Ethanolic Extract from Seed Residues of Sea Buckthorn (Hippophae rhamnoides L.) Ameliorates Oxidative Stress Damage and Prevents Apoptosis in Murine Cell and Aging Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HYD-SBSR
2.2. Cell Culture
2.3. Cell Viability
2.4. Establishment of the H2O2-Induced B16F10 Cell Oxidative Stress Model
2.5. Protective and Repair Effects of HYD-SBSR on the H2O2-Induced B16F10 Cell Oxidative Stress Model
2.6. GSH-Px, CAT, SOD, and MDA in B16F10 Cells
2.7. Flow Cytometry Analysis for Cell Cycle Distribution and Apoptosis
2.8. Animals and Treatment
2.9. Collection of Mouse Experimental Samples
2.10. Differentially Expressed Proteins (DEPs) and GO Enrichment and KEGG Pathway Enrichment Analyses
2.11. Statistical Analysis
3. Results
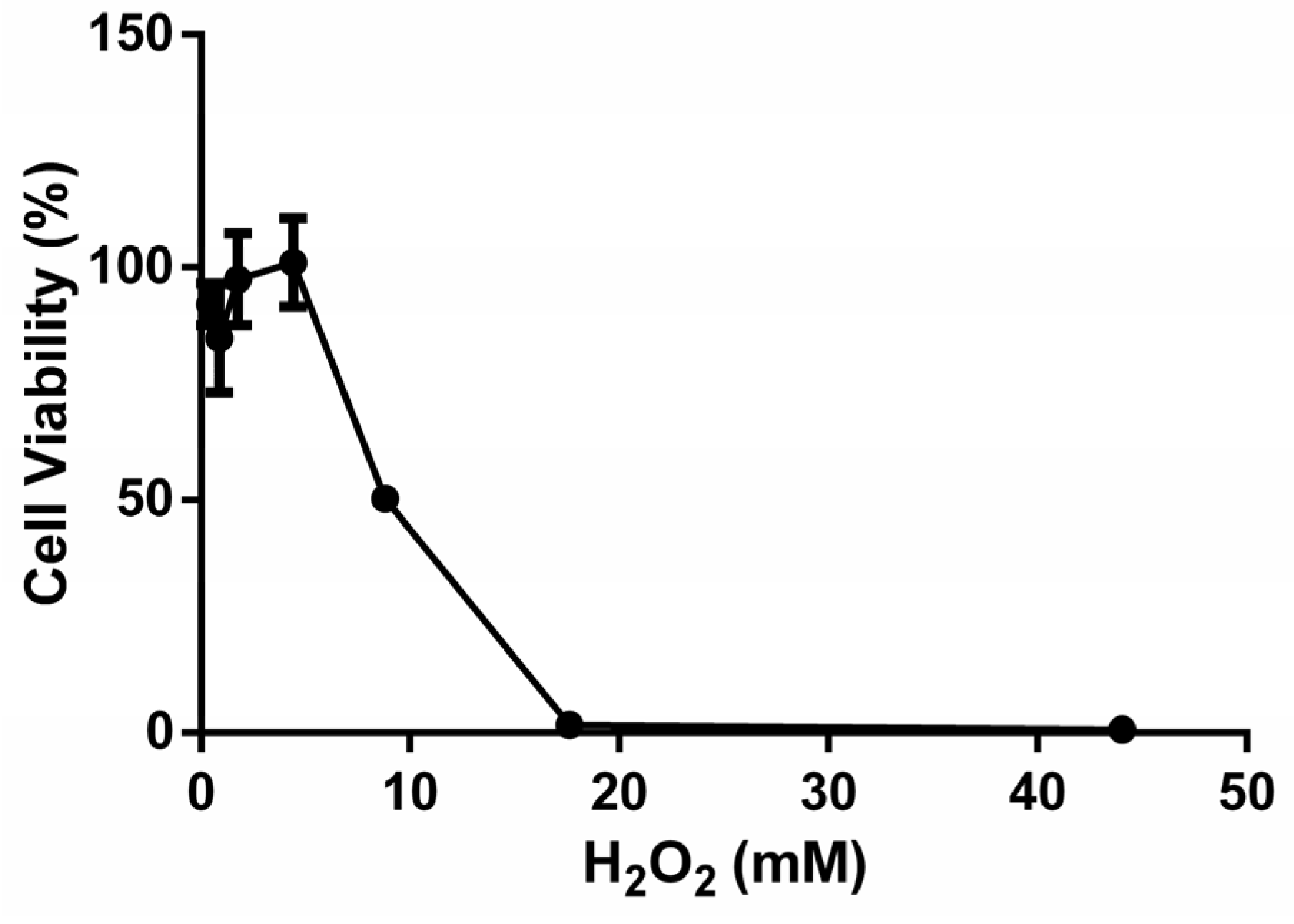
3.1. Establishment of the H2O2-Induced B16F10 Oxidative Stress Model
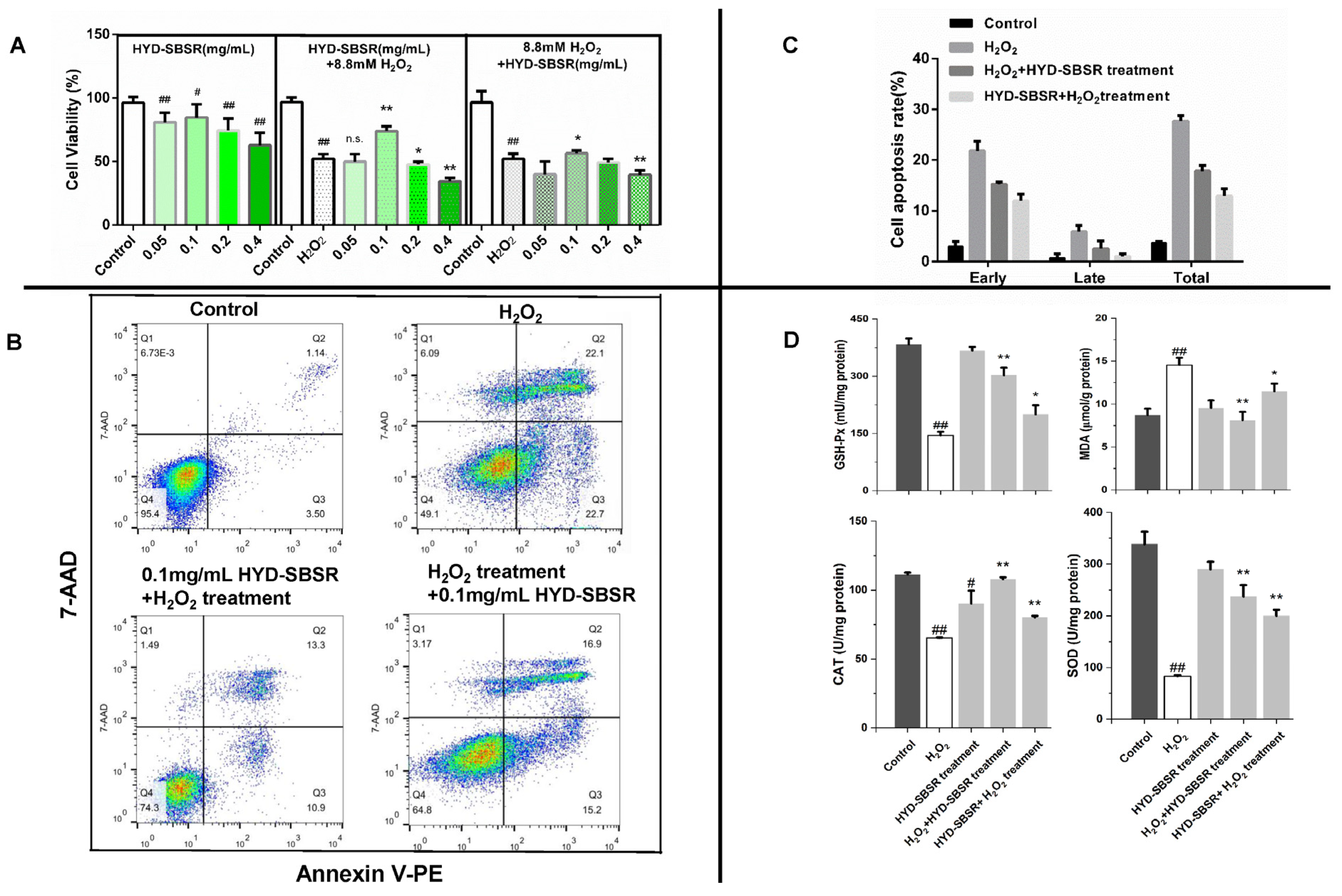
3.2. Protective and Repair Effects of HYD-SBSR on H2O2-Induced B16F10
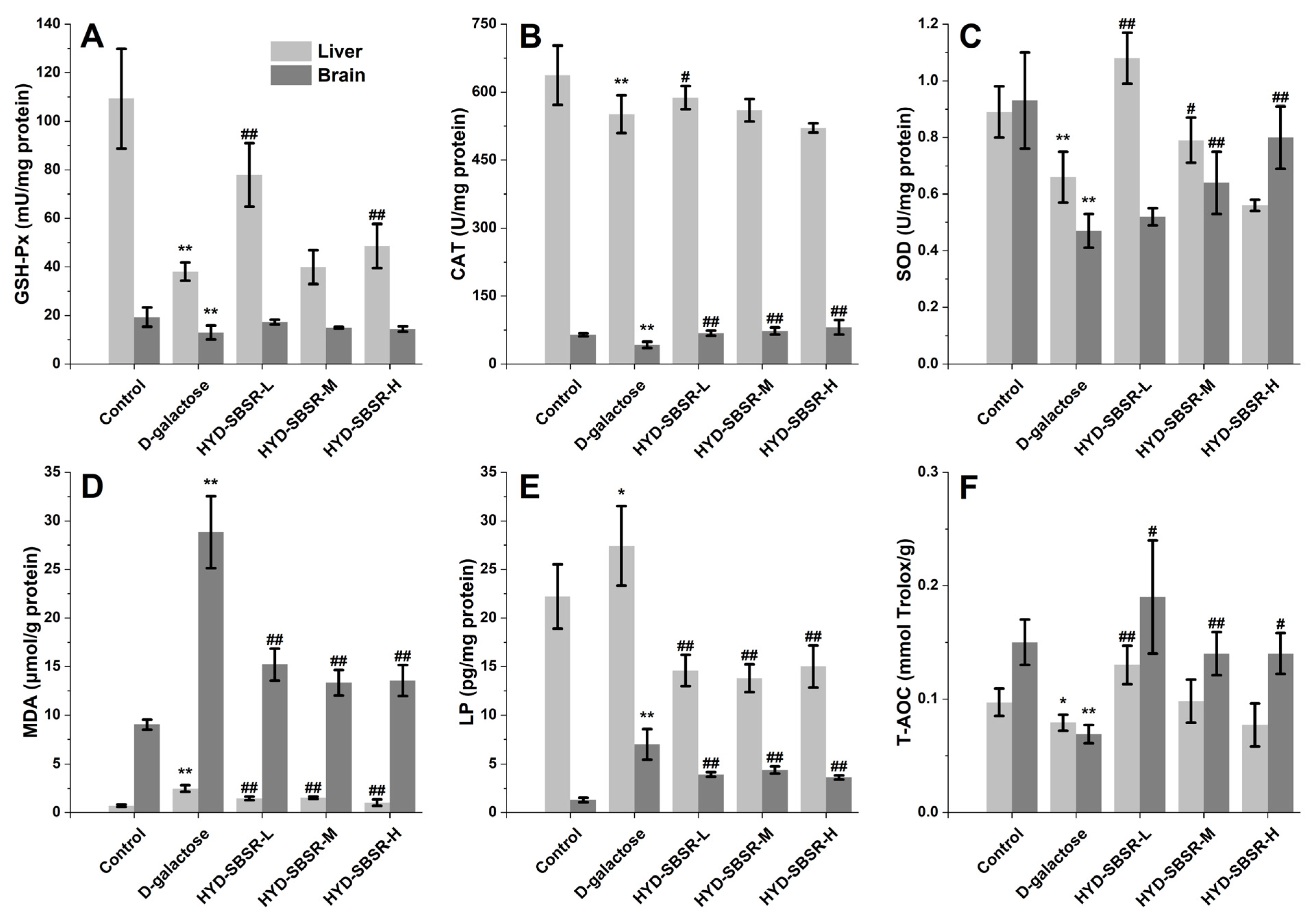
3.3. Antioxidant Enzyme and Peroxide Levels In Vivo
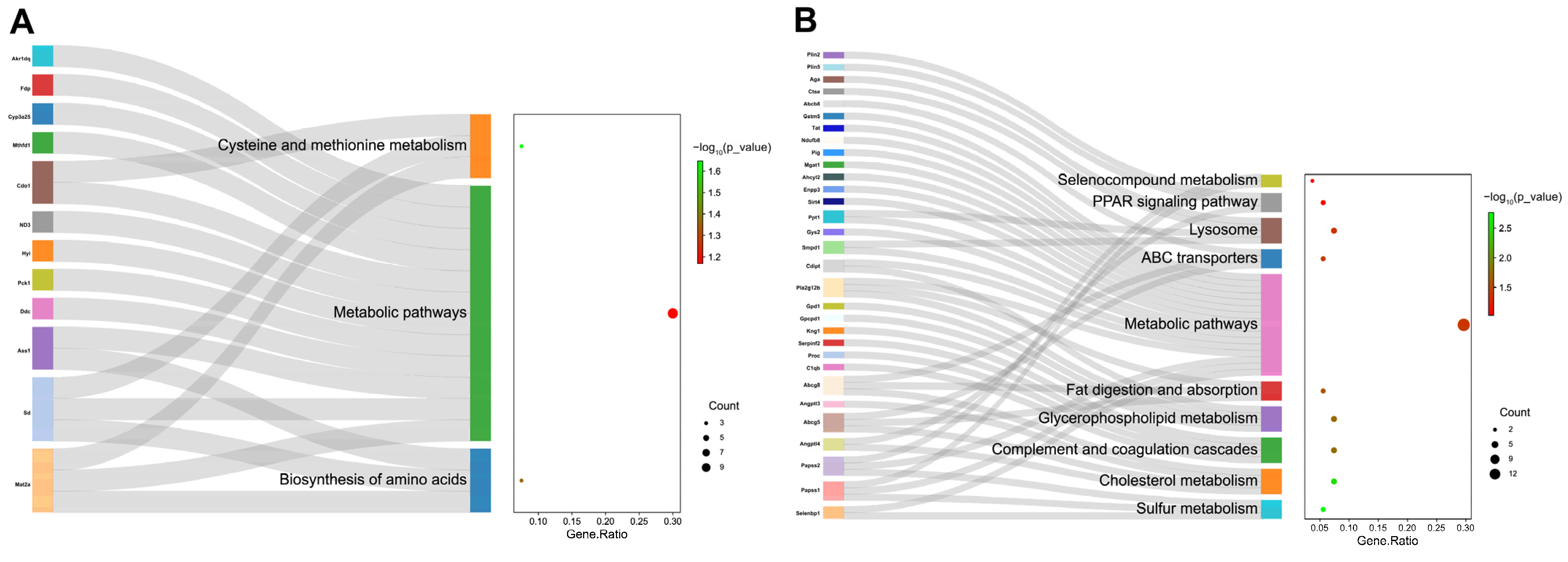
3.4. TMT-Based Quantitative Proteomics Analysis of Liver Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ivanisova, E.; Blaskova, M.; Terentjeva, M.; Grygorieva, O.; Vergun, O.; Brindza, J.; Kacaniova, M. Biological Properties of Sea buckthorn (Hippophae rhamnoides L.) Derived products. Acta Sci. Pol.-Technol. Aliment. 2020, 19, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, P.; Kuang, Y.; Hao, J.; Huang, T.; Liu, E. Flavonoids from sea buckthorn: A review on phytochemistry, pharmacokinetics and role in metabolic diseases. J. Food Biochem. 2021, 45, e13724. [Google Scholar] [CrossRef] [PubMed]
- Bal, L.M.; Meda, V.; Naik, S.N.; Satya, S. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res. Int. 2011, 44, 1718–1727. [Google Scholar] [CrossRef]
