Spotlight on the Multiscale Structural and Physicochemical Properties of Red Adzuki Bean Starch through Partial Amylose Removal Combined with Hydrochloric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Starch Separation
2.3. Deamylose Treatment
2.4. Hydrochloric Acid Modification Treatment
2.5. DA—HCl Treatment
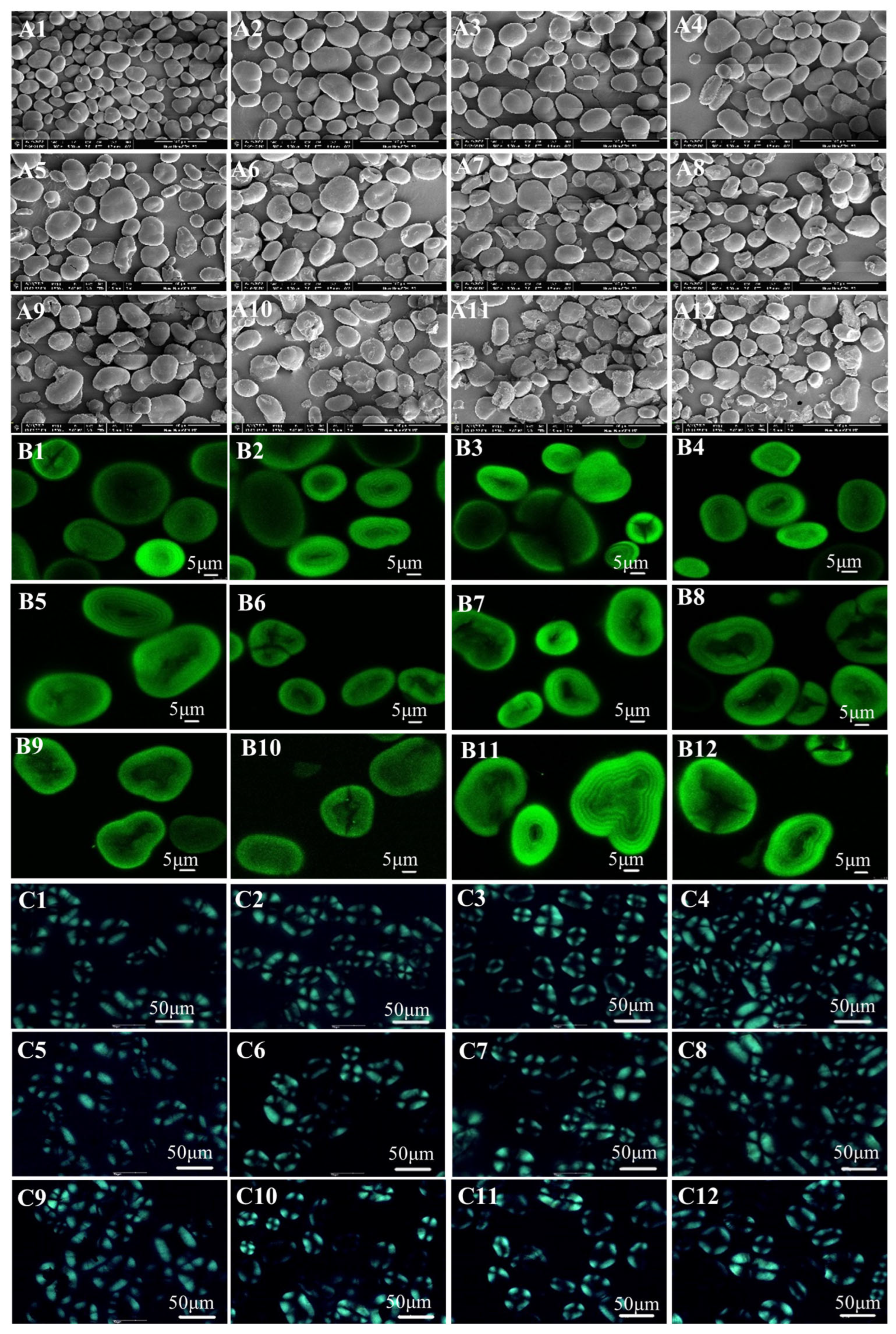
2.6. Microscopic Inspection
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. Confocal Laser Scanning Microscopy (CLSM)
2.6.3. Polarizing Light Microscope (PLM)
2.7. Chain Length Distribution (CLD) Measuring
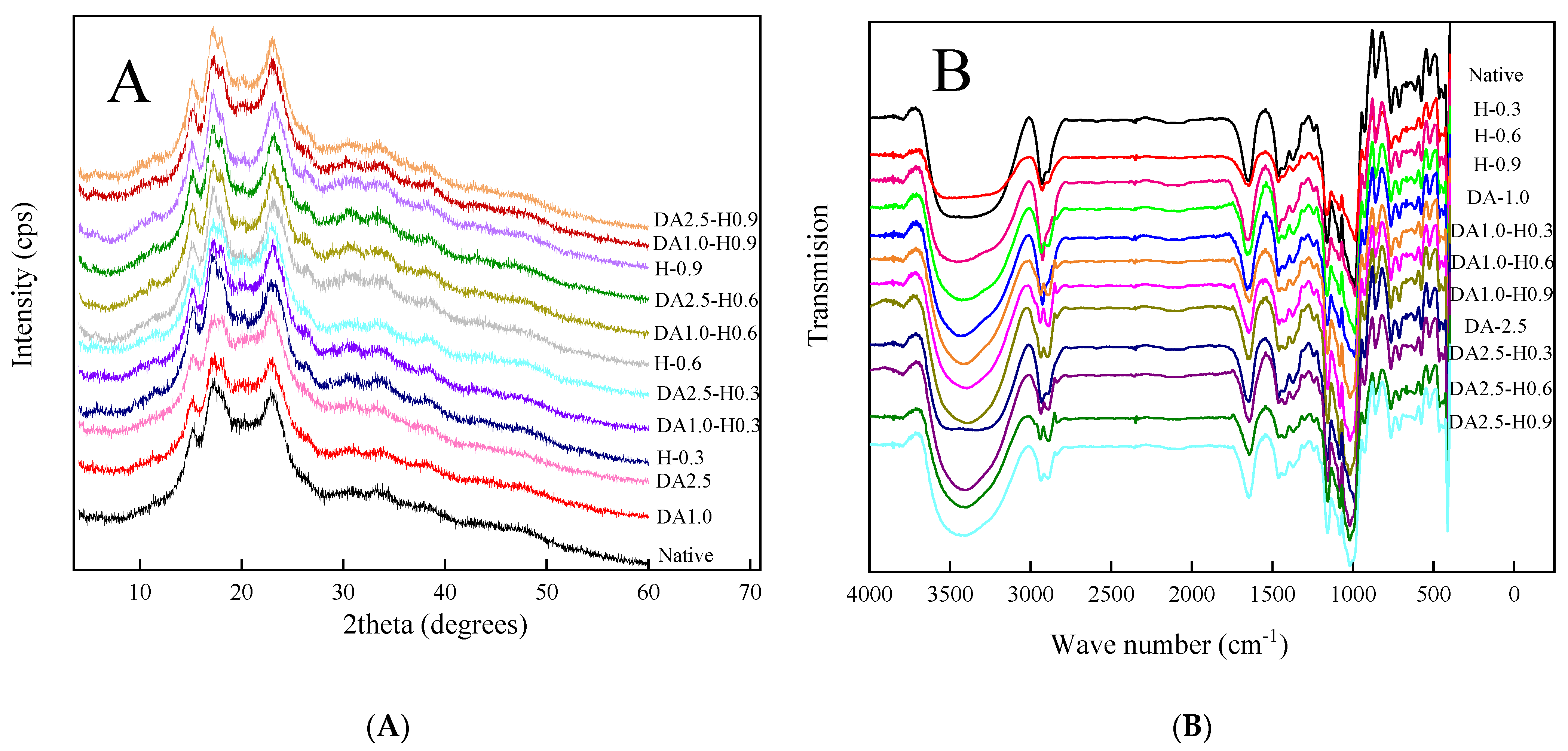
2.8. X-ray Diffraction (XRD) Measurement
2.9. Short-Range Ordering
2.10. Amylose Content
2.11. Measurement of Pasting Properties
2.12. Measurement of Solubility and Swelling Power
2.13. Statistics Analysis
3. Results
3.1. Morphological Characteristics
3.2. Chain Length Distribution
3.3. X-ray Diffraction (XRD)
3.4. FT-IR Spectra
3.5. Amylose Content
3.6. Pasting Properties
3.7. Solubility and Swelling Power
4. Discussion
4.1. Morphological Characteristics
4.2. Chain Length Distribution
4.3. X-ray Diffraction (XRD) Interpretation
4.4. FT-IR Spectra
4.5. Amylose Content Analysis
4.6. Pasting Property Evaluation
4.7. Solubility and Swelling Power Analysis
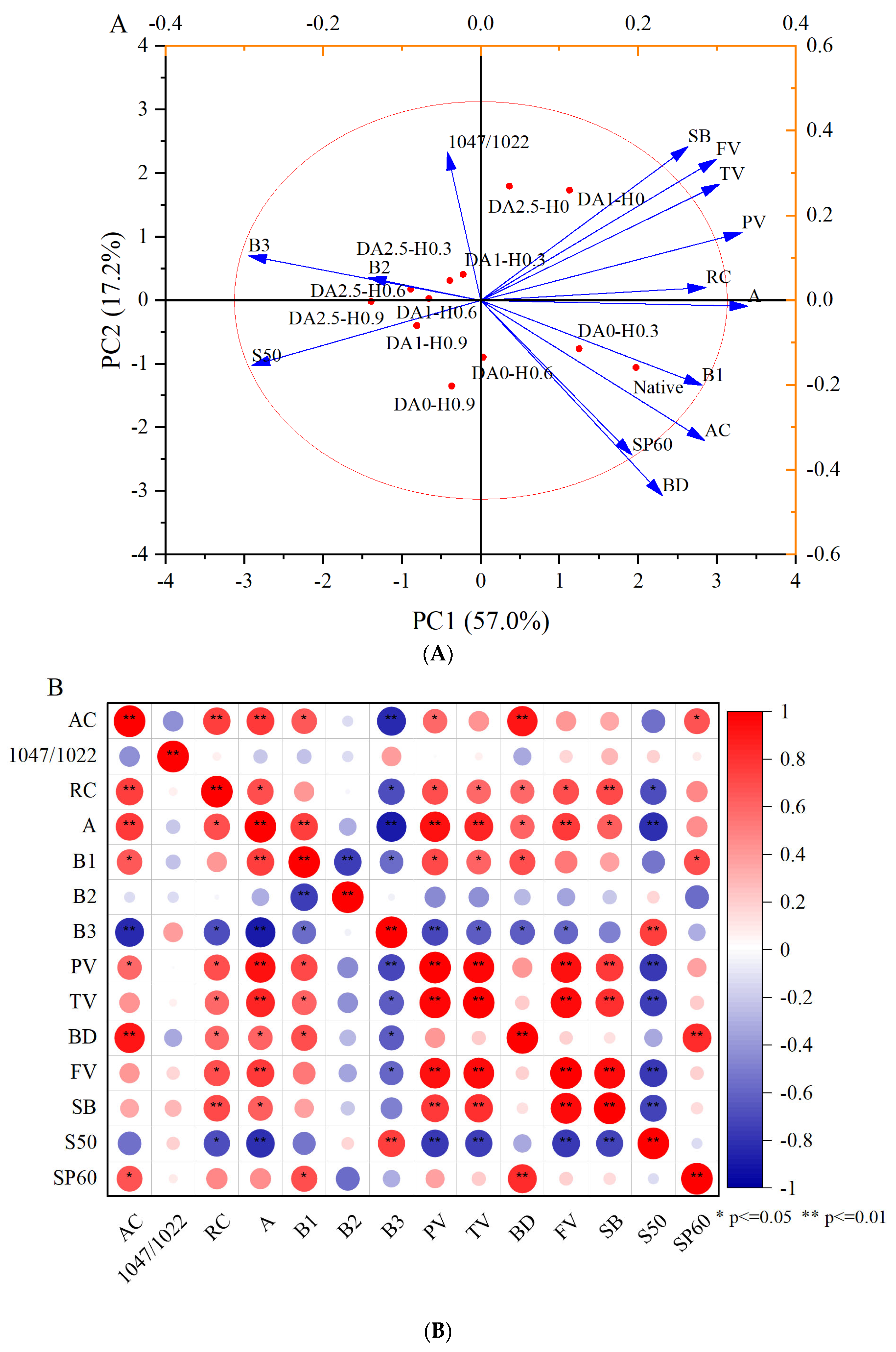
4.8. Principal Component Analysis
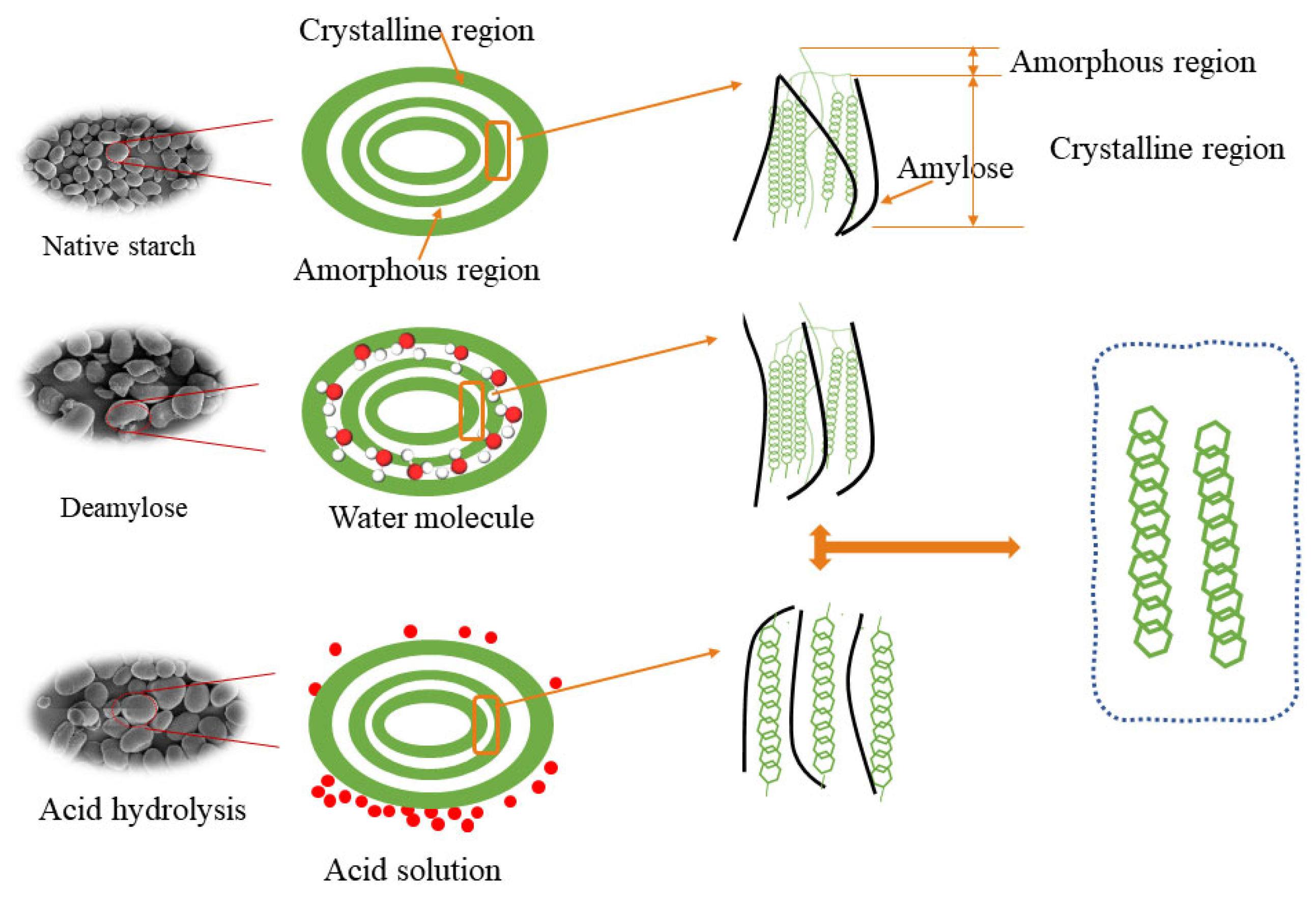
4.9. Mechanism of EBI Pretreatment Modification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Yang, Q.; Yang, Y.; Zhong, H. The effects of high-pressure treatment on the structure, physicochemical properties and digestive property of starch—A review. Int. J. Biol. Macromol. 2023, 244, 125376. [Google Scholar]
- Ge, X.; Shen, H.; Su, C.; Zhang, B.; Zhang, Q.; Jiang, H.; Li, W. The improving effects of cold plasma on multi-scale structure, physicochemical and digestive properties of dry heated red adzuki bean starch. Food Chem. 2021, 349, 129159. [Google Scholar] [PubMed]
