, P.; Salman, M.F. Antimicrobial Coating on Quality Attributes of Sausage during Refrigerated Storage. E3S Web Conf. 2018, 47, 01002. [Google Scholar] [CrossRef]
- Naufalin, R.; Wicaksono, R.; Erminawati; Arsil, P.; Gulo, K.I.T. Application of Concentrates Flower Kecombrang on Edible Coating as Antioxidant to Suppress Damage on Gourami Sausage. IOP Conf. Ser. Earth Environ. Sci. 2019, 255, 012040. [Google Scholar] [CrossRef]
- Dong, C.; Wang, B.; Li, F.; Zhong, Q.; Xia, X.; Kong, B. Effects of Edible Chitosan Coating on Harbin Red Sausage Storage Stability at Room Temperature. Meat Sci. 2020, 159, 107919. [Google Scholar] [CrossRef]
- Fatmawati, D.R.; Pangastuti, A.; Susilowati, A. Use of Edible Film Incorporated with Parijoto Fruit Extract (Medinilla Speciosa Blume) to Inhibit Microbiological and Oxidative Damages of Sausages. Appl. Food Biotechnol. 2021, 8, 319–328. [Google Scholar] [CrossRef]
- Kalkan, S.; Erginkaya, Z. Impact of Whey Protein Isolate Coatings Containing Different Antimicrobial Agents on Sliced Bologna-Type Sausage during Refrigerated Storage. Food Sci. Technol. 2020, 40, 136–145. [Google Scholar] [CrossRef]
- Qoeroti, B.; Pangastuti, A.; Susilowati, A. Application of Edible Film Incorporated with Portulaca Oleracea Extract to Inhibit Microbiological and Oxidative Damage in Sausages. Biodiversitas J. Biol. Divers. 2021, 22, 1–5. [Google Scholar] [CrossRef]
- Gita, R.S.D.; Waluyo, J.; Dafik; Indrawati. The Effectiveness of Using Chitosan as a Natural Antibacterial for Maintaining the Sausage Quality. Food Res. 2022, 6, 146–153. [Google Scholar] [CrossRef]
- Yasar, S.; Nizamlıoğlu, N.M.; Gücüş, M.O.; Bildik Dal, A.E.; Akgül, K. Origanum Majorana L. Essential Oil-Coated Paper Acts as an Antimicrobial and Antioxidant Agent against Meat Spoilage. ACS Omega 2022, 7, 9033–9043. [Google Scholar] [CrossRef]
- Koranne, V.; Cong Jonas, O.L.; Mitra, H.; Bapat, S.; Ardekani, A.M.; Sealy, M.P.; Rajurkar, K.; Malshe, A.P. Exploring Properties of Edible Hydrolyzed Collagen for 3D Food Printing of Scaffold for Biomanufacturing Cultivated Meat. Procedia CIRP 2022, 110, 186–191. [Google Scholar] [CrossRef]
- Andriani, V.; Abyor Handayani, N. Recent Technology of Edible Coating Production: A Review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- Santhosh, R.; Nath, D.; Sarkar, P. Novel Food Packaging Materials Including Plant-Based Byproducts: A Review. Trends Food Sci. Technol. 2021, 118, 471–489. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Mendes, C.V.T.; Pinela, S.R.; Moreira, R.; Carvalho, M.G.V.S.; Quina, M.J.; Braga, M.E.M.; Portugal, A.T. Assessment of Agroforestry Residues: Their Potential within the Biorefinery Context. ACS Sustain. Chem. Eng. 2019, 7, 17154–17165. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Gaspar, M.C.; Braga, M.E.M.; Quina, M.J. Integrated Management of Residues from Tomato Production: Recovery of Value-Added Compounds and Biogas Production in the Biorefinery Context. J. Environ. Manage. 2021, 299, 113505. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of Protein and Pectin from Pumpkin Industry By-Products and Their Utilization for Developing Edible Film. J. Food Sci. Technol. 2020, 57, 1807–1816. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Leocádio, J.; Mendes, C.V.T.; Cardeira, M.; Fernández, N.; Matias, A.; Carvalho, M.G.V.S.; Braga, M.E.M. Biodegradable Film Production from Agroforestry and Fishery Residues with Active Compounds. Food Packag. Shelf Life 2021, 28, 100661. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Systematic Review on Lignin Valorization in the Agro-Food System: From Sources to Applications. J. Environ. Manage. 2022, 317, 115258. [Google Scholar] [CrossRef] [PubMed]
