Chestnut Shell Polyphenols Inhibit the Growth of Three Food-Spoilage Bacteria by Regulating Key Enzymes of Metabolism
Abstract
:1. Introduction
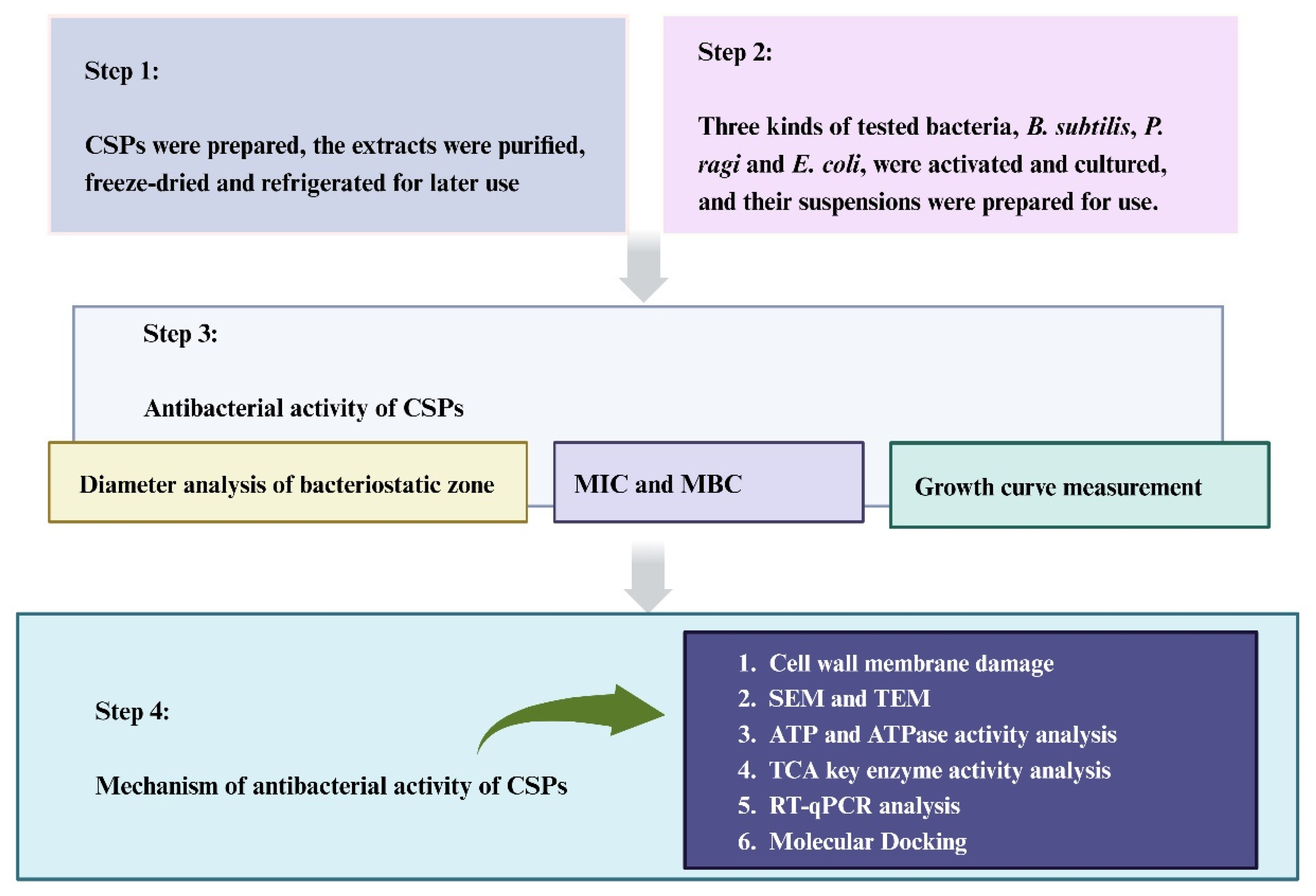
2. Materials and Methods
2.1. Raw Material Preparation
2.2. CSP Antibacterial Activity Assay
2.3. Bacterial Cell Wall and Membrane Damage Assay
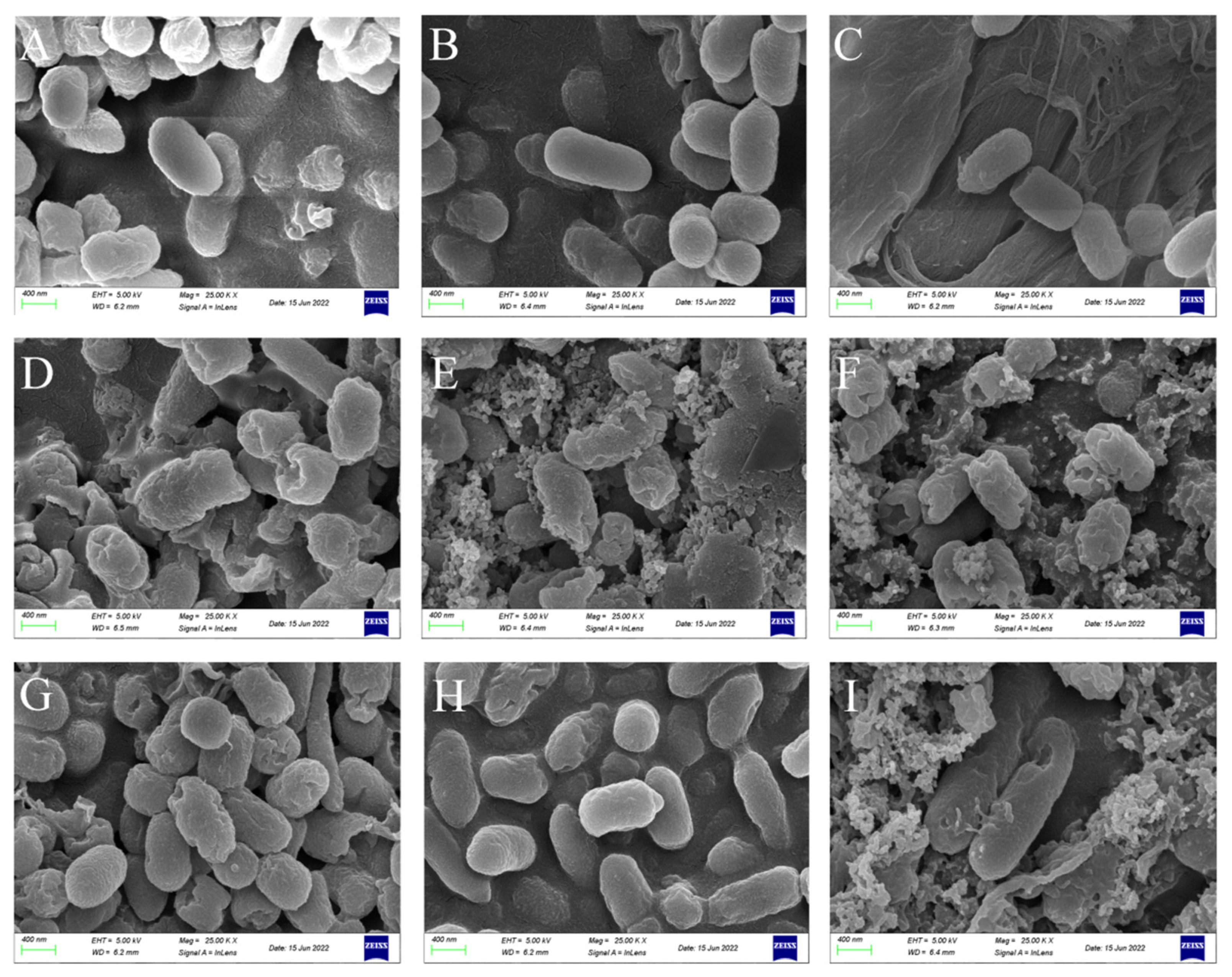
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
2.6. ATP Content and ATPase Activity Assay
2.7. Activity Measurements of Key Tricarboxylic Acid (TCA) Cycle Enzymes
2.8. RT-qPCR Assay
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results and Discussion
3.1. CSPs Inhibit the Activity of Three Food-Spoilage Bacteria
3.1.1. Diameter Analysis of Bacteriostatic Zone
3.1.2. MIC and MBC Analysis
3.1.3. Influence of CSPs on the Growth Curve of Food-Spoilage Bacteria
3.2. CSPs Damage the Structure of the Bacterial Cell Wall and Membrane
3.3. CSPs Damage Bacterial Morphology
3.4. CSPs Reduce the Content of ATP and the Activity of ATPases
3.5. CSPs Inhibit the Activity of Key TCA Cycle Enzymes in Bacteria
3.6. CSPs Regulate the Expression of Key TCA Cycle-Related Genes
3.7. Molecular Docking Simulation of Major CSPs Components and Key TCA Cycle Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive peptides: A promising alternative to chemical preservatives for food preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, X.; Feng, Z.; Miao, X.; Peng, X.; Wang, Y. Zanthoxylum molle Rehd. essential oil as a potential natural preservative in management of Aspergillus flavus. Ind. Crops Prod. 2014, 60, 151–159. [Google Scholar] [CrossRef]
- Wang, F.; Wei, F.; Song, C.; Jiang, B.; Tian, S.; Yi, J.; Yu, C.; Song, Z.; Sun, L.; Bao, Y.; et al. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind. Crops Prod. 2017, 109, 358–366. [Google Scholar] [CrossRef]
- Pang, D.; Liao, S.; Wang, W.; Mu, L.; Li, E.; Shen, W.; Liu, F.; Zou, Y. Destruction of the cell membrane and inhibition of cell phosphatidic acid biosynthesis in Staphylococcus aureus: An explanation for the antibacterial mechanism of morusin. Food Funct. 2019, 10, 6438–6446. [Google Scholar] [CrossRef]