- Yang, X.; Pan, Y.; Li, S.; Li, C.; Li, E. Effects of amylose and amylopectin molecular structures on rheological, thermal and textural properties of soft cake batters. Food Hydrocoll. 2022, 133, 107980. [Google Scholar]
- Shi, P.; Zhao, Y.; Qin, F.; Liu, K.; Wang, H. Understanding the multi-scale structure and physicochemical properties of millet starch with varied amylose content. Food Chem. 2023, 410, 135422. [Google Scholar]
- Soler, A.; Valenzuela-Díaz, E.D.; Velazquez, G.; Huerta-Ruelas, J.A.; Morales-Sanchez, E.; Hernandez-Gama, R.; Mendez-Montealvo, G. Double helical order and functional properties of acid-hydrolyzed maize starches with different amylose content. Carbohyd. Res. 2020, 490, 107956. [Google Scholar]
- Borah, A.; Das, D.K.; Mukhopadhyay, R.; Mahanta, C.L. Investigation of low amylose rice extrudates blended with germinated green gram and bhimkol flour. Appl. Food Res. 2023, 3, 100315. [Google Scholar]
- Borah, A.; Das, D.K.; Hazarika, M.K.; Mukhopadhyay, R.; Mahanta, C.L. Low-cost healthy extrudates of rice and bhimkol (Musa balbisiana, ABB) formulated through linear programming. J. Food Process Eng. 2019, 42, 13201. [Google Scholar]
- Jiang, M.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Effects of acid hydrolysis intensity on the properties of starch/xanthan mixtures. Int. J. Biol. Macromol. 2018, 106, 320–329. [Google Scholar]
- Li, H.; Wang, R.; Liu, J.; Zhang, Q.; Li, G.; Shan, Y.; Ding, S. Effects of heat-moisture and acid treatments on the structural, physicochemical, and in vitro digestibility properties of lily starch. Int. J. Biol. Macromol. 2020, 148, 956–968. [Google Scholar] [PubMed]
- Li, H.; Lei, N.; Yan, S.; Gao, M.; Yang, J.; Wang, J.; Sun, B. Molecular causes for the effect of cooking methods on rice stickiness: A mechanism explanation from the view of starch leaching. Int. J. Biol. Macromol. 2019, 128, 49–53. [Google Scholar]
- Ulbrich, M.; Daler, J.M.; Flöter, E. Acid hydrolysis of corn starch genotypes. I. Impact on morphological and molecular properties. Carbohyd. Polym. 2019, 219, 172–180. [Google Scholar]
