Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Enzymes
2.1.2. Other Reagents
2.2. Methods
2.2.1. Preparation of Egg Yolk Peptides
2.2.2. Amino Acid Composition
2.2.3. Protein Determination
2.2.4. Determination of Fat Content
2.2.5. Enzymatic Reaction
2.2.6. Degree of Hydrolysis (DH)
2.2.7. Peptide Identification by MALDI-TOF/MS
2.2.8. Antioxidative Potential Estimation
Ferric Reducing Antioxidant Power (FRAP)
Determination of DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical-Scavenging Activity
Determination of ABTS•+ (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) Radical Cation Decolorization Activity
2.2.9. Determination of Antibacterial Activity
2.2.10. Statistical Analysis
3. Results & Discussion
3.1. Amino Acid Composition/Nitrogen Analysis
3.2. Determination of Fat Content by Soxhlet Extraction
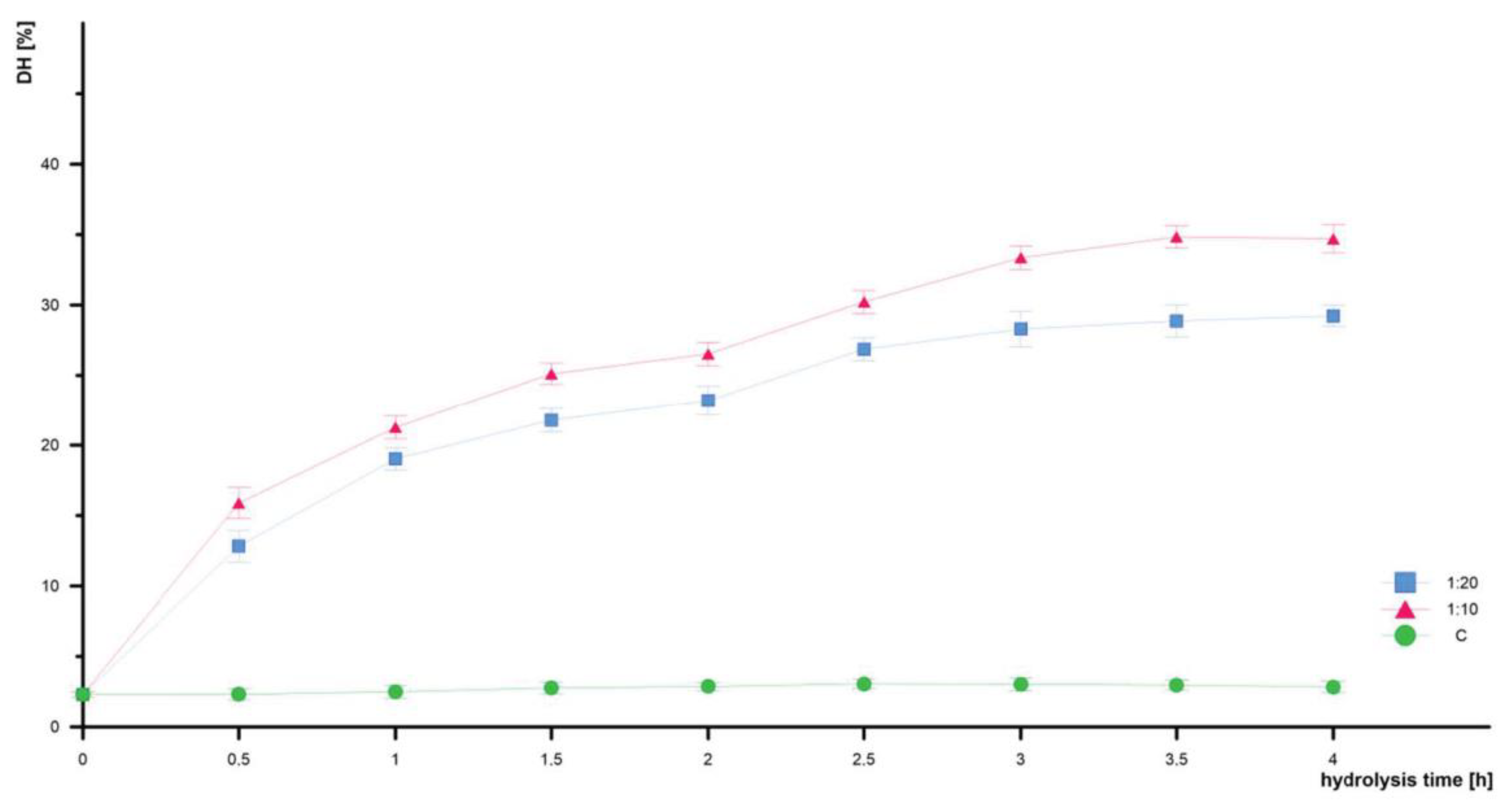
3.3. Degree of Hydrolysis DH
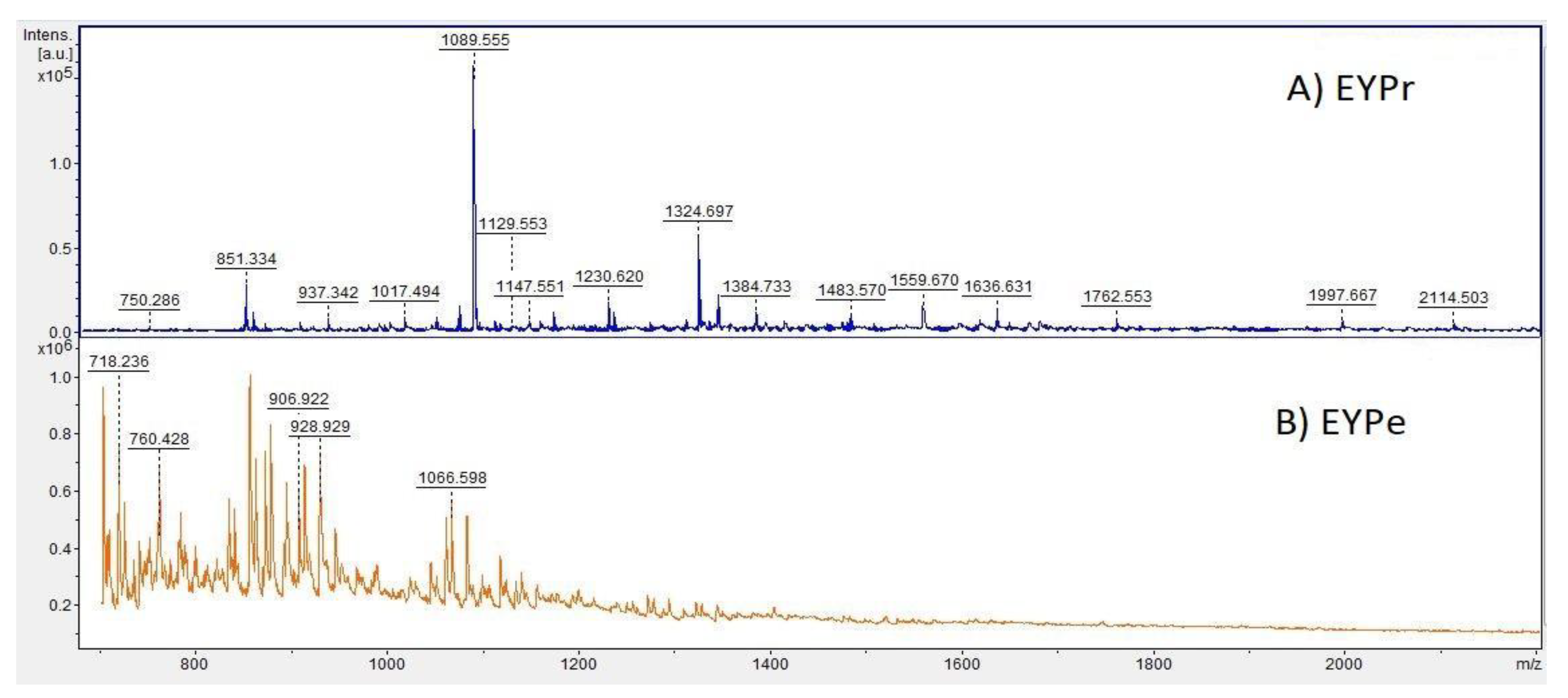
3.4. Investigation of the Peptide Profile by MALDI-TOF/MS Analysis
3.5. Determination of Antioxidant Activity
3.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent Bioactive Peptides in Milk Proteins: Proteolytic Activation and Significance in Dairy Processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.P.C. Review of methods for the analysis. of protein hydrolysates. Food Chem. 1997, 60, 263–271. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, Y.; Wang, X.; Chi, Y.-J. Effects of degree of hydrolysis (DH) on the functional properties of egg yolk hydrolysate with alcalase. J. Food Sci. Technol. 2017, 54, 669–678. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Dąbrowska, A.; Bobak, Ł.; Szołtysik, M. Egg yolk proteins and peptides with biological activity. Postepy Hig. Med. Dosw. 2014, 68, 1524–1529. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H.; Hartmann., R.; Meisel., H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom ( Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Zambrowicz, A.; Pokora, M.; Setner, B.; Dąbrowska, A.; Szołtysik, M.; Szewczuk, Z.; Polanowski, A.; Trziszka, T.; Chrzanowska, J. Egg-yolk protein by-product as a source of ACE-inhibitory peptides obtained with using unconventional proteinase from Asian pumpkin (Cucurbita ficifolia). J. Proteom. 2014, 110, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Laca, A.; Paredes, B.; Díaz, M. A method of egg yolk fractionation. Characterization of fractions. Food Hydrocoll. 2010, 24, 434–443. [Google Scholar] [CrossRef]
