Comparing Different Chemometric Approaches to Detect Adulteration of Cold-Pressed Flaxseed Oil with Refined Rapeseed Oil Using Differential Scanning Calorimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Melting Phase Transition Analysis via Differential Scanning Calorimetry (DSC)
2.2.2. Data Analysis
3. Results
3.1. DSC Melting Profiles of Cold-Pressed Flaxseed Oil Adulterated with Refined Rapeseed Oil
3.2. Changes in DSC Parameters of Flaxseed Oil Melting Phase Transition Depending on Adulterant Concentrations
4. Discussion
4.1. Classification Models for Predicting Cold-Pressed Flaxseed Oil Adulteration Levels
4.2. Regression Models for Predicting the Concentration of Refined Rapeseed Oil in Cold-Pressed Flaxseed Oil
Principle Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jović, O.; Jović, A. FTIR-ATR adulteration study of hempseed oil of different geographic origins. J. Chemom. 2017, 31, e2938. [Google Scholar] [CrossRef]
- Sim, S.F.; Chai, M.X.L.; Jeffrey Kimura, A.L. Prediction of lard in palm olein oil using simple linear regression (SLR), multiple linear regression (MLR), and partial least squares regression (PLSR) based on fourier-transform infrared (FTIR). J. Chem. 2018, 2018, 7182801. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J. Rapid quantitative determination of butter adulteration with palm oil using the DSC technique. Food Control 2016, 60, 629–635. [Google Scholar] [CrossRef]
- Carter, O.C.S. On the Detection of Adulterations in Oils; American Philosophical Society: Philadelphia, PA, USA, 1885; Volume 22. [Google Scholar]
- FDA. Economically Motivated Adulteration; Public Meeting; Request for Comment; FDA: College Park, MD, USA, 2009; Volume 74, pp. 69–71. Available online: https://www.federalregister.gov/documents/2009/04/06/E9-7843/economically-motivated-adulteration-public-meeting-request-for-comment (accessed on 2 June 2023).
- FAO. Food Fraud-Intention, Detection and Management. Food Safety Technical Toolkit for Asia and the Pacific No. 5. Bangkok. 2021. Available online: https://www.fao.org/3/cb2863en/cb2863en.pdf (accessed on 5 June 2023).
- Gelpí, E.; Posada de la Paz, M.; Terracini, B.; Abaitua, I.; Gómez de la Cámara, A.; Kilbourne, E.M.; Lahoz, C.; Nemery, B.; Philen, R.M.; Soldevilla, L.; et al. The spanish toxic oil syndrome 20 years after its onset: A multidisciplinary review of scientific knowledge. Environ. Health Perspect. 2002, 110, 457–464. [Google Scholar] [CrossRef]
- European Commission. European Commission The EU Food Fraud Network and the System for Administrative Assistance-Food Fraud. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2018:772:FIN (accessed on 27 May 2023).
- Rocha, W.F.C.; Prado, C.B.; Niksa, B. Comparison of Chemometric Problems in Food Analysis using Non-Linear Methods. Molecule 2020, 25, 3025. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Jing, B.; Dong, Y.; Yu, X. New Method for the Discrimination of Adulterated Flaxseed Oil Using Dielectric Spectroscopy. Food Anal. Methods 2019, 12, 2623–2629. [Google Scholar] [CrossRef]
- Rocha, W.F.C.; Vaz, B.G.; Sarmanho, G.F.; Leal, L.H.C.; Nogueira, R.; Silva, V.F.; Borges, C.N. Chemometric Techniques Applied for Classification and Quantification of Binary Biodiesel/Diesel Blends. Anal. Lett. 2012, 45, 2398–2411. [Google Scholar] [CrossRef]
- Peng, D.; Shi, C.; Nie, Q.; Xie, S.; Bi, Y.; Li, J. Qualitative and quantitative detection of peanut oils adulteration based on fatty acid information fusion coupled with chemometrics. Lwt 2023, 181, 114785. [Google Scholar] [CrossRef]
- Giese, E.; Rohn, S.; Fritsche, J. Chemometric tools for the authentication of cod liver oil based on nuclear magnetic resonance and infrared spectroscopy data. Anal. Bioanal. Chem. 2019, 411, 6931–6942. [Google Scholar] [CrossRef]
- Bao, R.; Tang, F.; Rich, C.; Hatzakis, E. A comparative evaluation of low-field and high-field NMR untargeted analysis: Authentication of virgin coconut oil adulterated with refined coconut oil as a case study. Anal. Chim. Acta 2023, 1273, 341537. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, L.; Wang, D.; Jiang, J.; Harrington, P.d.B.; Mao, J.; Zhang, Q.; Li, P. Detection of flaxseed oil multiple adulteration by near-infrared spectroscopy and nonlinear one class partial least squares discriminant analysis. LWT 2020, 125, 109247. [Google Scholar] [CrossRef]
- Hao, S.; Zhu, L.; Sui, R.; Zuo, M.; Luo, N.; Shi, J.; Zhang, W.; He, X.; Chen, Z. Identification and quantification of vegetable oil adulteration with waste frying oil by laser-induced fluorescence spectroscopy. OSA Contin. 2019, 2, 1148. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Eugenia Muñoz-Pérez, M.; Arce, L. Detection of adulteration in extra virgin olive oils by using UV-IMS and chemometric analysis. Food Control 2018, 85, 292–299. [Google Scholar] [CrossRef]