- Kaynarca, G.B.; Kamer, D.D.A.; Gumus, T.; Sagdıc, O. Characterization of Poly(Vinyl Alcohol)/Gelatin Films Made with Winery Solid by-Product (Vinasse) Extract. Food Packag. Shelf Life 2023, 35, 101013. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of Antioxidant Edible Films Based on Mung Bean Protein Enriched with Pomegranate Peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active Biodegradable Films Based on the Whole Potato Peel Incorporated with Bacterial Cellulose and Curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, W.; Zhao, K.; Ma, Y.; Cheng, S.; Zhou, J.; Wu, Z. Producing a Novel Edible Film from Mushrooms (L. edodes and F. velutipes) Byproducts with a Two-Stage Treatment Namely Grinding and Bleaching. J. Food Eng. 2020, 275, 109862. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk Derived Bioactive Peptides and Their Impact on Human Health—A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A Novel Active Bionanocomposite Film Incorporating Rosemary Essential Oil and Nanoclay into Chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Othman, S.H. Bio-Nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-Sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Makwana, S.; Choudhary, R.; Kohli, P. Advances in Antimicrobial Food Packaging with Nanotechnology and Natural Antimicrobials. Int. J. Food Sci. Nutr. Eng. 2015, 5, 169–175. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H. The Current Application of Nanotechnology in Food and Agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ullah, A. Recent Advances in Protein Derived Bionanocomposites for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 406–434. [Google Scholar] [CrossRef]
- de Azeredo, H.M.C.; Capparelli Mattoso, L.H.; Habig, T. Nanocomposites in Food Packaging—A Review. Adv. Divers. Ind. Appl. Nanocompos. 2011, 57–78. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial Bio-Nanocomposites and Their Potential Applications in Food Packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Therias, S.; Larché, J.-F.; Bussière, P.-O.; Gardette, J.-L.; Murariu, M.; Dubois, P. Photochemical Behavior of Polylactide/ZnO Nanocomposite Films. Biomacromolecules 2012, 13, 3283–3291. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Hosseinkhan, P.; Zand, A.M.; Imani, M.S.; Rezayi, M.; Rezaei Zarchi, S. Determining the Antibacterial Effect of ZnO Nanoparticle against the Pathogenic Bacterium, Shigella Dysenteriae (Type 1). Int. J. Nano Dimens. 2011, 1, 279–285. [Google Scholar]
- Uysal Unalan, I.; Cerri, G.; Marcuzzo, E.; Cozzolino, C.A.; Farris, S. Nanocomposite Films and Coatings Using Inorganic Nanobuilding Blocks (NBB): Current Applications and Future Opportunities in the Food Packaging Sector. RSC Adv. 2014, 4, 29393–29428. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites Materials for Food Packaging Applications: Concepts and Future Outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical Properties of Gelatin/Silver Nanoparticle Antimicrobial Composite Films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rafieian, F.; Shahedi, M.; Keramat, J.; Simonsen, J. Thermomechanical and Morphological Properties of Nanocomposite Films from Wheat Gluten Matrix and Cellulose Nanofibrils. J. Food Sci. 2014, 79, N100–N107. [Google Scholar] [CrossRef]
- Rouhi, J.; Mahmud, S.; Naderi, N.; Ooi, C.R.; Mahmood, M.R. Physical Properties of Fish Gelatin-Based Bio-Nanocomposite Films Incorporated with ZnO Nanorods. Nanoscale Res. Lett. 2013, 8, 364. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanotechnology Approaches for Increasing Nutrient Bioavailability. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–30. [Google Scholar]
- EFSA. Scientific Opinion on the Safety Evaluation of the Substance, Titanium Nitride, Nanoparticles, for Use in Food Contact Materials. EFSA J. 2012, 10, 2641. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Antimicrobial Poly(Lactic Acid)/Cellulose Bionanocomposite for Food Packaging Application: A Review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Choi, Y.-J.; Jung, E.-J.; Yin, H.-Q.; Kwon, J.-T.; Kim, J.-E.; Im, H.-T.; Cho, M.-H.; Kim, J.-H.; Kim, H.-Y.; et al. Genomics-Based Screening of Differentially Expressed Genes in the Brains of Mice Exposed to Silver Nanoparticles via Inhalation. J. Nanoparticle Res. 2010, 12, 1567–1578. [Google Scholar] [CrossRef]