- Mine, Y.; Kovacs-Nolan, J. New insights in biologically active proteins and peptides derived from hen egg. World Poultry Sci. J. 2006, 62, 87–95. [Google Scholar] [CrossRef]
- Roslan, J.; Yunos, K.F.M.; Abdullah, N.; Kamal, S.M.M. Characterization of Fish Protein Hydrolysate from Tilapia (Oreochromis niloticus) by-Product. Agric. Agric. Sci. Proc. 2014, 2, 312–319. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T. Egg yolk protein modification by controlled enzymatic hydrolysis for improved functionalities. Int. J. Food Sci. Technol. 2009, 44, 763–769. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and Modification of the Carcinogenic Response by bha, Bht, and Other Antioxidants. CRC Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Pokora, M.; Setner, B.; Dąbrowska, A.; Szołtysik, M.; Babij, K.; Szewczuk, Z.; Trziszka, T.; Lubec, G.; Chrzanowska, J. Multifunctional peptides derived from an egg yolk protein hydrolysate: Isolation and characterization. Amino Acids 2015, 47, 369–380. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sogo, N.; Iwao, R.; Miyamoto, T. Antioxidant effect of egg yolk on linoleate in emulsions. Agric. Biol. Chem. 1990, 54, 3099–3104. [Google Scholar]
- King, M.F.; Boyd, L.C.; Sheldon, B.W. Antioxidant properties of individual phospholipids in a salmon oil model system. J. Am. Oil Chem. Soc. 1992, 69, 545–551. [Google Scholar] [CrossRef]
- Sugino, H.; Ishikawa, M.; Nitoda, T.; Koketsu, M.; Juneja, L.R.; Kim, M.; Yamamoto, T. Antioxidative Activity of Egg Yolk Phospholipids. J. Agr. Food Chem. 1997, 45, 551–554. [Google Scholar] [CrossRef]
- Lee, S.K.; Han, J.H.; Decker, E.A. Antioxidant Activity of Phosvitin in Phosphatidylcholine Liposomes and Meat Model Systems. J. Food Sci. 2002, 67, 37–41. [Google Scholar] [CrossRef]
