Probiotic Yeasts and How to Find Them—Polish Wines of Spontaneous Fermentation as Source for Potentially Probiotic Yeasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
Yeast Identification
2.2. Evaluation of Potentially Probiotic Traits
2.2.1. Survival and Growth at 37 °C
2.2.2. Survival and Growth under Gastrointestinal Tract Conditions
concentration of living cells before in vitro digestion × 100%
2.2.3. Hydrophobicity of Cell Surface
2.2.4. Autoaggregation Assay
2.2.5. Antioxidant Activity
2.2.6. Antimicrobial Activity
2.3. Traits Related to Safety and Virulence
2.3.1. Hemolytic Activity
2.3.2. Biogenic Amine Production
2.4. Enzymatic Activity
2.5. Statistical Analysis
3. Results
3.1. Yeast Identification
3.2. Evaluation of Potentially Probiotic Traits
3.2.1. Survival and Growth at 37 °C
3.2.2. Survival and Growth under Gastrointestinal Tract Conditions
3.2.3. Hydrophobicity of Cell Surface
3.2.4. Autoaggregation Assay
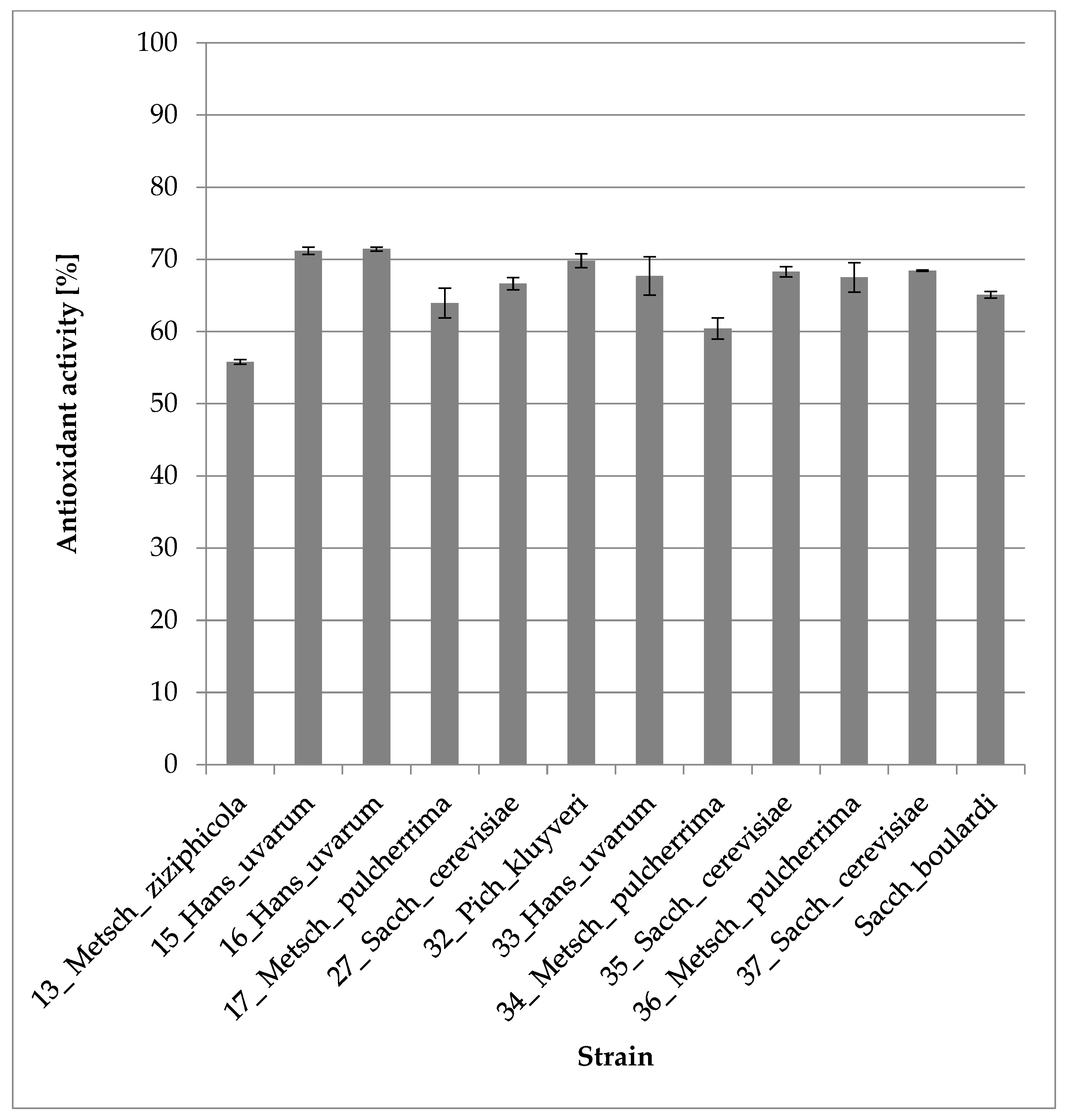
3.2.5. Antioxidant Activity
3.2.6. Antimicrobial Activity
3.3. Traits Related to Safety and Virulence
3.3.1. Hemolytic Activity
3.3.2. Biogenic Amine Production
3.4. Enzymatic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics: 10 Years Later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and Potentially Probiotic Yeasts—Characteristics and Food Application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- Jarocki, P.; Komoń-Janczara, E.; Młodzińska, A.; Sadurski, J.; Kołodzińska, K.; Łaczmański, Ł.; Panek, J.; Frąc, M. Occurrence and Genetic Diversity of Prophage Sequences Identified in the Genomes of L. Casei Group Bacteria. Sci. Rep. 2023, 13, 8603. [Google Scholar] [CrossRef]
- McFarland, L.V. From Yaks to Yogurt: The History, Development, and Current Use of Probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review Article: Yeast as Probiotics–Saccharomyces Boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The Gut Mycobiome in Health, Disease, and Clinical Applications in Association with the Gut Bacterial Microbiome Assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Ali, G.M.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Chen, L.S.; Ma, Y.; Maubois, J.L.; He, S.H.; Chen, L.J.; Li, H.M. Screening for the Potential Probiotic Yeast Strains from Raw Milk to Assimilate Cholesterol. Dairy Sci. Technol. 2010, 90, 537–548. [Google Scholar] [CrossRef]
- Arévalo-Villena, M.; Fernandez-Pacheco, P.; Castillo, N.; Bevilacqua, A.; Briones Pérez, A. Probiotic Capability in Yeasts: Set-up of a Screening Method. LWT 2018, 89, 657–665. [Google Scholar] [CrossRef]