- Cho, C.H.; Jang, H.; Lee, M.; Kang, H.; Heo, H.J.; Kim, D.-O. Sea Buckthorn (Hippophae rhamnoides L.) Leaf Extracts Protect Neuronal PC-12 Cells from Oxidative Stress. J. Microbiol. Biotechnol. 2017, 27, 1257–1265. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Kumar, R.; Mandotra, S.K.; Meena, R.N.; Siddiqui, M.S.; Sawhney, R.C.; Gupta, A. Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem. Toxicol. 2009, 47, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.; Li, T.S.C.; Oomah, B.D.; Smith, A. Sea buckthorn products: Manufacture and composition. J. Agric. Food Chem. 1999, 47, 3480–3488. [Google Scholar] [CrossRef]
- Gegotek, A.; Jastrzab, A.; Jarocka-Karpowicz, I.; Muszynska, M.; Skrzydlewska, E. The Effect of Sea Buckthorn (Hippophae rhamnoides L.) Seed Oil on UV-Induced Changes in Lipid Metabolism of Human Skin Cells. Antioxidants 2018, 7, 110. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Song, H.; Zhu, W.; Liu, Y. Antioxidant Activity and Phenolic Components of Sea Buckthorn (Hippophae rhamnoides) Seed Extracts. In Proceedings of the International Conference on Advanced Mechatronic Systems (ICAMechS), Luoyang, China, 25–27 September 2013; pp. 96–101. [Google Scholar]
- Cenkowski, S.; Yakimishen, R.; Przybylski, R.; Muir, W.E. Quality of extracted sea buckthorn seed and pulp oil. Can. Biosyst. Eng. 2006, 48, 3.9–3.16. [Google Scholar]
- Arimboor, R.; Kumar, K.S.; Arumughan, C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophae rhamnoides) using RP-HPLC with DAD. J. Pharm. Biomed. Anal. 2008, 47, 31–38. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Zhu, X.; Zhuang, X.; Pang, X.; Wang, J.; Qu, W. Antihyperglycemic effect of aqueous extract of sea buckthorn (Hippophae rhamnoides L.) Seed residues in streptozotocin-treated and high fat-diet-fed rats. J. Food Biochem. 2010, 34, 856–868. [Google Scholar] [CrossRef]