- Lu, C.-L.; Baker, R.C. Characteristics of Egg Yolk Phosvitin as an Antioxidant for Inhibiting Metal-Catalyzed Phospholipid Oxidations. Poultry Sci. 1986, 65, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Kitahata, K.; Mitsuya, T.; Gutierrez, M.A.; Luneja, L.R. Protein quality determination of delipidated egg-yolk. J. Food Compos. Anal. 2000, 13, 773–781. [Google Scholar] [CrossRef]
- Davies, M.G.; Thomas, A.J. An investigation of hydrolytic techniques for the amino acid analysis of foodstuffs. J. Sci. Food Agric. 1973, 24, 1525–1540. [Google Scholar] [CrossRef]
- Schram, E.; Moore, S.; Bigwood, E.J. Chromatographic determination of cystine as cysteic acid. Biochem. J. 1954, 59, 33–37. [Google Scholar] [CrossRef]
- Thiex, N.J.; Anderson, S.; Gildemeister, B. Crude fat, hexanes extraction, in feed, cereal grain, and forage (Randall/soxtec/submersion method): Collaborative study. J. AOAC Int. 2003, 86, 899–908. [Google Scholar] [CrossRef]
- Jehmlich, N.; Golatowski, C.; Murr, A.; Salazar, G.; Dhople, V.M.; Hammer, E.; Völker, U. Comparative evaluation of peptide desalting methods for salivary proteome analysis. Clin. Chim. Acta. 2014, 434, 16–20. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kosikowska, U.; Stepien-Pysniak, D.; Ozga, D.; Wernicki, A.; Malm, A. Identification of Bacillus spp. colonizing the nasal mucosa of healthy adults living in the suburban area using the matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) system. Curr. Issues Pharm. Med. Sci. 2014, 27, 137–141. [Google Scholar] [CrossRef]
- Pokora, M.; Eckert, E.; Zambrowicz, A.; Bobak, Ł.; Szołtysik, M.; Dąbrowska, A.; Chrzanowska, J.; Polanowski, A.; Trziszka, T. Biological and functional properties of proteolytic enzyme-modified egg protein by-products. Food Sci. Nutr. 2013, 1, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and Amino Acid Content in Four Brands of Commercial Table Eggs in Retail Markets in Relation to Human Requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, E.I.; Arifalo, M.K.O. Nutritional Qualities of the Amino Acid profile of the Yolk and Albumen of Chicken (Hen) Egg. Biosci. Biotechnol. Res. Asia 2011, 8, 483–490. [Google Scholar] [CrossRef]
- Jiang, Y.; Noh, S.K.; Koo, S.I. Egg Phosphatidylcholine Decreases the Lymphatic Absorption of Cholesterol in Rats. J. Nutr. 2001, 131, 2358–2363. [Google Scholar] [CrossRef]