- Han, W.; Yu, Y.; Li, N.; Wang, L. Application and Safety Assessment for Nano-Composite Materials in Food Packaging. Chinese Sci. Bull. 2011, 56, 1216–1225. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-Dependent Toxicity of Metal Oxide Particles—A Comparison between Nano- and Micrometer Size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.; Baeg, G. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver Nanoparticles Induce Oxidative Cell Damage in Human Liver Cells through Inhibition of Reduced Glutathione and Induction of Mitochondria-Involved Apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Li, X.; Zhou, W. Safety Assessment of Nanocomposite for Food Packaging Application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Sufian, M.M.; Khattak, J.Z.K.; Yousaf, S.; Rana, M.S. Safety Issues Associated with the Use of Nanoparticles in Human Body. Photodiagnosis Photodyn. Ther. 2017, 19, 67–72. [Google Scholar] [CrossRef]
| Fruit | Material Coating | Active Compounds | Coating Advantages | References |
|---|---|---|---|---|
| Apple | Soy protein Alginate Xanthan gum Carboxymethyl cellulose Chitosan | Thyme essential oil Cinnamon bark Tocopherol Ferulic acid Aloe vera Ascorbic acid Grape seed essential oil | Prevented water loss, showed antioxidants and antimicrobial properties, possess effective barrier properties and exhibit favorable mechanical and structural characteristics, physicochemical properties reserved, activity of PAP (phosphatidic acid phosphatase) and PPO (polyphenol oxidases) enzymes reduced and reduction in respiration rate observed, preserved the quality of food products, prolonging their shelf life and inhibiting browning. | [97,98,99,100,101,102] |
| Blackberries | Starch | Nystose Acetic acid or lactic acid | Antifungal effect, had beneficial impacts in slowing down pH increase, preserving firmness, and retaining anthocyanin content. | [80,99,103,104] |
| Blueberries | Chitosan | Blueberries leaf extract | Reduced microbial growth and decay rate, leading to an extended shelf-life. | [99,105] |
| Citrus fruit | Chitosan Gelatin | Pomegranate peel extract Cinnamaldeyde | Prolonged shelf life by inhibiting green mold development and exhibiting antifungal properties. Additionally, they reduce weight loss and decrease total acidity (TA) while enhancing total phenolic content (TPC) and antioxidant activity (AOC). Moreover, edible coatings maintain fruit firmness and glossiness throughout storage. | [80,99,100,106,107,108] |
| Peach | Carboxymethyl cellusose Chitosan Sodium alginate | Soybean oil Oleic acid Clorogenic acid Rhuborb extract Aloe vera | Regulated the degradation caused by Penicillium expansum, displaying antifungal characteristics, and preserved the physiological quality of the product. | [80,100,108,109,110] |
| Pears | Chitosan Carboxymethyl cellulose | Salicylic acid Soybean oil Oleic acid Cumin essential oils | Resulted in a reduction of PPO activity, effectively preventing the occurrence of internal browning during storage. It also minimized fungal infection, maintained fruit firmness, and significantly extended the product’s shelf life. | [99,111] |
| Strawberries | Sodium alginate Pectin Methyl cellulose Hydroxymethyl cellulose Chitosan Arabic gum Xanthan gum | Eugenol oil Citral essential oils Lactobacillus plantarum Curcumin Limonene Asparagus waste extract Grape seed essential oil Peony extracts Lemon essential oil | Slowed down the growth rate of molds and yeasts on the surface of strawberries. Additionally, it enhanced its functionality as a probiotic, resulting in reduced weight loss, pH, color changes, total acidity (TA), total phenolic content (TPC), and DPPH (a measure of antioxidant activity). Furthermore, the coating effectively reduced microbial growth on the strawberries. | [80,99,100,101,110,112,113,114,115] |
| Antimicrobial Edible Coating | Active Compound | Results | References |
|---|---|---|---|