- Vanstone, N.; Moore, A.; Martos, P.; Neethirajan, S. Detection of the adulteration of extra virgin olive oil by near-infrared spectroscopy and chemometric techniques. Food Qual. Saf. 2018, 2, 189–198. [Google Scholar] [CrossRef]
- Huang, K.; Zhong, P.; Xu, B. Discrimination on potential adulteration of extra virgin olive oils consumed in China by differential scanning calorimeter combined with dimensionality reduction classification techniques. Food Chem. 2023, 405, 134996. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.M.; De Santana, F.B.; Gontijo, L.C.; Mazivila, S.J.; Borges Neto, W. Quantification of adulterations in extra virgin flaxseed oil using MIR and PLS. Food Chem. 2015, 182, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Xin, J.X.; Sun, S.S.; Li, Y.; Wei, D.X.; Zhu, J.; Wang, X.L.; Wang, J.; Yao, Y.F. Rapid identification of adulteration in edible vegetable oils based on low-field nuclear magnetic resonance relaxation fingerprints. Foods 2021, 10, 3068. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Li, P.; Xu, B.; Ma, F.; Zhang, Q.; Zhang, W. Fatty acid profiles based adulteration detection for flaxseed oil by gas chromatography mass spectrometry. LWT 2015, 63, 430–436. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Wang, X.; Li, Y.; Wang, S.; Gan, S.; Dong, G.; Chen, X.; Wang, S. Adulteration detection of Qinghai-Tibet Plateau flaxseed oil using HPLC-ELSD profiling of triacylglycerols and chemometrics. LWT 2022, 160, 113300. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Chiavaro, E.; Vittadini, E.; Rodriguez-Estrada, M.T.; Cerretani, L.; Bendini, A. Differential scanning calorimeter application to the detectionof refined hazelnut oil in extra virgin olive oil. Food Chem. 2008, 110, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Kadivar, M.; Keramat, J. Detection of adulteration in Iranian olive oils using instrumental (GC, NMR, DSC) methods. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 103–110. [Google Scholar] [CrossRef]
- Van Wetten, I.A.; Van Herwaarden, A.W.; Splinter, R.; Boerrigter-Eenling, R.; Van Ruth, S.M. Detection of sunflower oil in extra virgin olive oil by fast differential scanning calorimetry. Thermochim. Acta 2015, 603, 237–243. [Google Scholar] [CrossRef]
- Karbasian, M.; Givianrad, M.H.; Ramezan, Y. A rapid method for detection of refined olive oil as adulterant in extra virgin olive oil by differential scanning calorimetry. Orient. J. Chem. 2015, 31, 1735–1739. [Google Scholar] [CrossRef]
- Marikkar, J.M.N. Differential Scanning Calorimetric Analysis of Virgin Coconut Oil, Palm Olein, and their Adulterated Blends. Cord 2019, 35, 9. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Monitoring the adulteration of virgin coconut oil by selected vegetable oils using differential scanning calorimetry. J. Food Lipids 2009, 16, 50–61. [Google Scholar] [CrossRef]
- Coni, E.; Di Pasquale, M.; Coppolelli, P.; Bocca, A. Detection of animal fats in butter by differential scanning calorimetry: A pilot study. J. Am. Oil Chem. Soc. 1994, 71, 807–810. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J. Multivariate analysis of seasonal variation in the composition and thermal properties of butterfat with an emphasis on authenticity assessment. Grasas y Aceites 2016, 67, 4. [Google Scholar] [CrossRef]
- Upadhyay, N.; Goyal, A.; Kumar, A.; Lal, D. Detection of adulteration by caprine body fat and mixtures of caprine body fat and groundnut oil in bovine and buffalo ghee using differential scanning calorimetry. Int. J. Dairy Technol. 2017, 70, 297–303. [Google Scholar] [CrossRef]
- Marikkar, J.M.N.; Ghazali, H.M.; Che Man, Y.B.; Lai, O.M. The use of cooling and heating thermograms for monitoring of tallow, lard and chicken fat adulterations in canola oil. Food Res. Int. 2002, 35, 1007–1014. [Google Scholar] [CrossRef]
- Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. Development of a method for qualitative detection of lead chromate adulteration in turmeric powder using X-ray powder diffraction. Food Control 2021, 126, 107992. [Google Scholar] [CrossRef]
- Clas, S.D.; Dalton, C.R.; Hancock, B.C. Differential scanning calorimetry: Applications in drug development. Pharm. Sci. Technol. Today 1999, 2, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Drzeżdżon, J.; Jacewicz, D.; Sielicka, A.; Chmurzyński, L. Characterization of polymers based on differential scanning calorimetry based techniques. TrAC Trends Anal. Chem. 2019, 110, 51–56. [Google Scholar] [CrossRef]
- Parniakov, O.; Bals, O.; Barba, F.J.; Mykhailyk, V.; Lebovka, N.; Vorobiev, E. Application of differential scanning calorimetry to estimate quality and nutritional properties of food products. Crit. Rev. Food Sci. Nutr. 2018, 58, 362–385. [Google Scholar] [CrossRef]
- FAO. Production/Yield Quantities of Oil of Linseed in World + (Total) [Internet]. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 11 June 2023).