- Fernandez-Pacheco Rodríguez, P.; Arévalo-Villena, M.; Zaparoli Rosa, I.; Briones Pérez, A. Selection of Potential Non-Sacharomyces Probiotic Yeasts from Food Origin by a Step-by-Step Approach. Food Res. Int. 2018, 112, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.; Ramos, A.; Reyes-Becerril, M.; Guerra, K.; Monreal-Escalante, E.; Angulo, C. Probiotic Debaryomyces hansenii CBS 8339 Yeast Enhanced Immune Responses in Mice. 3 Biotech 2023, 13, 28. [Google Scholar] [CrossRef]
- Homayouni-Rad, A.; Azizi, A.; Oroojzadeh, P.; Pourjafar, H. Kluyveromyces Marxianus as a Probiotic Yeast: A Mini-Review. Curr. Nutr. Food Sci. 2020, 16, 1163–1169. [Google Scholar] [CrossRef]
- Koshchayev, I.; Lavrinenko, K.; Medvedeva, P. Probiotic Drug Based on Kluyveromyces Marxianus for Poultry. E3S Web Conf. 2022, 363, 03070. [Google Scholar] [CrossRef]
- Ponomarova, O.; Gabrielli, N.; Sévin, D.C.; Mülleder, M.; Zirngibl, K.; Bulyha, K.; Andrejev, S.; Kafkia, E.; Typas, A.; Sauer, U.; et al. Yeast Creates a Niche for Symbiotic Lactic Acid Bacteria through Nitrogen Overflow. Cell Syst. 2017, 5, 345–357. [Google Scholar] [CrossRef]
- Sybesma, W.; Kort, R.; Lee, Y.K. Locally Sourced Probiotics, the next Opportunity for Developing Countries? Trends Biotechnol. 2015, 33, 197–200. [Google Scholar] [CrossRef]
- Tamang, J.P.; Lama, S. Probiotic Properties of Yeasts in Traditional Fermented Foods and Beverages. J. Appl. Microbiol. 2022, 132, 3533–3542. [Google Scholar] [CrossRef] [PubMed]
- Kunyeit, L.; Rao, R.P.; Anu-Appaiah, K.A. Yeasts Originating from Fermented Foods, Their Potential as Probiotics and Therapeutic Implication for Human Health and Disease. Crit. Rev. Food Sci. Nutr. 2023, 1–12. [Google Scholar] [CrossRef]
- de Miranda, N.M.Z.; de Souza, A.C.; de Souza Costa Sobrinho, P.; Dias, D.R.; Schwan, R.F.; Ramos, C.L. Novel Yeasts with Potential Probiotic Characteristics Isolated from the Endogenous Ferment of Artisanal Minas Cheese. Brazilian J. Microbiol. 2023, 54, 1021–1033. [Google Scholar] [CrossRef]
- Pytka, M.; Kordowska-Wiater, M.; Wajs, J.; Glibowski, P.; Sajnaga, E. Usefulness of Potentially Probiotic L. lactis Isolates from Polish Fermented Cow Milk for the Production of Cottage Cheese. Appl. Sci. 2022, 12, 12088. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Skotniczny, M. Biodiversity of Yeasts Isolated during Spontaneous Fermentation of Cool Climate Grape Musts. Arch. Microbiol. 2021, 203, 153–162. [Google Scholar] [CrossRef]
