Impact of Six Extraction Methods on Molecular Composition and Antioxidant Activity of Polysaccharides from Young Hulless Barley Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch and Protein Removal from Young Hulless Barley Leaf Powders
2.3. Extraction of Hulless Barley Leaf Polysaccharide through Different Methods
2.3.1. Hot-Water Extraction
2.3.2. High-Pressure Steam Extraction
2.3.3. Alkaline Extraction
2.3.4. Extraction with Xylanase
2.3.5. Extraction with Cellulase
2.3.6. Combined Extraction with Both Xylanase and Cellulase
2.4. Monosaccharide Composition of Different Extracted Fibers
2.5. Molecular Weight Determination
2.6. Fourier Transform Infrared (FTIR) Analysis
2.7. Bioactivity Analyses
2.8. Rheological Property
2.9. Statistical Analysis
3. Results and Discussion
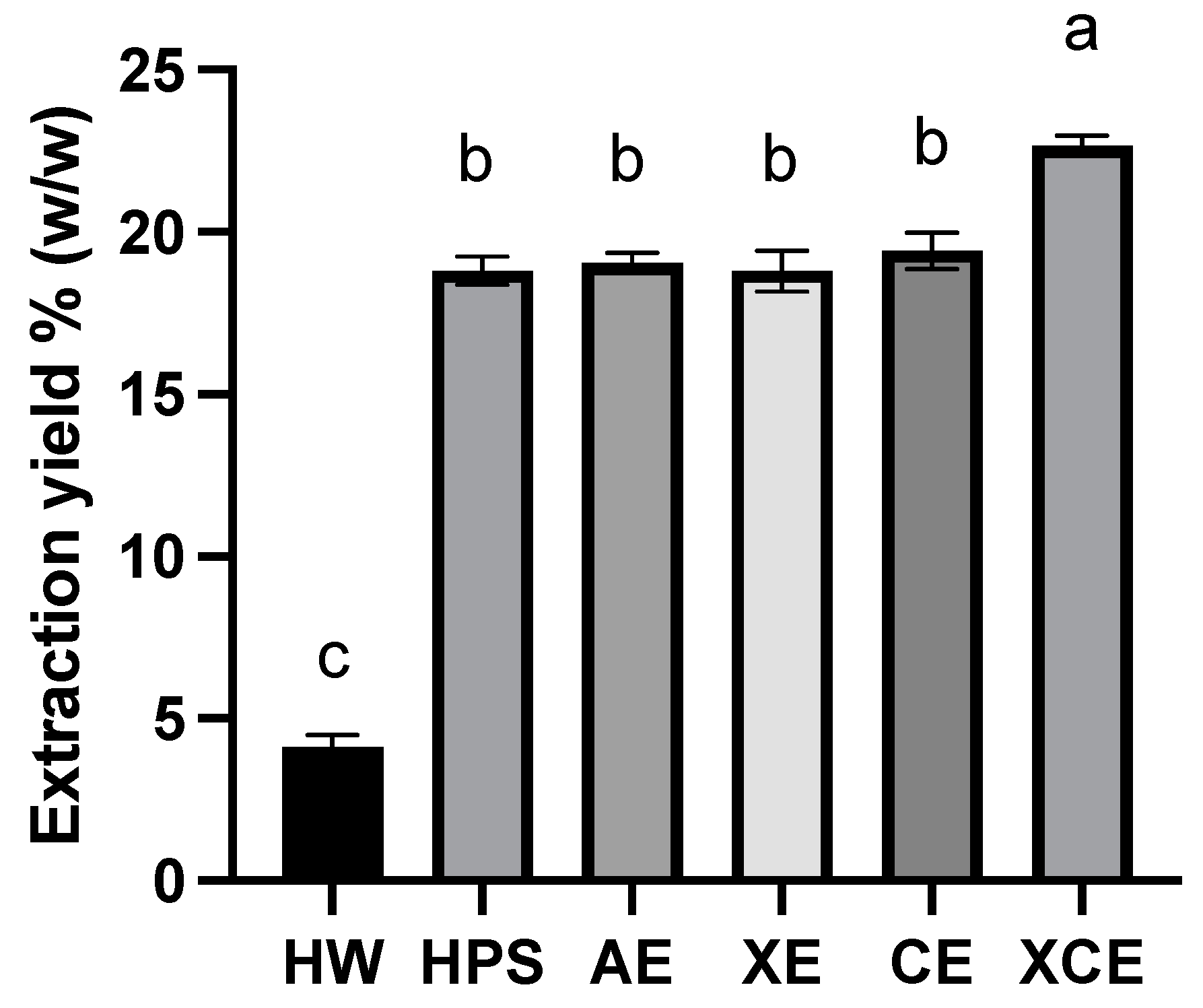
3.1. Extraction Yield
3.2. Monosaccharide Composition of Hulless Barley Leaf Polysaccharides Extracted Using Different Methods
3.3. Molecular Weight Distribution of Extracted Hulless Barley Leaf Polysaccharides
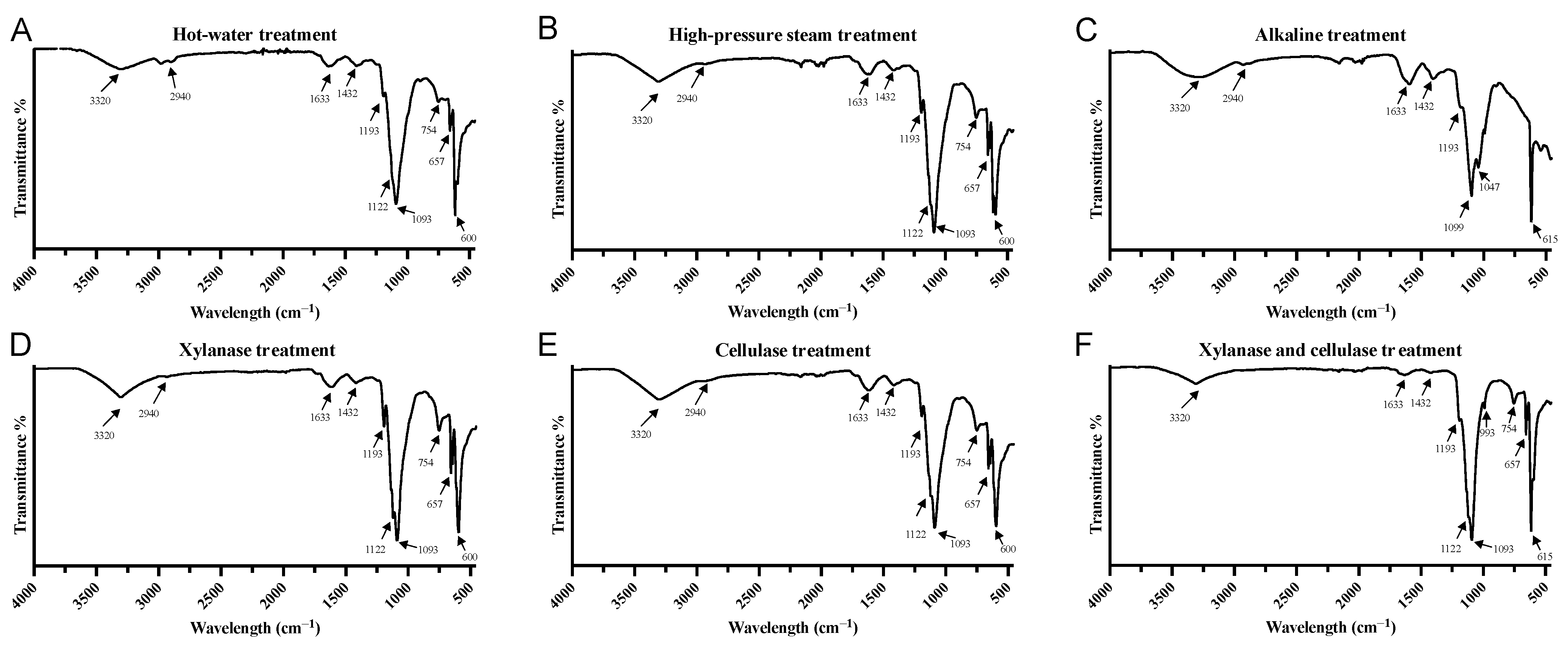
3.4. FTIR Spectrum Analysis
3.5. Radical Scavenging Bioactivities of Extracted Hulless Barley Leaf Fibers
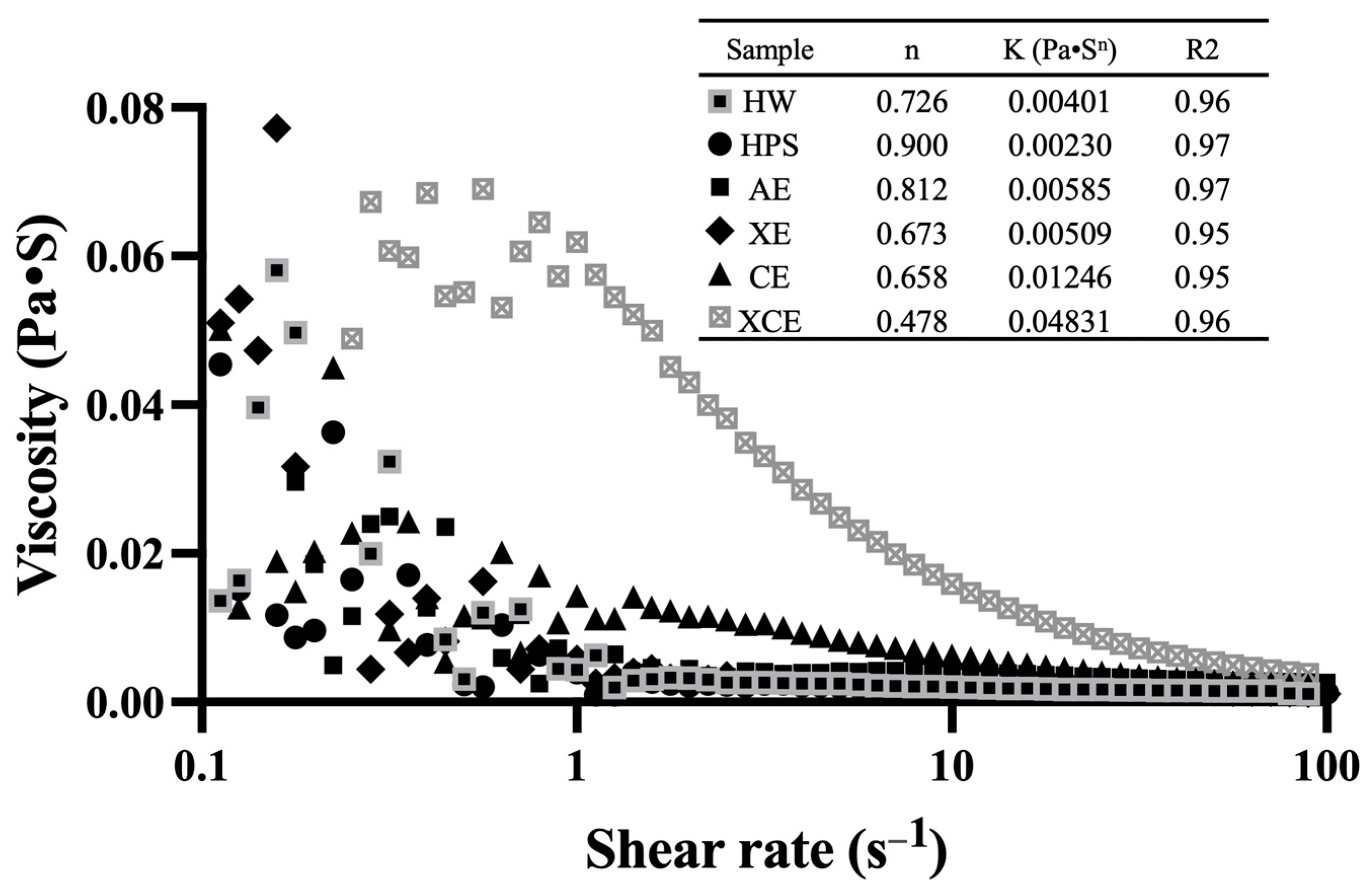
3.6. Rheological Property
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, X.; Kasapis, S.; Zhu, P.; Sui, Z.; Bao, J.; Corke, H. Physicochemical and Structural Characteristics of Starches from Chinese Hull-Less Barley Cultivars. Int. J. Food Sci. Technol. 2016, 51, 509–518. [Google Scholar] [CrossRef]
- Storsley, J.M.; Izydorczyk, M.S.; You, S.; Biliaderis, C.G.; Rossnagel, B. Structure and Physicochemical Properties of β-Glucans and Arabinoxylans Isolated from Hull-Less Barley. Food Hydrocoll. 2003, 17, 831–844. [Google Scholar] [CrossRef]