- Tomaszewska-Gras, J.; Islam, M.; Grzeca, L.; Kaczmarek, A.; Fornal, E. Comprehensive thermal characteristics of different cultivars of flaxseed oil (Linum usittatissimum L.). Molecules 2021, 26, 1958. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.Y.; Zeitoun, M.A. Quality evaluation of flaxseed oil obtained by different extraction techniques. LWT Food Sci. Technol. 2013, 53, 338–345. [Google Scholar] [CrossRef]
- Visentainer, J.V.; De Souza, N.E.; Makoto, M.; Hayashi, C.; Franco, M.R.B. Influence of diets enriched with flaxseed oil on the α-linolenic, eicosapentaenoic and docosahexaenoic fatty acid in Nile tilapia (Oreochromis niloticus). Food Chem. 2005, 90, 557–560. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Uygur, A.; Karagül Yüceer, Y. The Effects of Infrared Roasting on HCN Content, Chemical Composition and Storage Stability of Flaxseed and Flaxseed Oil. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 877–884. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.; Deng, Q.; Huang, Q.; Yang, J.; Huang, F. Flaxseed oil and α-lipoic acid combination reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis. 2012, 11, 148. [Google Scholar] [CrossRef]
- Dwivedi, C.; Natarajan, K.; Matthees, D.P. Chemopreventive effects of dietary flaxseed oil on colon tumor development. Nutr. Cancer 2005, 51, 52–58. [Google Scholar] [CrossRef]
- Popa, S.; Milea, M.S.; Boran, S.; Nițu, S.V.; Moșoarcă, G.E.; Vancea, C.; Lazău, R.I. Rapid adulteration detection of cold pressed oils with their refined versions by UV–Vis spectroscopy. Sci. Rep. 2020, 10, 16100. [Google Scholar] [CrossRef] [PubMed]