- Akhter, P.; Bhatti, T.Y.; Shafiq, I.; Jamil, F.; Nazar, R.; Nazir, M.S.; Ul Hassan, S.; Hussain, M.; Park, Y. Antioxidant activity of sea buckthorn (Hippophae rhamnoides) seed oil extracted using various organic solvents. Korean J. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Kitryte, V.; Povilaitis, D.; Kraujaliene, V.; Sulniute, V.; Pukalskas, A.; Venskutonis, P.R. Fractionation of sea buckthorn pomace and seeds into valuable components by using high pressure and enzyme-assisted extraction methods. LWT-Food Sci. Technol. 2017, 85, 534–538. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, X.; He, L.; Yan, Q.; Yuan, F.; Gao, Y. Optimization of subcritical water extraction parameters of antioxidant polyphenols from sea buckthorn (Hippophae rhamnoides L.) seed residue. J. Food Sci. Technol.-Mysore 2015, 52, 1534–1542. [Google Scholar] [CrossRef]
- Cho, S. The Role of Functional Foods in Cutaneous Anti-aging. J. Lifestyle Med. 2014, 4, 8–16. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Chen, J.; Chen, X.; Zhang, Y. Anti-aging and redox state regulation effects of A-type proanthocyanidins-rich cranberry concentrate and its comparison with grape seed extract in mice. J. Funct. Foods 2017, 30, 63–73. [Google Scholar] [CrossRef]
- Park, B.M.; Chun, H.; Chae, S.W.; Kim, S.H. Fermented garlic extract ameliorates monocrotaline-induced pulmonary hypertension in rats. J. Funct. Foods 2017, 30, 247–253. [Google Scholar] [CrossRef]