- García-Ruiz, A.; González de Llano, D.; Esteban-Fernández, A.; Requena, T.; Bartolomé, B.; Moreno-Arribas, M.V. Assessment of Probiotic Properties in Lactic Acid Bacteria Isolated from Wine. Food Microbiol. 2014, 44, 220–225. [Google Scholar] [CrossRef]
- Marzano, M.; Fosso, B.; Manzari, C.; Grieco, F.; Intranuovo, M.; Cozzi, G.; Mulè, G.; Scioscia, G.; Valiente, G.; Tullo, A.; et al. Complexity and Dynamics of the Winemaking Bacterial Communities in Berries, Musts, and Wines from Apulian Grape Cultivars through Time and Space. PLoS ONE 2016, 11, e0157383. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M.; Pytka, M.; Stój, A.; Kubik-Komar, A.; Wyrostek, J.; Waśko, A. A Metagenetic Insight into Microbial Diversity of Spontaneously Fermented Polish Red Wines and an Analysis of Selected Physicochemical Properties. Appl. Sci. 2022, 12, 4373. [Google Scholar] [CrossRef]
- Sirén, K.; Mak, S.S.T.; Melkonian, C.; Carøe, C.; Swiegers, J.H.; Molenaar, D.; Fischer, U.; Thomas, P.; Gilbert, M. Taxonomic and Functional Characterization of the Microbial Community During Spontaneous in Vitro Fermentation of Riesling Must. Front. Microbiol. 2019, 10, 697. [Google Scholar] [CrossRef]
- Vergara Alvarez, S.C.; Leiva Alaniz, M.J.; Mestre Furlani, M.V.; Vazquez, F.; Mancha Agresti, P.; Cristina Nally, M.; Paola Maturano, Y. Bioprospecting of the Probiotic Potential of Yeasts Isolated from a Wine Environment. Fungal Genet. Biol. 2023, 164, 103767. [Google Scholar] [CrossRef]
- Vilela, A.; Cosme, F.; Inês, A. Wine and Non-Dairy Fermented Beverages: A Novel Source of Pro-and Prebiotics. Fermentation 2020, 6, 113. [Google Scholar] [CrossRef]
- Rayavarapu, B.; Tallapragada, P. Evaluation of Potential Probiotic Characters of Lactobacillus fermentum. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2019, 20, 183–197. [Google Scholar]
- Agarbati, A.; Canonico, L.; Marini, E.; Zannini, E.; Ciani, M.; Comitini, F. Potential Probiotic Yeasts Sourced from Natural Environmental and Spontaneous Processed Foods. Foods 2020, 9, 287. [Google Scholar] [CrossRef]
- Przybek, P. Winnice w Polsce Mapa 581 Polskich Winnic Winogrodnicy.PL. Available online: http://winogrodnicy.pl/ (accessed on 11 July 2023).
- Drozdz, I.; Makarewicz, M.; Sroka, P.; Satora, P.; Jankowski, P. Comparison of the Yeast Microbiota of Different Varieties of Cool-Climate Grapes by PCR-RAPD. Potravin. Slovak J. Food Sci. 2015, 9, 293–298. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR-Protocols and Applications—A Laboratory Manual; Academic Press Inc.: Cambridge, MA, USA, 1990; pp. 315–322. ISBN 0-12-372180-6. [Google Scholar]