- Zeng, Y.; Pu, X.; Du, J.; Yang, X.; Li, X.; Mandal, M.S.N.; Yang, T.; Yang, J. Molecular Mechanism of Functional Ingredients in Barley to Combat Human Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, e3836172. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Ma, S.; Liu, J.; Wang, X. A Systematic Review of Highland Barley: Ingredients, Health Functions and Applications. Grain Oil Sci. Technol. 2022, 5, 35–43. [Google Scholar] [CrossRef]
- Takano, A.; Kamiya, T.; Tomozawa, H.; Ueno, S.; Tsubata, M.; Ikeguchi, M.; Takagaki, K.; Okushima, A.; Miyata, Y.; Tamaru, S.; et al. Insoluble Fiber in Young Barley Leaf Suppresses the Increment of Postprandial Blood Glucose Level by Increasing the Digesta Viscosity. Evid.-Based Complement. Altern. Med. 2013, 2013, e137871. [Google Scholar] [CrossRef] [PubMed]
- EunJu, Y.; YoungSook, C.; MyungSook, C.; MyoungNam, W.; MyungJoo, K.; MiYae, S.; MiKyung, L. Effect of young barley leaf on lipid contents and hepatic lipid-regulating enzyme activities in mice fed high-fat diet. Korean J. Nutr. 2009, 42, 14–22. [Google Scholar]
- Yamaura, K.; Nakayama, N.; Shimada, M.; Bi, Y.; Fukata, H.; Ueno, K. Antidepressant-like Effects of Young Green Barley Leaf (Hordeum vulgare L.) in the Mouse Forced Swimming Test. Pharmacogn. Res. 2012, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Panthi, M.; Subba, R.K.; Raut, B.; Khanal, D.P.; Koirala, N. Bioactivity Evaluations of Leaf Extract Fractions from Young Barley Grass and Correlation with Their Phytochemical Profiles. BMC Complement. Med. Ther. 2020, 20, 64. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with Potent Antioxidant Activity Found in Young Green Barley Leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Chang, W.-C.; Chang, C.-T.; Hsieh, C.-L.; Tsai, C.E. Effects of Young Barley Leaf Extract and Antioxidative Vitamins on LDL Oxidation and Free Radical Scavenging Activities in Type 2 Diabetes. Diabetes Metab. 2002, 28, 107–114. [Google Scholar]
- Zeng, Y.; Du, J.; Pu, X.; Yang, J.; Yang, T.; Yang, S.; Yang, X. Coevolution between Cancer Activities and Food Structure of Human Being from Southwest China. BioMed Res. Int. 2015, 2015, e497934. [Google Scholar] [CrossRef] [PubMed]