- American Oil Chemists’ Society. DSC Melting Properties of Fats and Oils; Cj 1-94; American Oil Chemists’ Society: Urbana, IL, USA, 2000. [Google Scholar]
- Efenberger-Szmechtyk, M.; Nowak, A.; Kregiel, D. Implementation of chemometrics in quality evaluation of food and beverages. Crit. Rev. Food Sci. Nutr. 2018, 58, 1747–1766. [Google Scholar] [CrossRef] [PubMed]
- Indriyani, L.; Rohman, A.; Riyanto, S. Authentication of avocado oil (Persea americana Mill.) Using differential scanning calorimetry and multivariate regression. Asian J. Agric. Res. 2016, 10, 78–86. [Google Scholar] [CrossRef]
- Friedman, J.H. Multivariate adaptive regression splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Karami, H.; Rasekh, M.; Mirzaee–Ghaleh, E. Comparison of chemometrics and AOCS official methods for predicting the shelf life of edible oil. Chemom. Intell. Lab. Syst. 2020, 206, 104165. [Google Scholar] [CrossRef]
- Firouz, M.S.; Omid, M.; Babaei, M.; Rashvand, M. Dielectric spectroscopy coupled with artificial neural network for classification and quantification of sesame oil adulteration. Inf. Process. Agric. 2022, 9, 233–242. [Google Scholar] [CrossRef]
- Hai, Z.; Wang, J. Detection of adulteration in camellia seed oil and sesame oil using an electronic nose. Eur. J. Lipid Sci. Technol. 2006, 108, 116–124. [Google Scholar] [CrossRef]
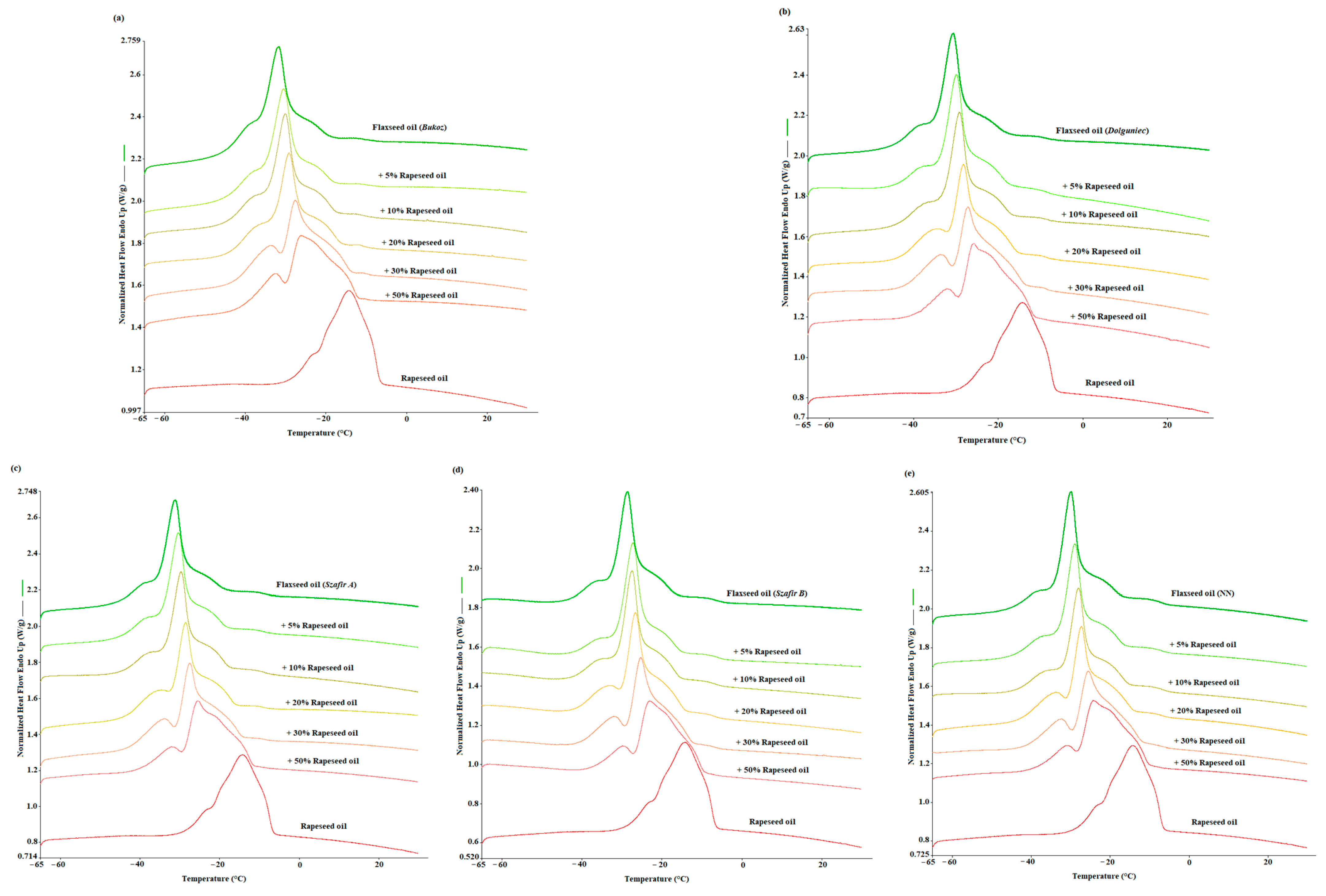
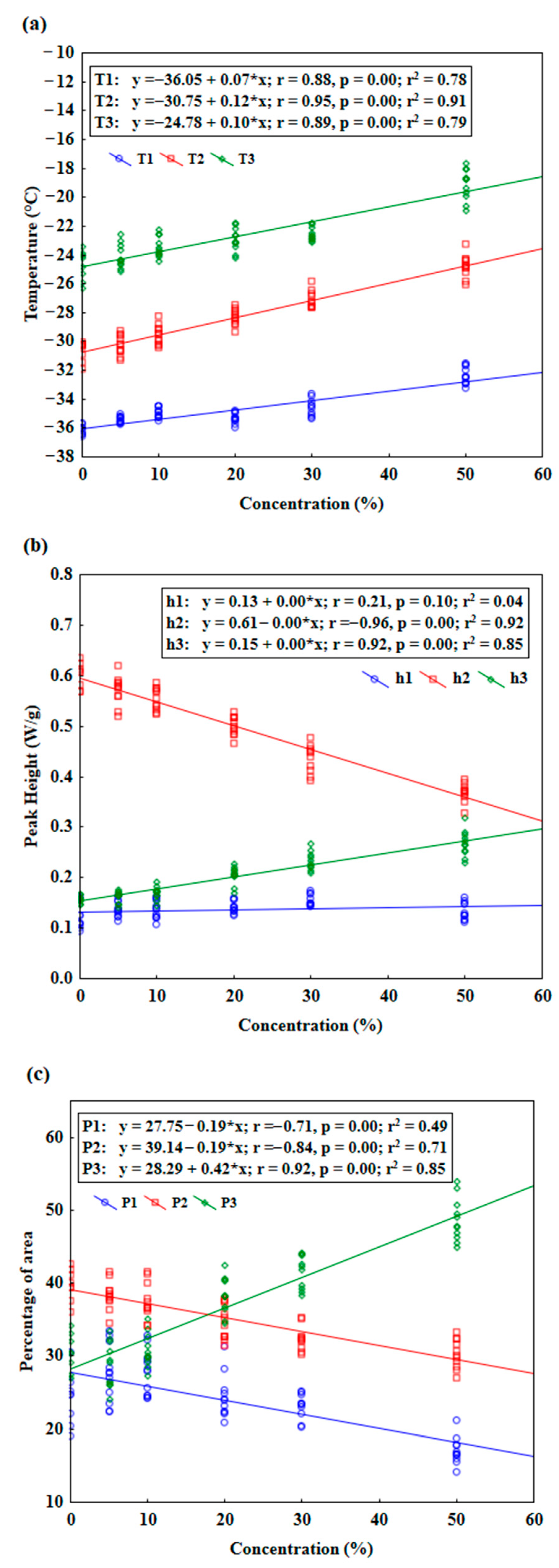

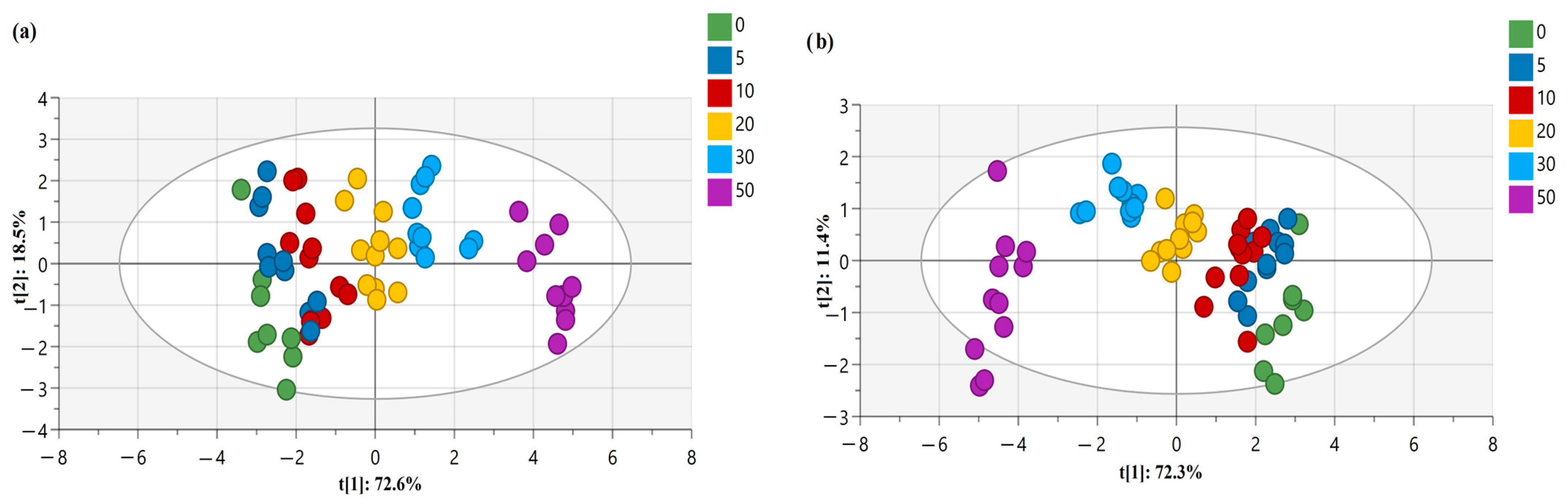
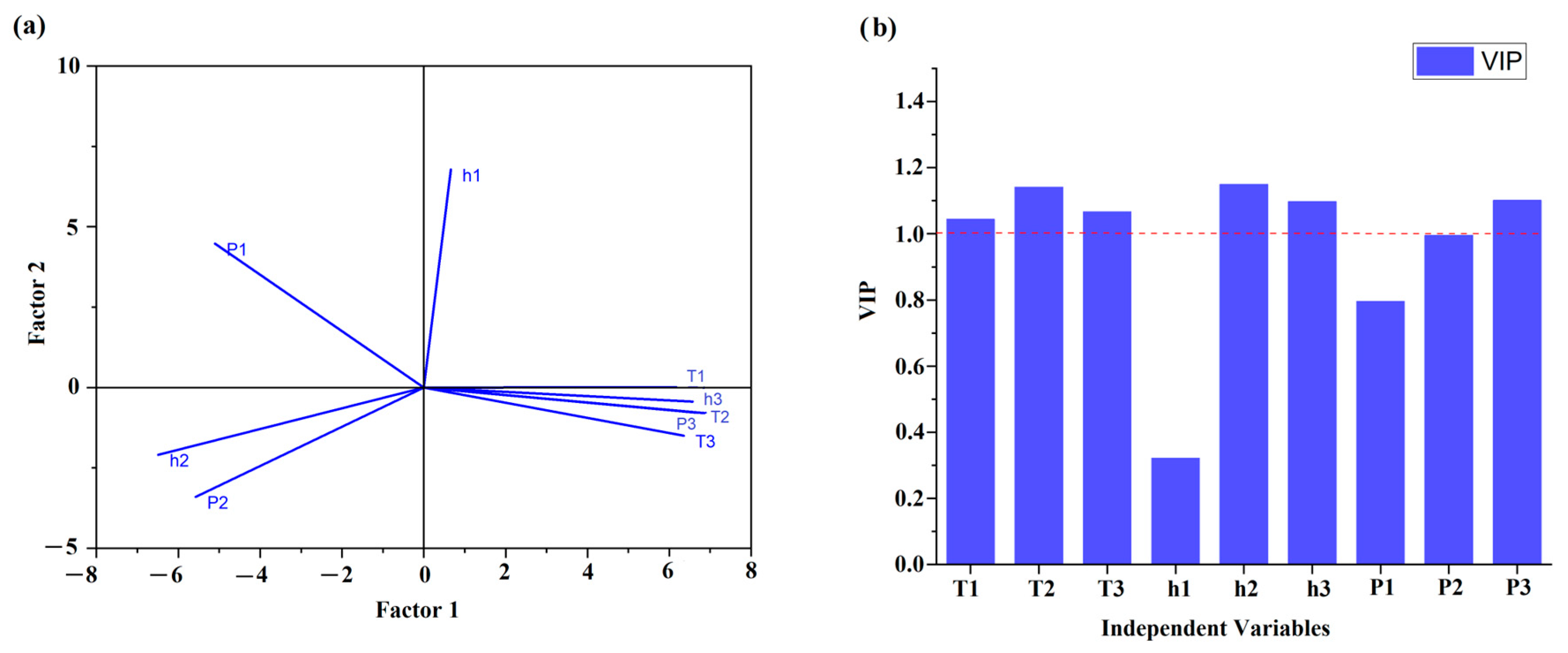

| DSC Parameters | Concentrations | |||||
|---|---|---|---|---|---|---|
| 0% | 5% | 10% | 20% | 30% | 50% | |