- Chay Pak Ting, B.P.; Mine, Y.; Juneja, L.R.; Okubo, T.; Gauthier, S.F.; Pouliot, Y. Comparative Composition and Antioxidant Activity of Peptide Fractions Obtained by Ultrafiltration of Egg Yolk Protein Enzymatic Hydrolysates. Membranes 2011, 1, 149–161. [Google Scholar] [CrossRef]
- Jung, S.; Murphy, P.A.; Johnson, L.A. Physicochemical and Functional Properties of Soy Protein Substrates Modified by Low Levels of Protease Hydrolysis. J. Food Sci. 2005, 70, C180–C187. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Reitmeier, C.; Murphy, P.A.; Johnson, L.A. Enzymatic hydrolysis of extruded-expelled soy flour and resulting functional properties. J. Am. Oil Chem. Soc. 2006, 83, 731–737. [Google Scholar] [CrossRef]
- Severin, S.; Xia, W.S. Enzymatic hydrolysis of whey proteins by two different proteases and their effect on the functional properties of resulting protein hydrolysates. J. Food Biochem. 2006, 30, 77–97. [Google Scholar] [CrossRef]
- Sathivel, S.; Bechtel, P.J.; Babbitt, J.; Smiley, S.; Crapo, C.; Reppond, K.D.; Prinyawiwatkul, W. Biochemical and Functional Properties of Herring (Clupea harengus) Byproduct Hydrolysates. J. Food Sci. 2003, 68, 2196–2200. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, H.; Qian, H. Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem. 2007, 102, 759–763. [Google Scholar] [CrossRef]
- Eckert, E.; Pokora, M.; Zambrowicz, A.; Szołtysik, M.; Dąbrowska, A.; Chrzanowska, J.; Trziszka, T. The application of microbial proteases to obtain egg yolk protein hydrolysates with antioxidant and antimicrobial activity. Zywn. Nauk. Technol. Ja. 2013, 1, 105–118. [Google Scholar] [CrossRef]
- Young, D.; Fan, M.Z.; Mine, Y. Egg Yolk Peptides Up-regulate Glutathione Synthesis and Antioxidant Enzyme Activities in a Porcine Model of Intestinal Oxidative Stress. J. Agric. Ford Chem. 2010, 58, 7624–7633. [Google Scholar] [CrossRef]
- Graszkiewicz, A.; Żelazko, M.; Trziszka, T.; Polanowski, A. Antioxidative capacity of hydrolysates of hen egg proteins. Pol. J. Food Nutr. Sci. 2007, 4, 195–199. [Google Scholar]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Mahaki, H.; Zare-Zardini, H. Antioxidant activity of protein hydrolysates and purified peptides from zizyphus jujube fruits. J. Funct. Foods 2013, 5, 62–70. [Google Scholar] [CrossRef]