- Potter, R.; Truelstrup Hansen, L.; Gill, T.A. Inhibition of foodborne bacteria by native and modified protamine: Importance of electrostatic interactions. Int. J. Food Microbiol. 2005, 103, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, Y.; Dou, X.; Zohaib Aslam, M.; Liu, Y.; Xia, X.; Yang, S.; Wang, X.; Qin, X.; Hirata, T.; et al. A review of potential antibacterial activities of nisin against Listeria monocytogenes: The combined use of nisin shows more advantages than single use. Food Res. Int. 2023, 164, 112363. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Li, H.; Guo, X.; Guo, D.; Yang, Y.; Wang, X.; Zhang, C.; Shan, Z.; Xia, X.; et al. Antibiofilm activity of shikonin against Listeria monocytogenes and inhibition of key virulence factors. Food Control 2021, 120, 107558. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Zhou, M.; Wu, Y.; Guan, X. Study on the interaction between grain polyphenols and intestinal microorganisms: A review. Food Biosci. 2023, 53, 102536. [Google Scholar] [CrossRef]
- Ding, J.; Dwibedi, V.; Huang, H.; Ge, Y.; Li, Y.; Li, Q.; Sun, T. Preparation and antibacterial mechanism of cinnamaldehyde/tea polyphenol/polylactic acid coaxial nanofiber films with zinc oxide sol to Shewanella putrefaciens. Int. J. Biol. Macromol. 2023, 237, 123932. [Google Scholar] [CrossRef]
- Fei, P.; Xu, Y.; Zhao, S.; Gong, S.; Guo, L. Olive oil polyphenol extract inhibits vegetative cells of Bacillus cereus isolated from raw milk. J. Dairy Sci. 2019, 102, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.M.; Zhu, J.L.; Fu, L.L.; Li, J.R. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int. J. Food Microbiol. 2010, 144, 111–117. [Google Scholar] [CrossRef]
- Borges, O.P.; Carvalho, J.S.; Correia, P.R.; Silva, A.P. Lipid and fatty acid profiles of Castanea sativa Mill. chestnuts of 17 native portuguese cultivars. J. Food Compos. Anal. 2007, 20, 80–89. [Google Scholar] [CrossRef]
- Alessandra, M.; Giuseppe, S.; Susana, M.P.; Alves, L.; Francesco, C.; Patrícia, M. Development of an energy biorefinery model for chestnut (Castanea sativa Mill.) shells. Energies 2017, 10, 1504. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Feo, V.D.; Pimentel, T.C.; Coppola, R.; Cruz, A.G. Chapter 13—Polyphenols applications in food industry sector. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Pintado, M.E., Saraira, J.M.A., Alexandre, E.M.C., Eds.; Academic Press: London, UK, 2022; pp. 301–336. ISBN 978-0-323-85273-9. [Google Scholar]