- Ulbrich, M.; Daler, J.M.; Flöter, E. Acid hydrolysis of corn starch genotypes. II. Impact on functional properties. Food Hydrocoll. 2020, 98, 105249. [Google Scholar]
- Li, H.; Xu, M.; Yan, S.; Liu, R.; Ma, Z.; Wen, Y.; Wang, J.; Sun, B. Insights into waxy maize starch degradation by sulfuric acid: Impact on starch structure, pasting, and rheological property. Int. J. Biol. Macromol. 2020, 165 Pt A, 214–221. [Google Scholar]
- Li, W.; Tian, X.; Liu, L.; Wang, P.; Wu, G.; Zheng, J.; Zhang, G. High pressure induced gelatinization of red adzuki bean starch and its effects on starch physicochemical and structural properties. Food Hydrocoll. 2015, 45, 132–139. [Google Scholar] [CrossRef]
- Su, C.; Saleh, A.S.M.; Zhang, B.; Zhao, K.; Ge, X.; Zhang, Q.; Li, W. Changes in structural, physicochemical, and digestive properties of normal and waxy wheat starch during repeated and continuous annealing. Carbohyd. Polym. 2020, 247, 116675. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Liu, N.; Wang, J.; Huang, Y.; Wang, F.; Cao, J.; Fan, R.; Zhang, X.; et al. The effects of dielectric barrier discharge plasma on physicochemical and digestion properties of starch. Int. J. Biol. Macromol. 2019, 138, 819–830. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, H.; Liu, P.; Wang, W.; Dong, H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocoll. 2019, 87, 173–179. [Google Scholar] [CrossRef]
- Włodarczyk-Stasiak, M.; Mazurek, A.; Jamroz, J.; Pikus, S.; Kowalski, R. Physicochemical properties and structure of hydrothermally modified starches. Food Hydrocoll. 2019, 95, 88–97. [Google Scholar] [CrossRef]
- Singh, M.; Adedeji, A.A. Characterization of hydrothermal and acid modified proso millet starch. LWT—Food Sci Technol. 2017, 79, 21–26. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. Effects of acid hydrolysis on the evolution of starch fine molecular structures and gelatinization properties. Food Chem. 2021, 353, 129449. [Google Scholar] [PubMed]