- Eckert, E.; Zambrowicz, A.; Bobak, Ł.; Zabłocka, A.; Chrzanowska, J.; Trziszka, T. Production and Identification of Biologically Active Peptides Derived from By-product of Hen Egg-Yolk Phospholipid Extraction. Int. J. Pept. Res. Ther. 2018, 25, 669–680. [Google Scholar] [CrossRef]
- Delgado, M.C.O.; Galleano, M.; Añón, M.C.; Tironi, V.A. Amaranth, Peptides from Simulated Gastrointestinal Digestion: Antioxidant Activity Against Reactive Species. Plant Foods Hum. Nutr. 2015, 70, 27–34. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Wu, T.-K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant Activity and Phenolic Compositions of Lentil (Lens culinaris var. Morton) Extract and Its Fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Hülsmeier, A.J.; Hunziker, P.; Thomas, U. Proteolytic fragments of ovalbumin display antimicrobial activity. Biochim. Biophys. Acta 2004, 1672, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Pimchan, T.; Tian, F.; Thumanu, K.; Rodtong, S.; Yongsawatdigul, J. Isolation, identification, and mode of action of antibacterial peptides derived from egg yolk hydrolysate. Poultry Sci. 2023, 102, 102695. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
| Energy Value | Fat | Carbohydrates | Proteins | Ash |
|---|---|---|---|---|
| 1155 kJ/ 279 kcal | 25.0 g ± 0.1 | 0.9 g ± 0.0 | 14.0 g ± 0.1 | 0.2 g ± 0.0 |
| Amino Acid | Egg Yolk Protein (EYPr) [mg/g] | Hydrolysates (EYPe) [mg/g] | ANOVA p Value |
|---|---|---|---|
| Asp | 54.3 ± 3.1 | 72.1 ± 4.1 | 0.000425 |
| Thr | 28.3 ± 1.9 | 38.5 ± 2.6 | 0.000728 |
| Ser | 48.4 ± 5.3 | 53.7 ± 5.9 | 0.223833 |
| Glu | 76.1 ± 4.2 | 103.4 ± 5.7 | 0.000240 |
| Pro | 23.3 ± 1.9 | 38.6 ± 3.2 | 0.000502 |
| Gly | 17.0 ± 0.1 | 33.9 ± 0.2 | <0.000000 |
| Ala | 29.7 ± 0.7 | 40.5 ± 1.0 | 0.000006 |
| Cys | 7.9 ± 3.2 | 27.3 ± 11.0 | 0.033286 |
| Val | 32.4 ± 0.9 | 43.7 ± 1.3 | 0.000008 |
| Met | 15.5 ± 5.3 | 16.8 ± 5.7 | 0.748595 |
| Ile | 29.3 ± 0.9 | 35.9 ± 1.1 | 0.000079 |
| Leu | 50.7 ± 0.3 | 58.5 ± 0.3 | <0.000000 |