- Blicharz-Kania, A.; Andrejko, D.; Kluza, F.; Rydzak, L.; Kobus, Z. Assessment of the Potential Use of Young Barley Shoots and Leaves for the Production of Green Juices. Sustainability 2019, 11, 3960. [Google Scholar] [CrossRef]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Smith, C.; Li, W. Extraction and Modification Technology of Arabinoxylans from Cereal By-Products: A Critical Review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Gong, L.; Huang, L.; Zhang, Y. Effect of Steam Explosion Treatment on Barley Bran Phenolic Compounds and Antioxidant Capacity. J. Agric. Food Chem. 2012, 60, 7177–7184. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Bourgine, J.; Thomsen, M. Closing the Loop of Cereal Waste and Residues with Sustainable Technologies: An Overview of Enzyme Production via Fungal Solid-State Fermentation. Sustain. Prod. Consum. 2021, 27, 845–857. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Liao, Z.; Li, Z.; Xu, X.; Sui, Z.; Corke, H. Optimization of Soluble Dietary Fiber Extraction from Hulless Barley Grass. Cereal Chem. 2022, 99, 482–492. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kawk, H.-W.; Kim, S.-H.; Lee, H.-J.; Seo, J.-W.; Kim, J.-T.; Jang, S.-H.; Kim, M.-J.; Kim, Y.-M. Anti-Obesity Effect of Hot Water Extract of Barley Sprout through the Inhibition of Adipocyte Differentiation and Growth. Metabolites 2021, 11, 610. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Z.; Dong, H.; Ma, C.; Qiao, Y.; Zheng, Z. Structural Characterization and Antioxidant Activities of One Neutral Polysaccharide and Three Acid Polysaccharides from the Roots of Arctium lappa L.: A Comparison. Int. J. Biol. Macromol. 2021, 182, 187–196. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, J.; Liu, X.; Li, X.; Sun, X.; Kou, Y.; Li, D.; Liu, Y.; Zhang, H.; Wu, Y. Pectic Polysaccharides from Biluochun Tea: A Comparative Study in Macromolecular Characteristics, Fine Structures and Radical Scavenging Activities in Vitro. Int. J. Biol. Macromol. 