- Yu, B.; Li, J.; Tao, H.; Zhao, H.; Liu, P.; Cui, B. Physicochemical properties and in vitro digestibility of hydrothermal treated Chinese yam (Dioscorea opposita Thunb.) starch and flour. Int. J. Biol. Macromol. 2021, 176, 177–185. [Google Scholar]
- Chen, P.; Xie, F.; Zhao, L.; Qiao, Q.; Liu, X. Effect of acid hydrolysis on the multi-scale structure change of starch with different amylose content. Food Hydrocoll. 2017, 69, 359–368. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, N.; Lim, S.T. A comparison of native and acid thinned normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT—Food Sci. Technol. 2007, 40, 1527–1536. [Google Scholar]
- Aparicio-Saguilán, A.; Aguirre-Cruz, A.; Méndez-Montealvo, G.; Rodriguez-Ambriz, S.L.; Garcia-Suarez, F.J.; Páramo-Calderón, D.E.; Bello-Pérez, L.A. The effect of the structure of native banana starch from two varieties on its acid hydrolysis. LWT—Food Sci Technol. 2014, 58, 381–386. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Yang, Y.; Yu, X.; Xu, L.; Jiao, A.; Jin, Z. Structure, physicochemical properties and in vitro digestibility of extruded starch-lauric acid complexes with different amylose contents. Food Hydrocoll. 2023, 136, 108239. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Navaf, M.; Bhasha, S.A.; George, J.; Mounir, S.; Kumar, S.; Sajeevkumar, V.A. Hydrothermal modifications of nonconventional kithul (Caryota urens) starch: Physico-chemical, rheological properties and in vitro digestibility. J. Food Sci. Technol. 2020, 57, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Amini, A.; Razavi, S.M.A. Ultrasound-assisted acid-thinning of corn starch: Morphological, physicochemical, and rheological properties. Stärke 2015, 67, 640–653. [Google Scholar] [CrossRef]
- Kasemwong, K.; Piyachomkwan, K.; Wansuksri, R.; Sriroth, K. Granule sizes of canna (Canna edulis) starches and their reactivity toward hydration, enzyme hydrolysis and chemical substitution. Stärke 2010, 60, 624–633. [Google Scholar] [CrossRef]