- Altschul, S.F.; Boguski, M.S.; Gish, W.; Wootton, J.C. Issues in Searching Molecular Sequence Databases. Nat. Genet. 1994, 6, 119–129. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Buck, J.D.; Cleverdon, R.C. The Spread Plate As A Method For The Enumeration Of Marine Bacteria. Limnol. Oceanogr. 1960, 5, 78–80. [Google Scholar] [CrossRef]
- Amorim, J.C.; Piccoli, R.H.; Duarte, W.F. Probiotic Potential of Yeasts Isolated from Pineapple and Their Use in the Elaboration of Potentially Functional Fermented Beverages. Food Res. Int. 2018, 107, 518–527. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Technological Characterization and Probiotic Traits of Yeasts Isolated from Altamura Sourdough to Select Promising Microorganisms as Functional Starter Cultures for Cereal-Based Products. Food Microbiol. 2014, 38, 26–35. [Google Scholar] [CrossRef]
- Syal, P.; Vohra, A. Probiotic Potential of Yeasts Isolated From Traditional Indian Fermented Foods. Int. J. Microbiol. Res. 2013, 5, 390–398. [Google Scholar] [CrossRef]
- Gil-Rodríguez, A.M.; Carrascosa, A.V.; Requena, T. Yeasts in Foods and Beverages: In Vitro Characterisation of Probiotic Traits. LWT-Food Sci. Technol. 2015, 64, 1156–1162. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.; Olaizola, C.; Ontiveros, Y.; Ellis, M.; Ain, A. Well Diffusion for Antifungal Susceptibility Testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, C.; Seo, M.J.; Roh, S.W.; Lee, S.H. Characterization of a Potential Probiotic Bacterium Lactococcus raffinolactis WiKim0068 Isolated from Fermented Vegetable Using Genomic and in Vitro Analyses. BMC Microbiol. 2020, 20, 136. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Rantsiou, K.; Jiménez-Díaz, R.; Garrido-Fernández, A.; Cocolin, L. Screening of Lactic Acid Bacteria Isolated from Fermented Table Olives with Probiotic Potential. Food Res. Int. 2013, 50, 135–142. [Google Scholar] [CrossRef]
- Pan, W.H.; Li, P.L.; Liu, Z. The Correlation between Surface Hydrophobicity and Adherence of Bifidobacterium Strains from Centenarians’ Faeces. Anaerobe 2006, 12, 148–152. [Google Scholar] [CrossRef]
- Pizzolitto, R.P.; Armando, M.R.; Salvano, M.A.; Dalcero, A.M.; Rosa, C.A. Evaluation of Saccharomyces cerevisiae as an Antiaflatoxicogenic Agent in Broiler Feedstuffs. Poult. Sci. 2013, 92, 1655–1663. [Google Scholar] [CrossRef]