| Tyr | 23.9 ± 2.2 | 32.5 ± 2.9 | 0.003252 |
| Phe | 25.6 ± 1.2 | 32.8 ± 1.6 | 0.000321 |
| His | 15.5 ± 2.2 | 18.3 ± 2.6 | 0.152067 |
| Lys | 41.9 ± 1.1 | 54.2 ± 1.4 | 0.000008 |
| Arg | 43.0 ± 1.2 | 51.4 ± 1.5 | 0.000146 |
| Trp | 11.4 ± 0.4 | 16.6 ± 0.6 | 0.000005 |
| Sample | EYPr | EYPe I Step | EYPe II Step | EYPe III Step | EYPe IV Step |
|---|---|---|---|---|---|
| Average [%] | 50.6 ± 2.3 | 43.9 ± 1.3 | 35.5 ± 1.4 | 27.5 ± 1.5 | 18.9 ± 1.2 |
| E:S Ratio | Time of Hydrolysis [h] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Papain | Pepsin | ||||||||
| 0.0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | |
| DH [%] | |||||||||
| Without enzyme | 2.30 ± 0.02 | 2.32 ± 0.04 * | 2.49 ± 0.05 * | 2.77 ± 0.04 * | 2.87 ± 0.03 | 3.06 ± 0.04 | 3.02 ± 0.05 | 2.97 ± 0.04 * | 2.84 ± 0.04 |
| 1:20 | 2.30 ± 0.02 | 12.82 ± 0.06 * | 19.04 ± 0.04 * | 21.78 ± 0.04 * | 23.20 ± 0.05 * | 26.87 ± 0.04 * | 28.29 ± 0.17 * | 28.87 ± 0.06 * | 29.23 ± 0.04 * |
| 1:10 | 2.30 ± 0.02 | 15.89 ± 0.06 * | 21.27 ± 0.04 * | 25.11 ± 0.04 * | 26.53 ± 0.04 * | 30.23 ± 0.04 * | 33.36 ± 0.04 * | 34.83 ± 0.04 * | 34.71 ± 0.05 * |
| Enzyme | Digestion Time [h] | Condition | DH [%] | Source |
|---|---|---|---|---|
| Neutrase | 3 | 200 U/mg, pH 7.0, 45 °C | 12 | [4] |
| Neutrase | 2 | Not available | 27 | [33] |
| Neutrase + Pronase | 3—each enzyme | Not available | 27 | [43] |
| Alcalase + Protease N | 3 | E:S 0.5% (w/w) | 25.3 | [37] |
| Trypsin | 3 | Egg white, 1 U/1 mg protein, 30 °C | 14 | [45] |
| Trypsin | 3 | 0.5% (w/w), 45 °C | 15.6 | [45] |
| Pepsin | 2 | Not available | 45.3 | [17] |
| m/z | Sequence | Activity |
|---|---|---|
| 849.381 | YPWTQR | opioid ACE inhibitor |
| 949.407 | ITMIAPSAF | ACE inhibitor |
| 990.481 | DSYEHGGEP | antibacterial antioxidant |
| 996.487 | VVSGPYIVY | ACE inhibitor |
| 1002.475 | QQGVEQGTR | antiproliferative |
| 1230.620 | KPQMTEEQIK | antiproliferative |
| 1236.595 | LMSYMWSTSM | antioxidant |
| 1324.697 | HVDLDEVANKIA | antibacterial |
| 1394.566 | YINQMPQKSRE | ACE inhibitor |
| 1483.570 | PGVTYPHPGQDTSAG | antioxidant |
| 1636.631 | FEDPERQESSRKE | antibacterial antiproliferative |
| 1670.622 | PTDQKVGWGGEGQIQ | antioxidant |