| T1 | −36.15 ± 0.35 a | −35.44 ± 0.23 bc | −34.95 ± 0.35 bc | −35.32 ± 0.36 b | −34.57 ± 0.59 c | −32.39 ± 0.6 d |
| T2 | −30.67 ± 0.69 a | −30.26 ± 0.67 ab | −29.55 ± 0.68 b | −28.31 ± 0.54 c | −27.08 ± 0.61 d | −24.78 ± 0.77 e |
| T3 | −24.73 ± 1.04 a | −24.08 ± 0.84 ab | −23.44 ± 0.72 bc | −22.92 ± 0.81 c | −22.53 ± 0.48 c | −19.12 ± 1.07 d |
| h1 | 0.12 ± 0.02 a | 0.14 ± 0.02 abc | 0.14 ± 0.02 abc | 0.14 ± 0.01 bc | 0.15 ± 0.01 c | 0.13 ± 0.02 ab |
| h2 | 0.60 ± 0.03 e | 0.57 ± 0.03 de | 0.55 ± 0.02 d | 0.50 ± 0.02 c | 0.44 ± 0.03 b | 0.37 ± 0.02 a |
| h3 | 0.16 ± 0.01 a | 0.16 ± 0.01 a | 0.17 ± 0.01 a | 0.21 ± 0.02 b | 0.23 ± 0.02 c | 0.27 ± 0.02 d |
| P1 | 24.12 ± 3.58 bc | 27.50 ± 4.00 c | 27.55 ± 3.17 c | 24.41 ± 3.04 bc | 23.09 ± 1.74 b | 17.08 ± 1.84 a |
| P2 | 39.77 ± 2.16 c | 38.32 ± 1.96 c | 37.49 ± 2.51 c | 34.52 ± 2.35 b | 32.43 ± 1.69 ab | 30.27 ± 2.00 a |
| P3 | 30.49 ± 2.57 a | 28.78 ± 3.07 a | 30.75 ± 2.31 a | 38.30 ± 2.52 b | 41.50 ± 2.26 b | 48.71 ± 2.96 c |
| Observed | 0% | 5% | 10% | 20% | 30% | 50% | |
|---|---|---|---|---|---|---|---|
| Predicted | |||||||
| 0% | LDA | 8 | 0 | 0 | 0 | 0 | 0 |
| ANN | 8 | 0 | 0 | 0 | 0 | 0 | |
| SVM | 6 | 2 | 0 | 0 | 0 | 0 | |
| MARS | 7 | 1 | 0 | 0 | 0 | 0 | |
| 5% | LDA | 0 | 10 | 1 | 0 | 0 | 0 |
| ANN | 0 | 10 | 1 | 0 | 0 | 0 | |
| SVM | 0 | 10 | 1 | 0 | 0 | 0 | |
| MARS | 0 | 9 | 2 | 0 | 0 | 0 | |
| 10% | LDA | 0 | 0 | 11 | 0 | 0 | 0 |
| ANN | 0 | 3 | 8 | 0 | 0 | 0 | |
| SVM | 0 | 2 | 9 | 0 | 0 | 0 | |
| MARS | 0 | 1 | 10 | 0 | 0 | 0 | |
| 20% | LDA | 0 | 0 | 0 | 11 | 0 | 0 |
| ANN | 0 | 0 | 0 | 11 | 0 | 0 | |
| SVM | 0 | 0 | 0 | 11 | 0 | 0 | |