- Nadai, C.; Giacomini, A.; Corich, V. The Addition of Wine Yeast Starmerella bacillaris to Grape Skin Surface Influences Must Fermentation and Glycerol Production. OENO One 2021, 55, 47–55. [Google Scholar] [CrossRef]
- Englezos, V.; Giacosa, S.; Rantsiou, K.; Rolle, L.; Cocolin, L. Starmerella bacillaris in Winemaking: Opportunities and Risks. Curr. Opin. Food Sci. 2017, 17, 30–35. [Google Scholar] [CrossRef]
- Masneuf-Pomarede, I.; Juquin, E.; Miot-Sertier, C.; Renault, P.; Laizet, Y.; Salin, F.; Alexandre, H.; Capozzi, V.; Cocolin, L.; Colonna-Ceccaldi, B.; et al. The Yeast Starmerella bacillaris (Synonym Candida zemplinina) Shows High Genetic Diversity in Winemaking Environments. FEMS Yeast Res. 2015, 15, 11. [Google Scholar] [CrossRef]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Yeast Biodiversity in Vineyard Environments Is Increased by Human Intervention. PLoS ONE 2016, 11, e0160579. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; Van der Westhuizen, T.J.; Augustyn, O.P.H. Yeast Biodiversity in Vineyards and Wineries and Its Importance to the South African Wine Industry: A Review. S. Afr. J. Enol. Vitic. 1999, 20, 61–70. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial Terroir and Food Innovation: The Case of Yeast Biodiversity in Wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Shen, Y.; Bai, X.; Zhang, Y.; Gao, Q.; Bu, X.; Xu, Y.; Guo, N. Evaluation of the Potential Probiotic Yeast Characteristics with Anti-MRSA Abilities. Probiotics Antimicrob. Proteins 2022, 14, 727–740. [Google Scholar] [CrossRef]
- Turk, M.; Abramović, Z.; Plemenitaš, A.; Gunde-Cimerman, N. Salt Stress and Plasma-Membrane Fluidity in Selected Extremophilic Yeasts and Yeast-like Fungi. FEMS Yeast Res. 2007, 7, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.G.T.; Ramos, C.L.; Cenzi, G.; Melo, D.S.; Dias, D.R.; Schwan, R.F. Probiotic Potential, Antioxidant Activity, and Phytase Production of Indigenous Yeasts Isolated from Indigenous Fermented Foods. Probiotics Antimicrob. Proteins 2020, 12, 280–288. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and Related Pulcherrimin-Producing Yeasts: Fuzzy Species Boundaries and Complex Antimicrobial Antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef] [PubMed]
- Tenea, G.N.; Anrango Cajas, B.; Carlosama Sanchez, B. Inhibitory-like Substances Produced by Yeasts Isolated from Andean Blueberries: Prospective Food Antimicrobials. Foods 2023, 12, 2435. [Google Scholar] [CrossRef]