- Liu, S.; Lu, Z.; Liu, C.; Chang, X.; Apuclureheman, B.; Chen, S.; Ye, X. Castanea mollissima shell polyphenols regulate JAK2 and PPARγ expression to suppress inflammation and lipid accumulation by inhibiting M1 macrophages polarization. J. Funct. Foods 2022, 92, 105046. [Google Scholar] [CrossRef]
- Sun, X.; Jia, P.; Zhe, T.; Bu, T.; Liu, Y.; Wang, Q.; Wang, L. Construction and multifunctionalization of chitosan-based three-phase nano-delivery system. Food Hydrocoll. 2019, 96, 402–411. [Google Scholar] [CrossRef]
- Yuan, Z.; Ouyang, P.; Gu, K.; Rehman, T.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; Shu, G.; et al. The antibacterial mechanism of oridonin against methicillin-resistant Staphylococcus aureus (MRSA). Pharm. Biol. 2019, 57, 710–716. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Figueiredo, R.; Laranjinha, J.; da Silva, G.J. Intestinal cytotoxicity induced by Escherichia coli is fully prevented by red wine polyphenol extract: Mechanistic insights in epithelial cells. Chem. Biol. Interact. 2019, 310, 108711. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, Y.; Liu, X.; Zheng, Y.; Li, B.; Han, Y.; Li, Z.; Yeung, K.W.K.; Liang, Y.; Zhu, S.; et al. Microwave assisted antibacterial action of garcinia nanoparticles on gram-negative bacteria. Nat. Commun. 2022, 13, 2461. [Google Scholar] [CrossRef]
- Choi, Y.R.; Kang, M.K. Evaluation of cytotoxic and antibacterial effect of methanolic extract of Paeonia lactiflora. Medicina 2022, 58, 1272. [Google Scholar] [CrossRef]
- Snoussi, M.; Ahmad, I.; Aljohani, A.M.A.; Patel, H.; Abdulhakeem, M.A.; Alhazmi, Y.S.; Tepe, B.; Adnan, M.; Siddiqui, A.J.; Sarikurkcu, C.; et al. Phytochemical analysis, antioxidant, and antimicrobial activities of Ducrosia flabellifolia: A combined experimental and computational approaches. Antioxidants 2022, 11, 2174. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Miazga-Karska, M.; Klimek, K.; Ekiert, H.; Szopa, A. Precursor-Boosted production of metabolites in Nasturtium officinale microshoots grown in plantform bioreactors, and antioxidant and antimicrobial activities of biomass extracts. Molecules 2021, 26, 4660. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Dang, S.; Han, S.; Yun, C.; Wang, W.; Wang, H. Optimized ultrasound-assisted extraction of total polyphenols from Empetrum nigrum and its bioactivities. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1173, 122699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Yao, Z.; Qi, J.; Wang, L. Isolation, fractionation and characterization of melanin-like pigments from chestnut (Castanea mollissima) shells. J. Food Sci. 2012, 77, C671–C676. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth rates made easy. Mol. Biol. Evol. 2014, 31, 232–238. [Google Scholar] [CrossRef]