| MARS | 0 | 0 | 0 | 9 | 2 | 0 | |
| 30% | LDA | 0 | 0 | 0 | 0 | 10 | 0 |
| ANN | 0 | 0 | 0 | 0 | 10 | 0 | |
| SVM | 0 | 0 | 0 | 0 | 10 | 0 | |
| MARS | 0 | 0 | 0 | 2 | 8 | 0 | |
| 50% | LDA | 0 | 0 | 0 | 0 | 0 | 11 |
| ANN | 0 | 0 | 0 | 0 | 0 | 11 | |
| SVM | 0 | 0 | 0 | 0 | 0 | 11 | |
| MARS | 0 | 0 | 0 | 0 | 0 | 11 |
| Intercept | Term 1 | Term 2 | Term 3 | Term 4 | Term 5 | Term 6 | |
|---|---|---|---|---|---|---|---|
| 0% | −1.01 | −3.39 | −9.46 × 10−1 | 8.85 × 10−1 | 2.39 × 10−2 | 9.37 × 10−1 | 8.68 |
| 5% | 1.27 | −1.39 | 1.35 | −9.96 × 10−1 | 3.12 × 10−1 | −1.28 | 4.00 |
| 10% | −1.02 × 10−1 | 1.81 × 101 | −3.68 × 10−1 | −2.01 × 10−1 | −7.60 × 10−2 | 2.72 × 10−1 | −2.12 × 101 |
| 20% | 3.38 × 10−1 | 2.92 | 1.83 × 10−1 | 3.18 × 10−1 | −3.07 × 10−1 | −2.26 × 10−1 | −9.92 |
| 30% | 5.08 × 10−1 | −1.58 × 101 | −6.08 × 10−1 | −1.67 × 10−2 | 4.51 × 10−2 | 2.55 × 10−1 | 1.79 × 101 |
| 50% | 1.39 | −2.29 | 5.25 × 10−1 | −1.03 × 10−1 | 3.13 × 10−2 | −8.92 × 10−2 | 4.43 |
| Knots T1 | −3.44 × 101 | −3.44 × 101 | −3.55 × 101 | ||||
| Knots T2 | −2.88 × 101 | ||||||
| Knots h2 | 4.83 × 10−1 | 5.28 × 10−1 |
| Performance Parameter | Accuracy | Misclassification Rate | Precision | Sensitivity | Specificity | F1-Score |
|---|---|---|---|---|---|---|
| Model | ||||||
| LDA | 99.46% | 0.54% | 98.39% | 98.39% | 99.68% | 98.39% |
| ANN | 97.85% | 2.15% | 93.55% | 93.55% | 98.71% | 93.55% |
| SVM | 97.31% | 2.69% | 91.94% | 91.94% | 98.39% | 91.94% |
| MARS | 95.70% | 4.30% | 87.10% | 87.10% | 97.42% | 87.10% |
| Net Architecture | Training Accuracy | Test Accuracy | Validation Accuracy | Training Algorithm | Error Function | Hidden Activation | Output Activation |