- Robinson, J.P. Flow cytometry: Past and future. Biotechniques 2022, 72, 159–169. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Yan, X.; Chen, X.; Si, L.; Liu, Y.; Han, J.; Hao, W.; Zheng, Q. Isoliquiritigenin Inhibits Proliferation and Induces Apoptosis via Alleviating Hypoxia and Reducing Glycolysis in Mouse Melanoma B16F10 Cells. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 215–227. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, K.; Wu, Y.; Chen, Y.; Chen, X.; Hu, C.W.; Hu, F. Pinocembrin induces ER stress mediated apoptosis and suppresses autophagy in melanoma cells. Cancer Lett. 2018, 431, 31–42. [Google Scholar] [CrossRef]
- Ho, S.C.; Liu, J.H.; Wu, R.Y.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Najimi, S.A. Pancreatic protective and hypoglycemic effects of Vitex agnus-castus L. fruit hydroalcoholic extract in D-galactose-induced aging mouse model. Res. Pharm. Sci. 2017, 12, 137–143. [Google Scholar] [CrossRef]
- Yong, X.; Qing, M.; Ai, Z.; Yingdong, Z.; Yumei, Z.; Peiyu, W.; Heikki, K.; Baoru, Y. Effects of sea buckthorn (Hippophae rhamnoides) juice and L-quebrachitol on type 2 diabetes mellitus in db/db mice. J. Funct. Foods 2015, 16, 223–233. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Wang, C.; Sun, B.; Qi, C. Understanding the role of extracts from sea buckthorn seed residues in anti- melanogenesis properties on B16F10 melanoma cells. Food Funct. 2018, 9, 5402–5416. [Google Scholar] [CrossRef]
- Yap, C.V.; Subramaniam, K.S.; Khor, S.W.; Chung, I. Annonacin Exerts Antitumor Activity through Induction of Apoptosis and Extracellular Signal-regulated Kinase Inhibition. Pharmacogn. Res. 2017, 9, 378–383. [Google Scholar] [CrossRef]
- Yang, G.; Qiu, J.; Wang, D.; Tao, Y.; Song, Y.; Wang, H.; Tang, J.; Wang, X.; Sun, Y.; Yang, Z.; et al. Traditional Chinese Medicine Curcumin Sensitizes Human Colon Cancer to Radiation by Altering the Expression of DNA Repair-related Genes. Anticancer Res. 2018, 38, 131–136. [Google Scholar] [CrossRef]