- Corbu, V.M.; Csutak, O. Molecular and Physiological Diversity of Indigenous Yeasts Isolated from Spontaneously Fermented Wine Wort from Ilfov County, Romania. Microorganisms 2023, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pacheco, P.; Ramos Monge, I.M.; Fernández-González, M.; Poveda Colado, J.M.; Arévalo-Villena, M. Safety Evaluation of Yeasts With Probiotic Potential. Front. Nutr. 2021, 8, 239. [Google Scholar] [CrossRef]
- Matukas, M.; Starkute, V.; Zokaityte, E.; Zokaityte, G.; Klupsaite, D.; Mockus, E.; Rocha, J.M.; Ruibys, R.; Bartkiene, E. Effect of Different Yeast Strains on Biogenic Amines, Volatile Compounds and Sensory Profile of Beer. Foods 2022, 11, 2317. [Google Scholar] [CrossRef] [PubMed]
- Stój, A.; Płotka-Wasylka, J.; Simeonov, V.; Kapłan, M. The Content of Biogenic Amines in Rondo and Zweigelt Wines and Correlations between Selected Wine Parameters. Food Chem. 2022, 371, 131172. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Acquaticci, L.; Molina-Hernandez, J.B.; Rantsiou, K.; Martuscelli, M.; Kamgang-Nzekoue, A.F.; Vittori, S.; Paparella, A.; Chaves-López, C. Exploring the Capability of Yeasts Isolated from Colombian Fermented Cocoa Beans to Form and Degrade Biogenic Amines in a Lab-Scale Model System for Cocoa Fermentation. Microorganisms 2020, 9, 28. [Google Scholar] [CrossRef]
- Caruso, M.; Fiore, C.; Contursi, M.; Salzano, G.; Paparella, A.; Romano, P. Formation of Biogenic Amines as Criteria for the Selection of Wine Yeasts. World J. Microbiol. Biotechnol. 2002, 18, 159–163. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Pardo, I. Biogenic Amine Production by Lactic Acid Bacteria, Acetic Bacteria and Yeast Isolated from Wine. Food Control 2007, 18, 1569–1574. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Strzempko, M.N.; Belsky, C.A.; McKinley, G.A. API ZYM and API An-Ident Reactions of Fastidious Oral Gram-Negative Species. J. Clin. Microbiol. 1985, 22, 333–335. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Caro, I.; Rodriguez-Aparicio, L.B.; Rua, J.; Ferrero, M.A.; Garcia-Armesto, M.R. Characterization of Certain Bacterial Strains for Potential Use as Starter or Probiotic Cultures in Dairy Products. J. Food Prot. 2011, 74, 1379–1386. [Google Scholar] [CrossRef]
- Valderrama, B.; Ruiz, J.J.; Gutiérrez, M.S.; Alveal, K.; Caruffo, M.; Oliva, M.; Flores, H.; Silva, A.; Toro, M.; Reyes-Jara, A.; et al. Cultivable Yeast Microbiota from the Marine Fish Species Genypterus chilensis and Seriolella violacea. J. Fungi 2021, 7, 515. [Google Scholar] [CrossRef]