| 1681.584 | YIEAVNKVSPRAGQ | ACE inhibitor |
| Sample | Ferric Reducing Ability (FRAP) [mmol Fe2+/kg] | Ferric Reducing Ability (FRAP) [µg Fe2+/mg] | DPPH Scavenging Activity [mg AA/100g] | DPPH Scavenging Activity [µmol Trolox/mg] | ABTS Radical Cation Decolorization Assay [mmol Trolox/kg] |
|---|---|---|---|---|---|
| EYPe | 16.45 ± 0.19 | 0.11 ± 0.02 | 1776.66 ± 32.99 | 0.92 ± 0.04 | 390.43 ± 8.92 |
| No. | Strain | Gram | Inhibition Zone [mm] | |
|---|---|---|---|---|
| EYPe [50 mg/mL] | EYPe [25 mg/mL] | |||
| 1. | Bacillus cereus 1 | (+) | 20.0 ± 1.0 | 10.7 ± 0.6 |
| 2. | Bacillus cereus 2 | (+) | 13.0 ± 1.0 | 9.0 ± 1.0 |
| 3. | Bacillus megaterium | (+) | 13.7 ± 0.6 | 9.7 ± 0.6 |
| 4. | Bacillus pumilus | (+) | 12.0 ± 1.0 | 9.3 ± 0.6 |
| 5. | Kocuria rhizophila | (+) | 11.7 ± 0.6 | 7.7 ± 0.6 |
| 6. | Serratia liquefaciens | (−) | 12.3 ± 0.6 | 8.3 ± 0.6 |
| 7. | Pseudomonas aeruginosa | (−) | 9.3 ± 0.6 | 8.0 ± 1.0 |
| 8. | Hafnia alvei | (−) | 9.3 ± 0.6 | 9.0 ± 1.0 |
| 9. | Acinetobacter radioresistans | (−) | 8.7 ± 0.6 | 7.7 ± 0.6 |
| 10. | Stenotrophomonas maltophila | (−) | 8.7 ± 0.6 | 7.7 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czelej, M.; Czernecki, T.; Garbacz, K.; Wawrzykowski, J.; Jamioł, M.; Michalak, K.; Walczak, N.; Wilk, A.; Waśko, A. Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties. Foods 2023, 12, 3394. https://doi.org/10.3390/foods12183394
Czelej M, Czernecki T, Garbacz K, Wawrzykowski J, Jamioł M, Michalak K, Walczak N, Wilk A, Waśko A. Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties. Foods. 2023; 12(18):3394. https://doi.org/10.3390/foods12183394
Chicago/Turabian StyleCzelej, Michał, Tomasz Czernecki, Katarzyna Garbacz, Jacek Wawrzykowski, Monika Jamioł, Katarzyna Michalak, Natalia Walczak, Agata Wilk, and Adam Waśko. 2023. "Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties" Foods 12, no. 18: 3394. https://doi.org/10.3390/foods12183394
APA StyleCzelej, M., Czernecki, T., Garbacz, K., Wawrzykowski, J., Jamioł, M., Michalak, K., Walczak, N., Wilk, A., & Waśko, A. (2023). Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties. Foods, 12(18), 3394. https://doi.org/10.3390/foods12183394