|---|---|---|---|---|---|---|---|
| MLP 9-9-6 | 88.636 | 100.000 | 77.778 | BFGS 10 | Entropy | Linear | Softmax |
| MLP 9-11-6 | 88.636 | 88.889 | 100.000 | BFGS 11 | Entropy | Linear | Softmax |
| MLP 9-8-6 | 81.818 | 88.889 | 100.000 | BFGS 9 | Entropy | Linear | Softmax |
| MLP 9-9-6 | 84.091 | 77.778 | 100.000 | BFGS 32 | SOS | Exponential | Exponential |
| MLP 9-4-6 | 84.091 | 88.889 | 88.889 | BFGS 15 | Entropy | Tanh | Softmax |
| DSC Parameters | b* (Standardized Co-Efficient) | b (Raw Co-Efficient) | p-Value |
|---|---|---|---|
| 145.3464 * | 0.000059 * | ||
| T1 | 0.090680 | 1.2225 | 0.126748 |
| T2 | −0.052531 | −0.4175 | 0.627294 |
| T3 | 0.273274 * | 2.3374 * | 0.000332 * |
| h1 | 0.080773 | 73.6583 | 0.056670 |
| h2 | −0.324189 * | −65.5228 * | 0.000014 * |
| h3 | 0.168666 * | 65.1633 * | 0.004121 * |
| P1 | −0.128623 | −0.4718 | 0.151392 |
| P2 | −0.145236 | −0.6314 | 0.083058 |
| P3 | 0.019714 | 0.0435 | 0.896428 |
| Model | R | R² | Adjusted R2 | AIC | BIC | RMSE |
|---|---|---|---|---|---|---|
| ANN | 0.996 | 0.992 | 0.992 | 233 | 240 | 1.51 |
| MARS | 0.995 | 0.990 | 0.990 | 244 | 251 | 1.65 |
| SVM | 0.992 | 0.985 | 0.984 | 274 | 280 | 2.10 |
| MLR | 0.992 | 0.984 | 0.984 | 275 | 281 | 2.12 |
| Net Architecture | Training Accuracy | Test Accuracy | Validation Accuracy | Training Algorithm | Error Function | Hidden Activation | Output Activation |
|---|---|---|---|---|---|---|---|
| MLP 9-9-1 | 0.9957 | 0.9919 | 0.9901 | BFGS 43 | SOS | Logistic | Logistic |
| MLP 9-13-1 | 0.9964 | 0.9961 | 0.9941 | BFGS 46 | SOS | Logistic | Tanh |