- Li, K.; Guan, G.; Zhu, J.; Wu, H.; Sun, Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan-gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control 2019, 96, 234–243. [Google Scholar] [CrossRef]
- Balamayooran, G.; Batra, S.; Fessler, M.B.; Happel, K.I.; Jeyaseelan, S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Respir. Cell Mol. Biol. 2010, 43, 5–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Tang, Z.X.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Foods 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, X.; Xu, L.; Wang, S. Antibacterial properties and possible action mechanism of chelating peptides-zinc nanocomposite against Escherichia coli. Food Control 2019, 106, 106675. [Google Scholar] [CrossRef]
- Xiang, Q.; Kang, C.; Niu, L.; Zhao, D.; Li, K.; Bai, Y. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT 2018, 96, 395–401. [Google Scholar] [CrossRef]
- Hou, J.; Miao, L.; Wang, C.; Wang, P.; Ao, Y.; Qian, J.; Dai, S. Inhibitory effects of ZnO nanoparticles on aerobic wastewater biofilms from oxygen concentration profiles determined by microelectrodes. J. Hazard. Mater. 2014, 276, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Alshuniaber, M.A.; Krishnamoorthy, R.; AlQhtani, W.H. Antimicrobial activity of polyphenolic compounds from Spirulina against food-borne bacterial pathogens. Saudi J. Biol. Sci. 2021, 28, 459–464. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Hyun, B.; Cha, H.G.; Lee, N.; Yum, S.; Baek, S.; Shin, K. Development of an ATP assay for rapid onboard testing to detect living microorganisms in ballast water. J. Sea Res. 2018, 133, 73–80. [Google Scholar] [CrossRef]
- Shi, C.; Sun, Y.; Zheng, Z.; Zhang, X.; Song, K.; Jia, Z.; Chen, Y.; Yang, M.; Liu, X.; Dong, R.; et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem. 2016, 197, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Nazarenko, Y.; Lioy, P.J.; Mainelis, G. Collection efficiencies of an electrostatic sampler with superhydrophobic surface for fungal bioaerosols. Indoor Air 2011, 21, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010, 46, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 29, 102838. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Madzak, C.; Du, G.; Zhou, J. Mutagenesis of conserved active site residues of dihydrolipoamide succinyltransferase enhances the accumulation of α-ketoglutarate in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2016, 100, 649–659. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Thompson, C.B. Cellular metabolism and disease: What do metabolic outliers teach us. Cell 2012, 148, 1132–1144. [Google Scholar] [CrossRef]
- Kusuda, M.; Inada, K.; Ogawa, T.O.; Yoshida, T.; Shiota, S.; Tsuchiya, T.; Hatano, T. Polyphenolic constituent structures of Zanthoxylum piperitum fruit and the antibacterial effects of its polymeric procyanidin on methicillin-resistant Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2006, 70, 1423–1431. [Google Scholar] [CrossRef]





| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | |
|---|---|---|---|
| Reference genes | rpsj | GAAACGGCAAAACGTTCTGG | GTGTTGGGTTCACAATGTCG |
| 16S rRNA | GTCACCGGCAGTCTCCTTAG | ATTGGTGCCTTCGGGAACAT | |
| GAPDH | ACTTACGAGCAGATCAAAGC | AGTTTCACGAAGTTGTCGTT | |
| Bacillus subtilis | citA | CGGTTGTTTCAGCATTGGGG | TAACGTGACTCGTGCCGAAA |
| icd | GCCTTTTGCATACTCGATGCC | TTTACGCCGGAGAAAAGGCT | |
| odhA | TCGCACCGCCTACTTGATAC | CAAAGCGGATACCCGGTACA | |
| atpA | GACAATCCGGCATTGATCGC | GCTTACCGTGAGCTGTCCTT | |
| Pseudomonas fragi | gltA | AGAAGACCTGCGACGAAGTG | CTCGATGAAGTACGGGTCGG |
| IS178_RS16090 | GCAAAAGGTCGAAGAAGGCG | GTCACCGAGATGGTGTCCAG | |
| IS178_RS16290 | TGGTCGATTACAACCTGGGC | GACTCGTTGCTGTCCGAAGA | |
| atpA | ATTGTGCGGATTCACGGTCT | GACTGCATCAGTCTCGGTGT | |
| Escherichia coli | gltA | ACCCGTCTGTTCCATGCTTT | TTGCGCGGGTAAACAAATGG |
| icd | CGATGATCGCGTCACCAAAC | CCGAAATATGCCGGTCAGGA | |
| sucA | GTACCGTACCGCCAACTTCA | TATCGGTTCTGTTCGTGCCC | |
| atpA | GTGTTATCCGCATTCACGGC | TCCAGGATACGGCCAGTACA |
| Treatment Method | MIC (mg/mL) | MBC (mg/mL) | ||||
|---|---|---|---|---|---|---|
| B. subtilis | P. fragi | E. coli | B. subtilis | P. fragi | E. coli | |
| CSPs | 0.313 | 0.313 | 0.313 | 0.625 | 0.625 | 0.625 |
| SDA | 0.625 | 0.313 | 1.250 | 10 | 10 | 5 |
| Potassium sorbate | 5 | 2.5 | 5 | 10 | 10 | 10 |
| Sodium nitrite | 2.5 | 0.625 | 5 | 10 | 10 | 10 |