- Chen, L.; Ma, R.; Zhang, Z.; Huang, M.; Cai, C.; Zhang, R.; Jin, Z. Comprehensive investigation and comparison of surface microstructure of fractionated potato starches. Food Hydrocoll. 2019, 89, 11–19. [Google Scholar] [CrossRef]
- Gao, H.; Cai, J.; Han, W.; Huai, H.; Chen, Y.; Wei, C. Comparison of starches isolated from three different Trapa species. Food Hydrocoll. 2014, 37, 174–181. [Google Scholar] [CrossRef]
- Huang, T.T.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocoll. 2016, 54, 202–210. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, X.; Si, F.; Xiong, L. Effect of acid hydrolysis combined with heat moisture treatment on structure and physicochemical properties of corn starch. J. Food Sci. Technol. 2015, 52, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Blazek, J.; Gilbert, E.; Copeland, L. New insights on the mechanism of acid degradation of pea starch. Carbohyd. Polym. 2012, 87, 1941–1949. [Google Scholar] [CrossRef]
- Qiao, D.; Yu, L.; Liu, H.; Zou, W.; Xie, F.; Simon, G.; Petinakis, E.; Shen, Z.; Chen, L. Insights into the hierarchical structure and digestion rate of alkali-modulated starches with different amylose contents. Carbohyd. Polym. 2016, 144, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Hu, X.; Zhang, B.; Li, H.; Xu, X.; Jin, Z.; Tian, Y. Effect of defatting on acid hydrolysis rate of maize starch with different amylose contents. Int. J. Biol. Macromol. 2013, 62, 652–656. [Google Scholar] [CrossRef]
| Samples | AC (%) | 1047 cm−1/1022 cm−1 | RC (%) | Chain Length Distributions (%) | ||||
|---|---|---|---|---|---|---|---|---|
| DA | HCl | DP6–12 (A) | DP13–24 (B1) | DP25–36 (B2) | DP > 36 (B3) | |||
| 0 | 0 | 29.74 ± 0.07 a | 1.025 ± 0.000 f | 25.53 ± 0.19 c | 44.85 ± 0.93 a | 39.17 ± 0.94 a | 14.73 ± 0.61 f | 1.26 ± 0.62 g |
| 0.3 | 29.27 ± 0.03 b | 1.049 ± 0.01 bcd | 27.28 ± 0.26 a | 42.36 ± 0.51 bc | 37.25 ± 0.35 b | 16.26 ± 0.36 de | 4.14 ± 1.22 ef | |