- Riikonen, A.; Rouvinen, J.; Tikkanen, R.; Julkunen, I.; Peltonen, L.; Jalanko, A. Primary folding of aspartylglucosaminidase. Significance of disulfide bridges and evidence of early multimerization. J. Biol. Chem. 1996, 271, 21340–21344. [Google Scholar] [CrossRef]
- Tenhunen, K.; Laan, M.; Manninen, T.; Palotie, A.; Peltonen, L.; Jalanko, A. Molecular cloning, chromosomal assignment, and expression of the mouse aspartylglucosaminidase gene. Genomics 1995, 30, 244–250. [Google Scholar] [CrossRef]
- Dunder, U.; Kaartinen, V.; Valtonen, P.; Vaananen, E.; Kosma, V.M.; Heisterkamp, N.; Groffen, J.; Mononen, I. Enzyme replacement therapy in a mouse model of aspartylglycosaminuria. FASEB J. 2000, 14, 361–367. [Google Scholar] [CrossRef]
- Kotov, D.I.; Mitchell, J.S.; Pengo, T.; Ruedl, C.; Way, S.S.; Langlois, R.A.; Fife, B.T.; Jenkins, M.K. TCR Affinity Biases Th Cell Differentiation by Regulating CD25, Eef1e1, and Gbp2. J. Immunol. 2019, 202, 2535–2545. [Google Scholar] [CrossRef]
- Mirzaei, M.; Pushpitha, K.; Deng, L.T.; Chitranshi, N.; Gupta, V.; Rajput, R.; Mangani, A.; Dheer, Y.; Godinez, A.; McKay, M.J.; et al. Upregulation of Proteolytic Pathways and Altered Protein Biosynthesis Underlie Retinal Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6017–6034. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Kang, J.W.; Lee, S.W.; Choi, S.J.; Shin, Y.K.; Ahn, Y.H.; Choi, Y.H.; Choi, D.H.; Lee, K.S.; Kim, S. The haploinsufficient tumor suppressor p18 upregulates p53 via interactions with ATM/ATR. Cell 2005, 120, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Wang, L.H. FARP1 Facilitates Cell Proliferation Through Modulating MAPK Signaling Pathway in Cutaneous Melanoma. Am. J. Dermatopathol. 2019, 41, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, L.; Biederer, T. Activity-Dependent Regulation of Dendritic Complexity by Semaphorin 3A through Farp1. J. Neurosci. 2014, 34, 7999–8009. [Google Scholar] [CrossRef]