- Lee, N.-K.; Hong, J.-Y.; Yi, S.-H.; Hong, S.-P.; Lee, J.-E.; Paik, H.-D. Bioactive Compounds of Probiotic Saccharomyces cerevisiae Strains Isolated from Cucumber Jangajji. J. Funct. Foods 2019, 58, 324–329. [Google Scholar] [CrossRef]
- Patil, R.S.; Ghormade, V.; Deshpande, M.V. Chitinolytic Enzymes: An Exploration. Enzyme Microb. Technol. 2000, 26, 473–483. [Google Scholar] [CrossRef] [PubMed]
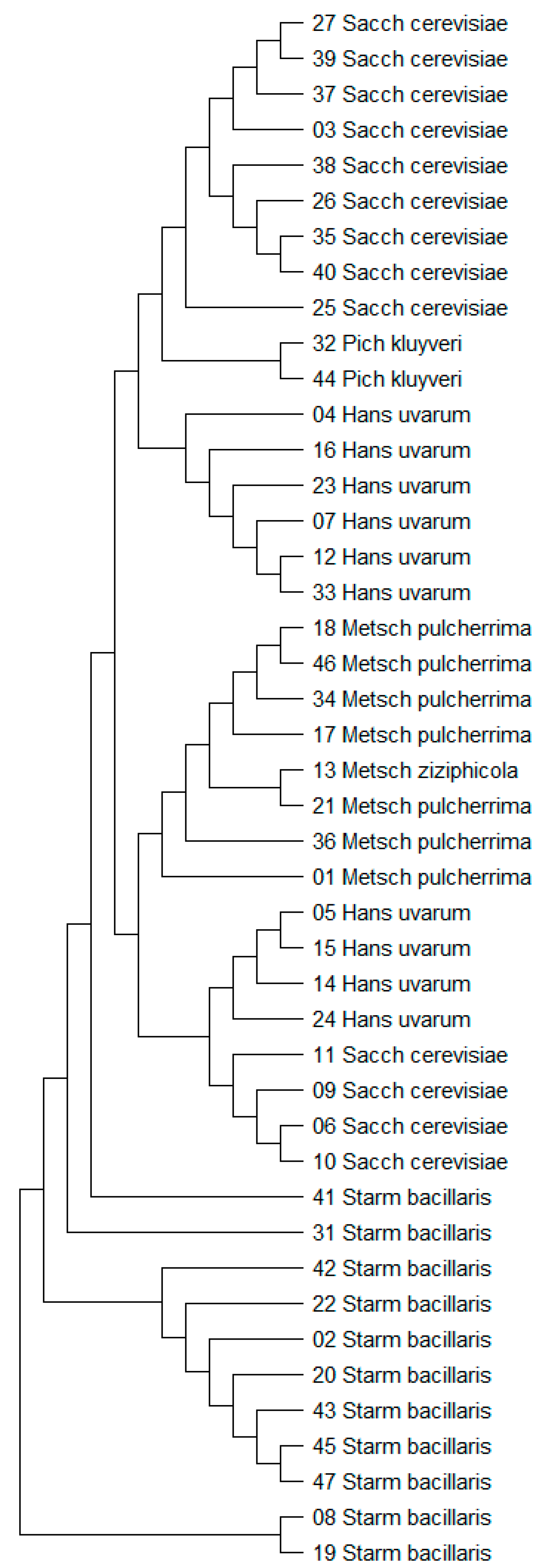
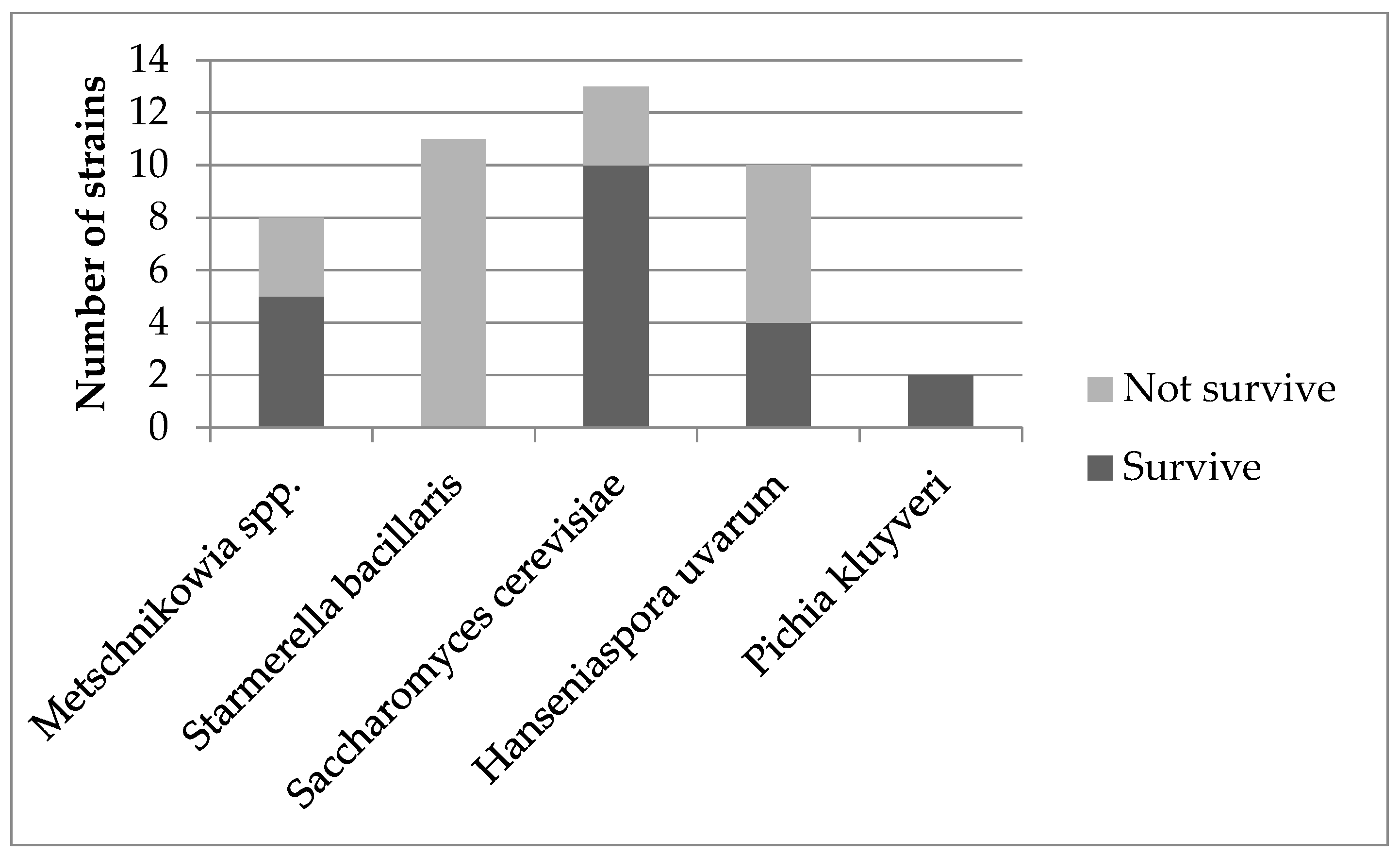
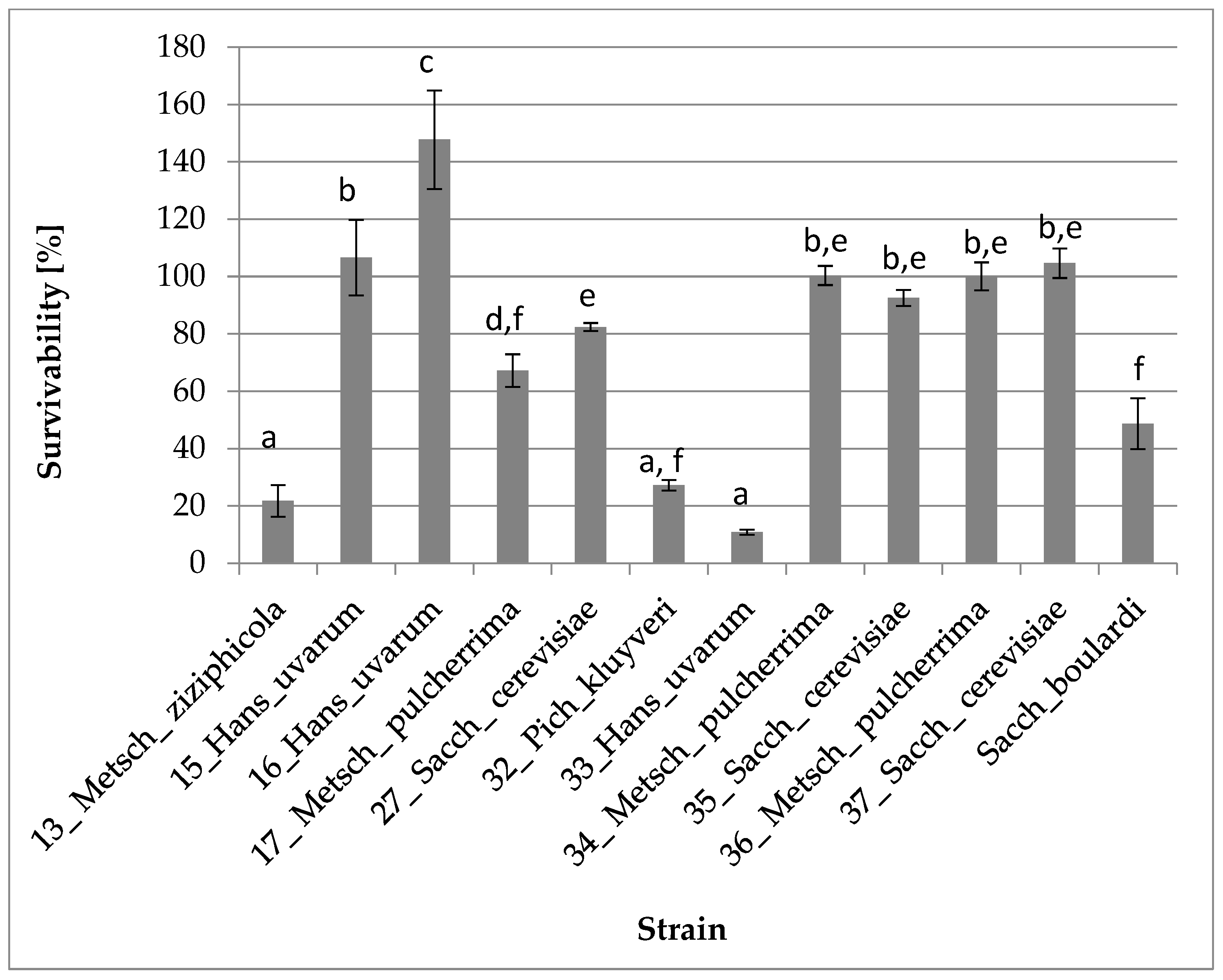

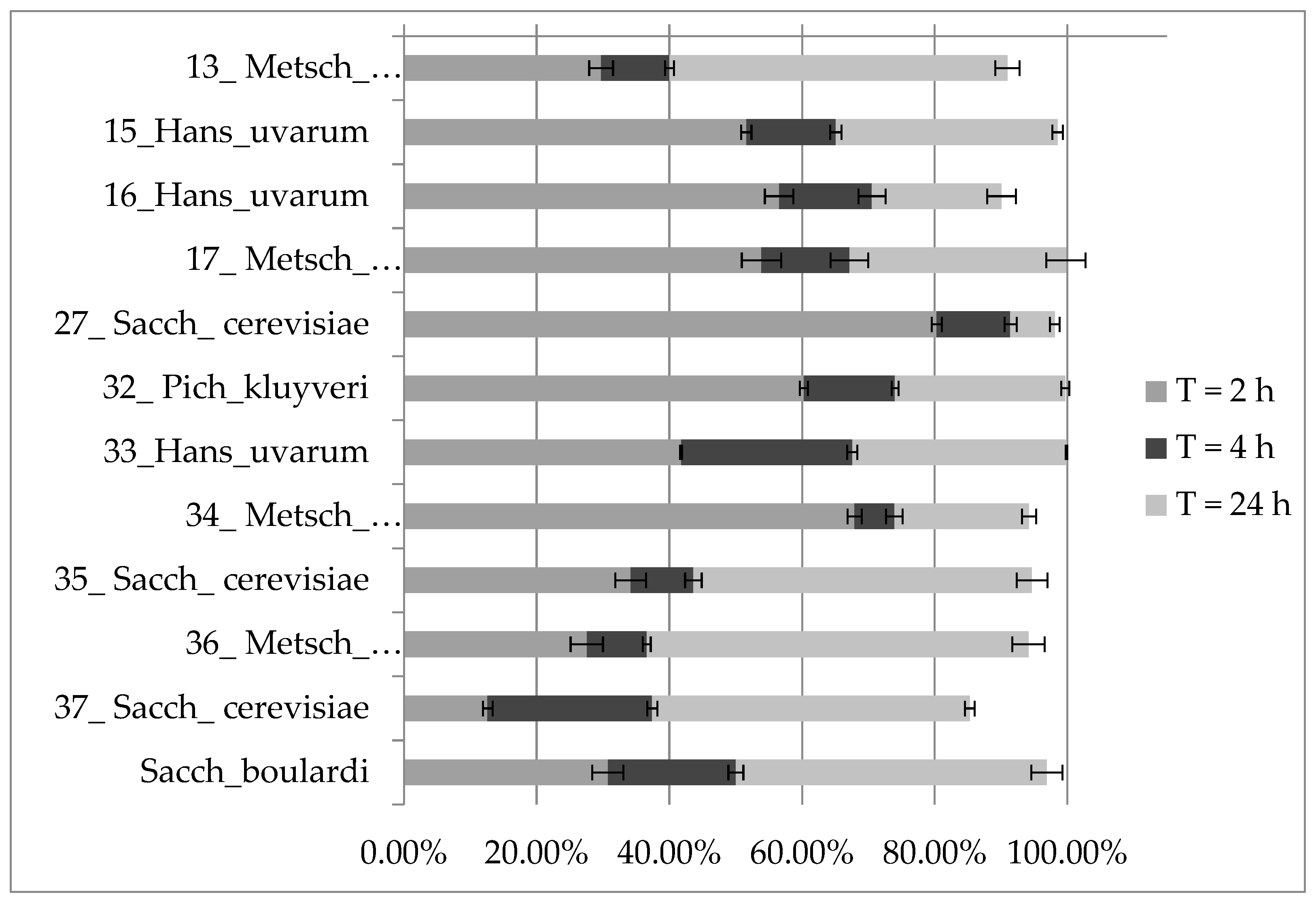
| Strain Number | Species | Code Name | Vineyard 1 |
|---|---|---|---|
| 01 | Metschnikowia pulcherrima | 01_Metsch_pulcherrima | DB |
| 02 | Starmerella bacillaris | 02_Starm_bacillaris | DB |
| 03 | Saccharomyces cerevisiae | 03_Sacch_cerevisiae | DB |
| 04 | Hanseniaspora uvarum | 04_Hans_uvarum | DB |
| 05 | Hanseniaspora uvarum | 05_Hans_uvarum | DB |
| 06 | Saccharomyces cerevisiae | 06_Sacch_cerevisiae | DB |
| 07 | Hanseniaspora uvarum | 07_Hans_uvarum | DB |
| 08 | Starmerella bacillaris | 08_Starm_bacillaris | DB |
| 09 | Saccharomyces cerevisiae | 09_Sacch_cerevisiae | DB |
| 10 | Saccharomyces cerevisiae | 10_Sacch_cerevisiae | DB |
| 11 | Saccharomyces cerevisiae | 11_Sacch_cerevisiae | DB |
| 12 | Hanseniaspora uvarum | 12_Hans_uvarum | DB |
| 13 | Metschnikowia ziziphicola | 13_Metsch_ziziphicola | MD |
| 14 | Hanseniaspora uvarum | 14_Hans_uvarum | MD |
| 15 | Hanseniaspora uvarum | 15_Hans_uvarum | MD |
| 16 | Hanseniaspora uvarum | 16_Hans_uvarum | MD |
| 17 | Metschnikowia pulcherrima | 17_Metsch_pulcherrima | MD |
| 18 | Metschnikowia pulcherrima | 18_Metsch_pulcherrima | MD |
| 19 | Starmerella bacillaris | 19_Starm_bacillaris | MD |
| 20 | Starmerella bacillaris | 20_Starm_bacillaris | MD |
| 21 | Metschnikowia pulcherrima | 21_Metsch_pulcherrima | MD |
| 22 | Starmerella bacillaris | 22_Starm_bacillaris | WJ |
| 23 | Hanseniaspora uvarum | 23_Hans_uvarum | WJ |
| 24 | Hanseniaspora uvarum | 24_Hans_uvarum | WJ |
| 25 | Saccharomyces cerevisiae | 25_Sacch_cerevisiae | DB |
| 26 | Saccharomyces cerevisiae | 26_Sacch_cerevisiae | DB |
| 27 | Saccharomyces cerevisiae | 27_Sacch_cerevisiae | DB |
| 31 | Starmerella bacillaris | 31_Starm_bacillaris | DB |
| 32 | Pichia kluyveri | 32_Pich_kluyveri | DB |
| 33 | Hanseniaspora uvarum | 33_Hans_uvarum | DB |
| 34 | Metschnikowia pulcherrima | 34_Metsch_pulcherrima | DB |