| 0.6 | 28.63 ± 0.03 c | 1.043 ± 0.002 cde | 26.09 ± 0.17 b | 41.46 ± 0.20 cd | 33.71 ± 0.4 de | 19.96 ± 1.35 b | 4.88 ± 0.74 de | |
| 0.9 | 28.21 ± 0.00 d | 1.025 ± 0.002 f | 24.61 ± 0.15 d | 40.30 ± 0.56 de | 34.89 ± 0.54 cd | 18.52 ± 0.68 bc | 6.30 ± 0.41 cd | |
| 1 | 0 | 27.64 ± 0.03 e | 1.046 ± 0.003 bcd | 26.23 ± 0.23 b | 42.90 ± 0.15 bcde | 35.57 ± 0.66 c | 18.84 ± 0.48 bc | 2.70 ± 0.99 fg |
| 0.3 | 27.27 ± 0.07 f | 1.041 ± 0.004 de | 25.37 ± 0.20 c | 41.54 ± 0.66 cd | 33.00 ± 1.28 e | 21.69 ± 0.97 a | 3.79 ± 0.97 ef | |
| 0.6 | 26.80 ± 0.03 g | 1.037 ± 0.002 e | 24.08 ± 0.16 e | 40.16 ± 0.63 ef | 34.15 ± 0.21 cde | 19.00 ± 0.01 bc | 6.70 ± 0.42 bc | |
| 0.9 | 26.46 ± 0.10 h | 1.021 ± 0.008 f | 22.20 ± 0.21 f | 40.40 ± 0.71 de | 34.43 ± 0.89 cde | 18.44 ± 0.79 bc | 6.73 ± 0.61 bc | |
| 2.5 | 0 | 26.39 ± 0.07 h | 1.051 ± 0.011 abc | 25.54 ± 0.22 c | 41.40 ± 0.56 cde | 34.19 ± 0.27 cde | 16.55 ± 0.64 de | 7.87 ± 0.19 abc |
| 0.3 | 26.01 ± 0.10 i | 1.058 ± 0.004 a | 24.37 ± 0.21 de | 40.45 ± 0.35 de | 35.48 ± 0.67 c | 15.83 ± 0.24 ef | 8.25 ± 0.78 ab | |
| 0.6 | 25.67 ± 0.03 j | 1.053 ± 0.006 ab | 22.12 ± 014 f | 40.42 ± 0.17 de | 34.20 ± 0.57 cde | 17.53 ± 0.11 cd | 7.85 ± 0.49 abc | |
| 0.9 | 25.61 ± 0.03 j | 1.049 ± 0.001 bcd | 21.57 ± 0.06 g | 39.06 ± 0.36 f | 33.83 ± 0.24 de | 17.72 ± 0.40 cd | 9.40 ± 0.28 a | |
| DA | HCl/ | PV (cP) | TV (cP) | BD (cP) | FV (cP) | SB (cP) | PT (min) | GT (°C) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 4510 ± 76 a | 3798 ± 42 a | 712 ± 34 b | 5094 ± 122 c | 1296 ± 81 d | 4.57 ± 0.05 e | 73.93 ± 0.60 fg |
| 0.3 | 2599 ± 2 d | 1836 ± 15 d | 763 ± 13 a | 4169 ± 5 d | 2333 ± 20 c | 4.44 ± 0.05 ef | 73.45 ± 0.00 g | |
| 0.6 | 1552 ± 40 f | 1045 ± 32 f | 507 ± 8 c | 1725 ± 42 g | 681 ± 11 f | 4.30 ± 0.04 f | 74.70 ± 0.57 ef | |
| 0.9 | 1049 ± 8 g | 618 ± 2 h | 431 ± 10 d | 1003 ± 8 i | 386 ± 11 g | 4.27 ± 0.00 f | 75.10 ± 0.07 e | |
| 1 | 0 | 3514 ± 7 b | 3426 ± 11 b | 88 ± 18 gh | 6705 ± 34 a | 3279 ± 45 a | 5.63 ± 0.14 b | 77.90 ± 0.56 d |