- Fan, L.F.; Yan, H.J.; Pellegrin, S.; Morigen; Mellor, H. The Rif GTPase regulates cytoskeletal signaling from plexinA4 to promote neurite retraction. Neurosci. Lett. 2015, 590, 178–183. [Google Scholar] [CrossRef]
- Hirano, T.; Shinsato, Y.; Tanabe, K.; Higa, N.; Kamil, M.; Kawahara, K.; Yamamoto, M.; Minami, K.; Shimokawa, M.; Arigami, T.; et al. FARP1 boosts CDC42 activity from integrin alpha v beta 5 signaling and correlates with poor prognosis of advanced gastric cancer. Oncogenesis 2020, 9, 13. [Google Scholar] [CrossRef]
- Lin, H.W.; Lin, J.; Pan, T.T.; Li, T.; Jiang, H.M.; Fang, Y.; Wang, Y.X.; Wu, F.L.; Huang, J.; Zhang, H.D.; et al. Polymeric immunoglobulin receptor deficiency exacerbates autoimmune hepatitis by inducing intestinal dysbiosis and barrier dysfunction. Cell Death Dis. 2023, 14, 68. [Google Scholar] [CrossRef]
- Kim, T.; Yang, Q. Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J. Cardiol. 2013, 5, 164–174. [Google Scholar] [CrossRef]
- Yu, L.Q.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Investig. 2002, 110, 671–680. [Google Scholar] [CrossRef]
- de Oliveira, P.R.B.; da Costa, C.A.; de Bem, G.F.; Cordeiro, V.S.C.; Santos, I.B.; de Carvalho, L.C.R.M.; da Conceicao, E.P.S.; Lisboa, P.C.; Ognibene, D.T.; Sousa, P.J.C.; et al. Euterpe oleracea Mart.-Derived Polyphenols Protect Mice from Diet-Induced Obesity and Fatty Liver by Regulating Hepatic Lipogenesis and Cholesterol Excretion. PLoS ONE 2015, 10, e0143721. [Google Scholar] [CrossRef]


| Accession | Symbol | Gene_ID | Control Group vs. Model Group | Model Group vs. HYD-SBSR Group | ||||
|---|---|---|---|---|---|---|---|---|
| Fold Change | p-Value | Regulated Type in the Model Group | Fold Change | p-Value | Regulated Type in HYD-SBSR Group | |||