2022, 195, 598–608. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.J.T.J.; Sebastiaan Dallinga, J.; Voss, H.-P.; Haenen, G.R.M.M.; Bast, A. A New Approach to Assess the Total Antioxidant Capacity Using the TEAC Assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Ballesteros, I.; Negro, M.J.; Castro, E. Enhanced Enzymatic Hydrolysis of Olive Tree Wood by Steam Explosion and Alkaline Peroxide Delignification. Process Biochem. 2006, 41, 423–429. [Google Scholar] [CrossRef]
- Fincher, G.B.; Stone, B.A. Cell Walls and Their Components in Cereal Grain Technology. Adv. Cereal Sci. Technol. 1986, 8, 207–295. [Google Scholar]
- Höije, A.; Gröndahl, M.; Tømmeraas, K.; Gatenholm, P. Isolation and Characterization of Physicochemical and Material Properties of Arabinoxylans from Barley Husks. Carbohydr. Polym. 2005, 61, 266–275. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.; Galarza, S.; Iza, D.; Silva, E. Nutritional Value and Bioactive Compounds of Leaves and Grains from Quinoa (Chenopodium quinoa Willd.). Plants 2022, 11, 213. [Google Scholar] [CrossRef]
- Paice, M.G.; Bourbonnais, R.; Desrochers, M.; Jurasek, L.; Yaguchi, M. A Xylanase Gene from Bacillus Subtilis: Nucleotide Sequence and Comparison with B. Pumilus Gene. Arch. Microbiol. 1986, 144, 201–206. [Google Scholar] [CrossRef]
- Escarnot, E.; Aguedo, M.; Paquot, M. Enzymatic Hydrolysis of Arabinoxylans from Spelt Bran and Hull. J. Cereal Sci. 2012, 55, 243–253. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Saeed, F.; Ahmad, N.; Imran, A.; Niaz, B.; Afzaal, M.; Imran, M.; Tufail, T.; Javed, A. Functional and Health-Endorsing Properties of Wheat and Barley Cell Wall’s Non-Starch Polysaccharides. Int. J. Food Prop. 2018, 21, 1463–1480. [Google Scholar] [CrossRef]
- Kim, H.; Kwak, B.-S.; Hong, H.-D.; Suh, H.-J.; Shin, K.-S. Structural Features of Immunostimulatory Polysaccharide Purified from Pectinase Hydrolysate of Barley Leaf. Int. J. Biol. Macromol. 2016, 87, 308–316. [Google Scholar] [CrossRef]