| 35 | Saccharomyces cerevisiae | 35_Sacch_cerevisiae | DB |
| 36 | Metschnikowia pulcherrima | 36_Metsch_pulcherrima | DB |
| 37 | Saccharomyces cerevisiae | 37_Sacch_cerevisiae | DB |
| 38 | Saccharomyces cerevisiae | 38_Sacch_cerevisiae | DB |
| 39 | Saccharomyces cerevisiae | 39_Sacch_cerevisiae | DB |
| 40 | Saccharomyces cerevisiae | 40_Sacch_cerevisiae | DB |
| 41 | Starmerella bacillaris | 41_Starm_bacillaris | MD |
| 42 | Starmerella bacillaris | 42_Starm_bacillaris | MD |
| 43 | Starmerella bacillaris | 43_Starm_bacillaris | MD |
| 44 | Pichia kluyveri | 44_Pich_kluyveri | MD |
| 45 | Starmerella bacillaris | 45_Starm_bacillaris | MD |
| 46 | Metschnikowia pulcherrima | 46_Metsch_pulcherrima | MD |
| 47 | Starmerella bacillaris | 47_Starm_bacillaris | MD |
| - | Saccharomyces cerevisiae var. bouardi | Sacch_boulardi | - |
| Enzyme | 13 1 | 15 1 | 16 1 | 17 1 | 27 1 | 32 1 | 33 1 | 34 1 | 35 1 | 36 1 | 37 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipase (C14) | - | - | - | - | + | - | - | - | - | - | - |
| Leucine arylamidase | - | + | - | + | + | + | - | + | + | + | + |
| Valine arylamidase | - | + | - | + | + | + | - | + | - | + | + |
| Cystine arylamidase | - | - | - | + | - | + | - | + | - | + | - |
| Chymotrypsin | - | + | - | + | - | + | - | - | - | + | - |
| Acid phosphatase | + | + | + | + | + | + | + | + | + | + | + |
| Naphthol-AS-BI-phosphohydrolase | + | + | + | + | + | + | + | - | + | + | - |
| β-Galactosidase | + | - | + | - | - | - | + | + | + | - | - |
| α-Glucosidase | + | + | + | + | + | + | - | + | + | + | + |
| β-Glucosidase | + | - | + | + | + | + | - | - | - | + | + |
| N-acetyl-β-glucosaminidase | + | + | - | - | - | - | - | + | + | - | - |
| α-Mannosidase | - | + | - | + | + | + | - | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniszewski, A.; Kordowska-Wiater, M. Probiotic Yeasts and How to Find Them—Polish Wines of Spontaneous Fermentation as Source for Potentially Probiotic Yeasts. Foods 2023, 12, 3392. https://doi.org/10.3390/foods12183392
Staniszewski A, Kordowska-Wiater M. Probiotic Yeasts and How to Find Them—Polish Wines of Spontaneous Fermentation as Source for Potentially Probiotic Yeasts. Foods. 2023; 12(18):3392. https://doi.org/10.3390/foods12183392
Chicago/Turabian StyleStaniszewski, Adam, and Monika Kordowska-Wiater. 2023. "Probiotic Yeasts and How to Find Them—Polish Wines of Spontaneous Fermentation as Source for Potentially Probiotic Yeasts" Foods 12, no. 18: 3392. https://doi.org/10.3390/foods12183392