| 0.3 | 1791 ± 11 e | 1523 ± 71 e | 203 ± 10 e | 2393 ± 115 e | 870 ± 44 e | 5.27 ± 0.00 cd | 78.30 ± 0.00 d | |
| 0.6 | 1043 ± 13 g | 908 ± 1 g | 135 ± 12 f | 1265 ± 8 h | 357 ± 7 g | 5.20 ± 0.00 d | 78.65 ± 0.56 cd | |
| 0.9 | 719 ± 1 i | 605 ± 4 h | 114 ± 4 fg | 810 ± 2 j | 205 ± 6 h | 5.14 ± 0.09 d | 78.68 ± 0.53 cd | |
| 2.5 | 0 | 3151 ± 17 c | 3121 ± 16 c | 30 ± 1 i | 5614 ± 120 b | 2493 ± 104 b | 6.44 ± 0.33 a | 79.53 ± 0.60 bc |
| 0.3 | 1598 ± 16 f | 1480 ± 10 e | 118 ± 6 fg | 2177 ± 13 f | 697 ± 23 f | 5.67 ± 0.00 b | 80.30 ± 0.49 ab | |
| 0.6 | 969 ± 14 h | 889 ± 16 g | 81 ± 2 h | 1190 ± 18 h | 302 ± 2 g | 5.57 ± 0.05 b | 81.15 ± 0.64 a | |
| 0.9 | 700 ± 9 i | 632 ± 5 h | 68 ± 4 h | 829 ± 12 j | 197 ± 7 h | 5.50 ± 0.04 bc | 80.73 ± 0.04 a |
| Treatment | Solubility (%) | Swelling Power (g/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | HCl | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C |
| 0 | 0 | 0.57 ± 0.23 de | 2.40 ± 0.08 c | 6.96 ± 0.66 de | 8.55 ± 0.17 g | 8.48 ± 0.32 h | 2.41 ± 0.04 d | 4.44 ± 0.06 ab | 9.73 ± 0.54 ab | 14.80 ± 0.08 a | 23.43 ± 0.24 a |
| 0.3 | 0.62 ± 0.08 cde | 3.37 ± 0.49 b | 7.82 ± 0.63 cd | 6.41 ± 0.49 j | 4.25 ± 0.28 j | 2.41 ± 0.07 d | 4.63 ± 0.18 a | 10.29 ± 0.11 a | 14.21 ± 0.28 b | 14.61 ± 0.80 e | |
| 0.6 | 0.93 ± 0.09 bcd | 3.77 ± 0.48 b | 7.05 ± 0.03 de | 10.09 ± 0.17 f | 12.03 ± 0.47 f | 2.26 ± 0.28 d | 4.29 ± 0.13 bc | 9.12 ± 0.75 bc | 12.28 ± 0.00 c | 15.72 ± 0.11 e | |
| 0.9 | 1.07 ± 0.27 b | 4.85 ± 0.10 a | 8.06 ± 0.69 bcd | 7.95 ± 0.46 gh | 21.79 ± 0.37 b | 2.18 ± 0.42 d | 4.08 ± 0.09 cd | 8.73 ± 0.55 cd | 10.54 ± 0.72 d | 17.89 ± 0.59 d | |
| 1 | 0 | 0.51 ± 0.06 e | 0.52 ± 0.04 f | 7.10 ± 0.01 de | 7.45 ± 0.75 hi | 3.78 ± 0.21 j | 3.31 ± 0.23 bc | 3.62 ± 0.16 efg | 8.23 ± 0.59 cde | 10.52 ± 0.16 d | 19.73 ± 0.42 c |
| 0.3 | 0.84 ± 0.30 bcde | 1.34 ± 0.17 e | 7.36 ± 0.28 d | 13.00 ± 0.12 d | 9.72 ± 0.23 g | 3.41 ± 0.26 abc | 3.42 ± 0.07 g | 8.32 ± 0.25 cde | 10.36 ± 0.23 de | 20.09 ± 0.35 c | |