| Gelatin coating solution | _ | Resulted in decreased thiobarbituric acid-reactive substances and peroxide value in traditional sausages. Additionally, it significantly reduced moisture loss by 32.6%. These findings demonstrate its effectiveness as a viable option for extending the quality and shelf life of the traditional sausages. | [120] |
| Chitosan | Green tea extract | The study revealed that incorporating green tea extract into the chitosan film enhanced its antioxidant and antimicrobial properties, leading to the preservation of sausage quality and prolonging its shelf life. | [121,122] |
| Whey protein | Origanum virens essential oils | The results strongly suggest that O. virens essential oil (EO) holds great potential as a food preservative for processed meat products. | [123,124] |
| Carboxymethyl cellulose (CMC) | Kecombrang (Nicolaia speciosa) extract | Coating traditional sausages with CMC effectively inhibits the growth of Bacillus cereus, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The findings highlight CMC’s dual role as a natural antioxidant and moisture barrier coating, which contributes to preserving sausage quality and extending its shelf life. | [125] |
| Chitosan | Garlic and oregano essential oils | The application of the coating effectively suppressed the growth of Salmonella and L. monocytogenes bacteria while also decreasing the count of S. aureus. | [88] |
| Carboxymethyil with glycerol | Kecombrang flower extract | Resulted in a deceleration of the oxidative degradation process in traditional sausages throughout storage. | [126] |
| Chitosan coating with glycerol | _ | Chitosan coating with glycerol was beneficial to improve the storage stability of traditional sausage at room temperature. This was evident through its ability to prevent the decline in pH, stabilize the L* value and water migration, and slow down the growth of aerobic bacteria and lactic acid bacteria. | [127] |
| Chitosan with sorbitol | Medinilla spesiosa extract | Study demonstrated that the Medinilla speciosa extract edible film inhibited microbiological and oxidative damages. | [128] |
| Whey protein isolates | Thyme, coriander, pepper, rosemary, basil | The research showcased the coatings’ ability to effectively deactivate Listeria innocua bacteria. | [129] |
| Carboxymethyil with glycerol | Kekombrang flower extract | To prevent and slow down the oxidative damage of traditional sausage products during storage. | [126] |
| Chitosan with sorbitol | Portulaca oleracea extract | Prevented both microbiological and oxidative damage in traditional sausages. | [130] |
| Chitosan | _ | This study showed that among the different concentrations tested, 1% chitosan concentration proved to be the most effective in preventing bacterial growth in traditional sausages. | [131] |
| Cationic starch | Origanum majorana L. essential oil | Extended the product’s refrigerated shelf life to six days while preserving its color and odor without any impact. | [132] |
| Collagen with bacterial cellulose, chitosan | Essential oil | The findings of this study indicate that using the applied coating can be a viable and effective option to improve product quality and prolong its shelf life. | [133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, C.; Silva, M.; Farinha, D.; Sales, H.; Pontes, R.; Nunes, J. Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing. Foods 2023, 12, 3308. https://doi.org/10.3390/foods12173308
Nunes C, Silva M, Farinha D, Sales H, Pontes R, Nunes J. Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing. Foods. 2023; 12(17):3308. https://doi.org/10.3390/foods12173308
Chicago/Turabian StyleNunes, Catarina, Mafalda Silva, Diana Farinha, Hélia Sales, Rita Pontes, and João Nunes. 2023. "Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing" Foods 12, no. 17: 3308. https://doi.org/10.3390/foods12173308
APA StyleNunes, C., Silva, M., Farinha, D., Sales, H., Pontes, R., & Nunes, J. (2023). Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing. Foods, 12(17), 3308. https://doi.org/10.3390/foods12173308