| Strain | Gene | Control | CSPs 1× MIC | CSPs 2× MIC | SDA 1× MIC | SDA 2× MIC |
|---|---|---|---|---|---|---|
| Bacillus subtilis | odhA | 1.00 ± 0.08 aA | 0.55 ± 0.05 bB | 0.40 ± 0.03 cA | 0.45 ± 0.05 cB | 0.30 ± 0.03 dB |
| icd | 1.00 ± 0.11 aA | 0.60 ± 0.04 bB | 0.30 ± 0.01 dB | 0.48 ± 0.01 cB | 0.23 ± 0.01 dC | |
| citA | 1.00 ± 0.11 aA | 0.70 ± 0.05 bA | 0.42 ± 0.05 cA | 0.58 ± 0.07 bA | 0.40 ± 0.05 cA | |
| atpA | 1.00 ± 0.11 aA | 0.61 ± 0.06 bB | 0.41 ± 0.03 cA | 0.53 ± 0.04 bAB | 0.18 ± 0.01 dC | |
| Pseudomonas fragi | gltA | 1.00 ± 0.04 aA | 0.60 ± 0.04 bC | 0.29 ± 0.02 dC | 0.39 ± 0.03 cB | 0.19 ± 0.01 eD |
| IS178_RS16090 | 1.00 ± 0.05 aA | 0.75 ± 0.01 bA | 0.37 ± 0.04 dAB | 0.54 ± 0.02 cA | 0.27 ± 0.02 eB | |
| IS178_RS16290 | 1.00 ± 0.11 aA | 0.51 ± 0.04 bD | 0.34 ± 0.04 cBC | 0.40 ± 0.03 cB | 0.23 ± 0.03 dC | |
| atpA | 1.00 ± 0.06 aA | 0.67 ± 0.04 bB | 0.43 ± 0.01 dA | 0.52 ± 0.05 cA | 0.32 ± 0.03 eA | |
| Escherichia coli | gltA | 1.00 ± 0.06 aA | 0.72 ± 0.08 bAB | 0.40 ± 0.01 dB | 0.51 ± 0.05 cBC | 0.28 ± 0.02 eB |
| icd | 1.00 ± 0.06 aA | 0.68 ± 0.03 bB | 0.41 ± 0.04 dB | 0.53 ± 0.08 cAB | 0.24 ± 0.02 eBC | |
| sucA | 1.00 ± 0.05 aA | 0.61 ± 0.02 bB | 0.35 ± 0.03 dB | 0.41 ± 0.01 cC | 0.23 ± 0.01 eC | |
| atpA | 1.00 ± 0.05 aA | 0.83 ± 0.09 bA | 0.51 ± 0.05 cA | 0.62 ± 0.05 cA | 0.34 ± 0.04 dA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Y.; Liu, S.; Wang, H.; Chang, X.; Zhang, J. Chestnut Shell Polyphenols Inhibit the Growth of Three Food-Spoilage Bacteria by Regulating Key Enzymes of Metabolism. Foods 2023, 12, 3312. https://doi.org/10.3390/foods12173312
Wang X, Li Y, Liu S, Wang H, Chang X, Zhang J. Chestnut Shell Polyphenols Inhibit the Growth of Three Food-Spoilage Bacteria by Regulating Key Enzymes of Metabolism. Foods. 2023; 12(17):3312. https://doi.org/10.3390/foods12173312
Chicago/Turabian StyleWang, Xinfang, Yue Li, Suwen Liu, Hao Wang, Xuedong Chang, and Jingzheng Zhang. 2023. "Chestnut Shell Polyphenols Inhibit the Growth of Three Food-Spoilage Bacteria by Regulating Key Enzymes of Metabolism" Foods 12, no. 17: 3312. https://doi.org/10.3390/foods12173312
APA StyleWang, X., Li, Y., Liu, S., Wang, H., Chang, X., & Zhang, J. (2023). Chestnut Shell Polyphenols Inhibit the Growth of Three Food-Spoilage Bacteria by Regulating Key Enzymes of Metabolism. Foods, 12(17), 3312. https://doi.org/10.3390/foods12173312