| MLP 9-11-1 | 0.9965 | 0.9913 | 0.9885 | BFGS 61 | SOS | Logistic | Exponential |
| MLP 9-13-1 | 0.9961 | 0.9899 | 0.9866 | BFGS 54 | SOS | Logistic | Exponential |
| MLP 9-11-1 | 0.9963 | 0.9937 | 0.9972 | BFGS 37 | SOS | Tanh | Tanh |
| Model | R2X (cum) | R2 (cum) | Q2 (cum) |
|---|---|---|---|
| PCA | 0.973 | 0.897 | |
| OPLS-DA | 0.986 | 0.465 | 0.330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Kaczmarek, A.; Montowska, M.; Tomaszewska-Gras, J. Comparing Different Chemometric Approaches to Detect Adulteration of Cold-Pressed Flaxseed Oil with Refined Rapeseed Oil Using Differential Scanning Calorimetry. Foods 2023, 12, 3352. https://doi.org/10.3390/foods12183352
Islam M, Kaczmarek A, Montowska M, Tomaszewska-Gras J. Comparing Different Chemometric Approaches to Detect Adulteration of Cold-Pressed Flaxseed Oil with Refined Rapeseed Oil Using Differential Scanning Calorimetry. Foods. 2023; 12(18):3352. https://doi.org/10.3390/foods12183352
Chicago/Turabian StyleIslam, Mahbuba, Anna Kaczmarek, Magdalena Montowska, and Jolanta Tomaszewska-Gras. 2023. "Comparing Different Chemometric Approaches to Detect Adulteration of Cold-Pressed Flaxseed Oil with Refined Rapeseed Oil Using Differential Scanning Calorimetry" Foods 12, no. 18: 3352. https://doi.org/10.3390/foods12183352
APA StyleIslam, M., Kaczmarek, A., Montowska, M., & Tomaszewska-Gras, J. (2023). Comparing Different Chemometric Approaches to Detect Adulteration of Cold-Pressed Flaxseed Oil with Refined Rapeseed Oil Using Differential Scanning Calorimetry. Foods, 12(18), 3352. https://doi.org/10.3390/foods12183352