| 0.6 | 0.82 ± 0.07 bcde | 1.29 ± 0.04 e | 9.00 ± 0.42 ab | 12.05 ± 0.23 e | 13.27 ± 0.56 e | 3.56 ± 0.14 ab | 3.44 ± 0.02 fg | 7.88 ± 0.49 de | 9.43 ± 0.06 fg | 22.58 ± 0.99 ab | |
| 0.9 | 0.80 ± 0.07 bcde | 1.62 ± 0.28 de | 9.23 ± 0.21 a | 14.35 ± 0.16 bc | 17.56 ± 0.67 c | 3.11 ± 0.06 c | 3.45 ± 0.31 fg | 7.61 ± 0.43 e | 8.83 ± 0.10 h | 15.06 ± 0.34 e | |
| 2.5 | 0 | 0.71 ± 0.15 bcde | 0.54 ± 0.08 f | 6.17 ± 0.66 e | 6.93 ± 0.12 ij | 6.51 ± 0.36 i | 3.63 ± 0.14 a | 3.70 ± 0.11 efg | 8.59 ± 0.37 cde | 10.26 ± 0.06 de | 21.65 ± 0.24 b |
| 0.3 | 0.99 ± 0.00 bc | 1.29 ± 0.01 e | 8.70 ± 0.19 abc | 13.99 ± 0.27 c | 8.02 ± 0.40 h | 3.49 ± 0.13 ab | 4.08 ± 0.21 cd | 7.80 ± 0.14 de | 9.86 ± 0.01 ef | 15.67 ± 0.23 e | |
| 0.6 | 1.00 ± 0.22 bc | 2.18 ± 0.57 cd | 8.98 ± 0.05 ab | 14.92 ± 0.05 b | 15.97 ± 0.53 d | 3.36 ± 0.05 abc | 3.89 ± 0.04 de | 8.03 ± 0.19 de | 9.61 ± 0.03 fg | 18.34 ± 0.55 d | |
| 0.9 | 1.43 ± 0.06 a | 2.31 ± 0.27 c | 9.38 ± 0.70 a | 16.62 ± 0.02 a | 29.43 ± 0.10 a | 3.41 ± 0.01 abc | 3.77 ± 0.06 def | 7.65 ± 0.09 e | 9.20 ± 0.02 gh | 15.48 ± 0.30 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Sun, Z.; Zhao, W.; Zheng, J.; Liang, W.; Li, W. Spotlight on the Multiscale Structural and Physicochemical Properties of Red Adzuki Bean Starch through Partial Amylose Removal Combined with Hydrochloric Acid. Foods 2023, 12, 3366. https://doi.org/10.3390/foods12183366
Liu X, Sun Z, Zhao W, Zheng J, Liang W, Li W. Spotlight on the Multiscale Structural and Physicochemical Properties of Red Adzuki Bean Starch through Partial Amylose Removal Combined with Hydrochloric Acid. Foods. 2023; 12(18):3366. https://doi.org/10.3390/foods12183366
Chicago/Turabian StyleLiu, Xinyue, Zhuangzhuang Sun, Wenqing Zhao, Jiayu Zheng, Wei Liang, and Wenhao Li. 2023. "Spotlight on the Multiscale Structural and Physicochemical Properties of Red Adzuki Bean Starch through Partial Amylose Removal Combined with Hydrochloric Acid" Foods 12, no. 18: 3366. https://doi.org/10.3390/foods12183366