- Roubroeks, J.P.; Andersson, R.; Åman, P. Structural Features of (1→3),(1→4)-β-d-Glucan and Arabinoxylan Fractions Isolated from Rye Bran. Carbohydr. Polym. 2000, 42, 3–11. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Din, A. Physicochemical and Functional Properties of Barley β-Glucan as Affected by Different Extraction Procedures. Int. J. Food Sci. Technol. 2009, 44, 181–187. [Google Scholar] [CrossRef]
- Kale, M.S.; Hamaker, B.R.; Campanella, O.H. Alkaline Extraction Conditions Determine Gelling Properties of Corn Bran Arabinoxylans. Food Hydrocoll. 2013, 31, 121–126. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Buchanan, N.L.; Debenham, J.S.; Gatenholm, P.; Jacobsson, M.; Shelton, M.C.; Watterson, T.L.; Wood, M.D. Preparation and Characterization of Arabinoxylan Esters and Arabinoxylan Ester/Cellulose Ester Polymer Blends. Carbohydr. Polym. 2003, 52, 345–357. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Kim, H.; Yu, K.-W.; Hong, H.-D.; Shin, K.-S. Effect of Arabinoxylan- and Rhamnogalacturonan I-Rich Polysaccharides Isolated from Young Barley Leaf on Intestinal Immunostimulatory Activity. J. Funct. Foods 2017, 35, 384–390. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A Comparison of a Polysaccharide Extracted from Ginger (Zingiber officinale) Stems and Leaves Using Different Methods: Preparation, Structure Characteristics, and Biological Activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Li, X.; Zhang, L. Molecular Mass and Chain Conformations of Rhizoma Panacis Japonici Polysaccharides. Carbohydr. Polym. 2009, 78, 596–601. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Qi, M.; Huang, W.; Sui, Z.; Corke, H. Chemical Characterization and In Vitro Anti-Cancer Activities of a Hot Water Soluble Polysaccharide from Hulless Barley Grass. Foods 2022, 11, 677. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Xin, Y.; Yin, J.-Y.; Huang, X.-J.; Wang, J.-Q.; Hu, J.-L.; Geng, F.; Nie, S.-P. Revealing the Architecture and Solution Properties of Polysaccharide Fractions from Macrolepiota albuminosa (Berk.) Pegler. Food Chem. 2022, 368, 130772. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the Contribution of ATR-FTIR Spectroscopy to Characterize Plant Cell Wall Polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Han, Q.; Liu, N.; Robinson, H.; Cao, L.; Qian, C.; Wang, Q.; Xie, L.; Ding, H.; Wang, Q.; Huang, Y.; et al. Biochemical Characterization and Crystal Structure of a GH10 Xylanase from Termite Gut Bacteria Reveal a Novel Structural Feature and Significance of Its Bacterial Ig-like Domain. Biotechnol. Bioeng. 2013, 110, 3093–3103. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH Methods as a Tool for Studying Antioxidant Capacity of Spring Barley and Malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free Radical Scavenging Properties of Wheat Extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Evageliou, V. Shear and Extensional Rheology of Selected Polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 1853–1861. [Google Scholar] [CrossRef]

| Mole Ratio (%) | HW | HPS | AE | XE | CE | XCE |
|---|---|---|---|---|---|---|
| Fucose | 0.00 ± 0.00 b | 0.57 ± 0.10 a | 0.49 ± 0.07 a | 0.62 ± 0.13 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Rhamnose | 2.57 ± 0.11 d | 4.62 ± 0.32 c | 2.14 ± 0.19 d | 6.82 ± 0.14 b | 7.77 ± 1.91 ab | 9.24 ± 1.09 a |
| Arabinose | 16.20 ± 0.90 b | 18.49 ± 1.81 ab | 17.91 ± 0.80 ab | 19.02 ± 0.36 ab | 18.01 ± 3.76 ab | 20.94 ± 0.95 a |
| Galactose | 31.00 ± 1.19 bc | 35.05 ± 4.38 bc | 24.54 ± 0.84 c | 39.38 ± 5.94 ab | 47.84 ± 0.71 a | 46.34 ± 9.70 a |
| Glucose | 34.00 ± 0.70 a | 13.70 ± 3.24 b | 13.73 ± 2.05 b | 7.06 ± 4.48 b | 13.58 ± 12.41 b | 9.65 ± 5.26 b |
| Xylose | 11.50 ± 0.40 c | 20.57 ± 0.03 b | 36.24 ± 2.29 a | 13.45 ± 0.21 c | 6.17 ± 2.00 d | 6.60 ± 0.27 d |