| NP_035385.1 | Rbp4 | 19662 | 1.14615057 | 0.027117863 | up | 1.12640839 | 0.033390243 | up |
| NP_079656.1 | Eef1e1 | 66143 | 1.163316017 | 0.03599657 | up | 0.854203188 | 0.030938576 | down |
| XP_006518978.1 | Farp1 | 223254 | 1.255319503 | 0.000803871 | up | 0.834798262 | 0.019648087 | down |
| XP_006509312.2 | Aga | 11593 | 1.195095275 | 0.002335059 | up | 0.821681249 | 0.025899789 | down |
| NP_035212.2 | Pigr | 18703 | 0.868934766 | 0.020919521 | down | 1.198358739 | 0.0253557 | up |
| XP_017176596.1 | Zbed5 | 71970 | 0.866735033 | 0.049608279 | down | 0.76157783 | 0.00203081 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Z.; Zhang, J.; Cheng, W.; Wang, C.; Zhao, D. Ethanolic Extract from Seed Residues of Sea Buckthorn (Hippophae rhamnoides L.) Ameliorates Oxidative Stress Damage and Prevents Apoptosis in Murine Cell and Aging Animal Models. Foods 2023, 12, 3322. https://doi.org/10.3390/foods12173322
Hua Z, Zhang J, Cheng W, Wang C, Zhao D. Ethanolic Extract from Seed Residues of Sea Buckthorn (Hippophae rhamnoides L.) Ameliorates Oxidative Stress Damage and Prevents Apoptosis in Murine Cell and Aging Animal Models. Foods. 2023; 12(17):3322. https://doi.org/10.3390/foods12173322
Chicago/Turabian StyleHua, Zhongjie, Jiachan Zhang, Wenjing Cheng, Changtao Wang, and Dan Zhao. 2023. "Ethanolic Extract from Seed Residues of Sea Buckthorn (Hippophae rhamnoides L.) Ameliorates Oxidative Stress Damage and Prevents Apoptosis in Murine Cell and Aging Animal Models" Foods 12, no. 17: 3322. https://doi.org/10.3390/foods12173322
APA StyleHua, Z., Zhang, J., Cheng, W., Wang, C., & Zhao, D. (2023). Ethanolic Extract from Seed Residues of Sea Buckthorn (Hippophae rhamnoides L.) Ameliorates Oxidative Stress Damage and Prevents Apoptosis in Murine Cell and Aging Animal Models. Foods, 12(17), 3322. https://doi.org/10.3390/foods12173322