| Mannose | 0.00 ± 0.00 b | 0.01 ± 0.01 b | 0.16 ± 0.22 b | 2.56 ± 0.74 a | 0.23 ± 0.16 b | 0.00 ± 0.00 b |
| GalA | 3.56 ± 0.93 b | 5.12 ± 3.35 ab | 3.65 ± 1.58 b | 9.30 ± 2.02 a | 4.43 ± 3.73 ab | 5.05 ± 2.65 ab |
| GlcA | 1.16 ± 0.15 b | 1.87 ± 0.03 a | 1.15 ± 0.14 b | 1.78 ± 0.41 a | 1.98 ± 0.16 a | 2.18 ± 0.51 a |
| Sample | Mw (Da) | Mn (Da) | PDI |
|---|---|---|---|
| HW | 7.035 × 104 (±1.531%) | 6.449 × 104 (±1.622%) | 1.091 |
| HPS | 5.919 × 104 (±1.641%) | 5.122 × 104 (±1.605%) | 1.156 |
| AE | 6.987 × 104 (±14.466%) | 5.905 × 104 (±15.736%) | 1.183 |
| XE | 3.773 × 105 (±1.323%) | 3.332 × 105 (±1.598%) | 1.132 |
| CE | 2.842 × 105 (±1.201%) | 2.708 × 105 (±1.332%) | 1.050 |
| XCE | 1.441 × 105 (±2.071%) | 8.164 × 104 (±3.152%) | 1.765 |
| DPPH | ABTS A | FRAP | |
|---|---|---|---|
| Fucose | 0.16 | 0.5 | 0.12 |
| Rhamnose | −0.22 | −0.87 * | −0.12 |
| Arabinose | −0.03 | −0.56 | −0.54 |
| Galactose | −0.18 | −0.87 * | 0.11 |
| Glucose | −0.09 | 0.22 | 0.23 |
| Xylose | 0.43 | 0.92 ** | −0.21 |
| Mannose | −0.66 | −0.24 | 0.1 |
| GalA | −0.54 | −0.41 | 0.09 |
| GlcA | 0.07 | −0.74 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, C.; Xu, Y.; Ma, M.; Yao, T.; Sui, Z. Impact of Six Extraction Methods on Molecular Composition and Antioxidant Activity of Polysaccharides from Young Hulless Barley Leaves. Foods 2023, 12, 3381. https://doi.org/10.3390/foods12183381
Wang M, Zhang C, Xu Y, Ma M, Yao T, Sui Z. Impact of Six Extraction Methods on Molecular Composition and Antioxidant Activity of Polysaccharides from Young Hulless Barley Leaves. Foods. 2023; 12(18):3381. https://doi.org/10.3390/foods12183381
Chicago/Turabian StyleWang, Mingming, Chuangchuang Zhang, Yuting Xu, Mengting Ma, Tianming Yao, and Zhongquan Sui. 2023. "Impact of Six Extraction Methods on Molecular Composition and Antioxidant Activity of Polysaccharides from Young Hulless Barley Leaves" Foods 12, no. 18: 3381. https://doi.org/10.3390/foods12183381





