Metabolome and Microbiome Analysis to Study the Flavor of Summer Black Tea Improved by Stuck Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Tea Preparation
2.3. Analysis of Sensory Evaluation
2.4. Analysis of Non-Volatile Metabolites by UPLC-MS/MS
2.4.1. Sample Preparation and Extraction
2.4.2. UPLC Conditions
2.4.3. ESI-QTRAP-MS/MS
2.5. Analysis of Volatile Metabolites by GC-MS/MS
2.5.1. Sample Preparation and Treatment
2.5.2. GC-MS Conditions
2.6. Analysis of Microbial Community by 16S rRNA and ITS Sequencing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Stuck Fermentation Improved the Sensory Quality of Summer Black Tea
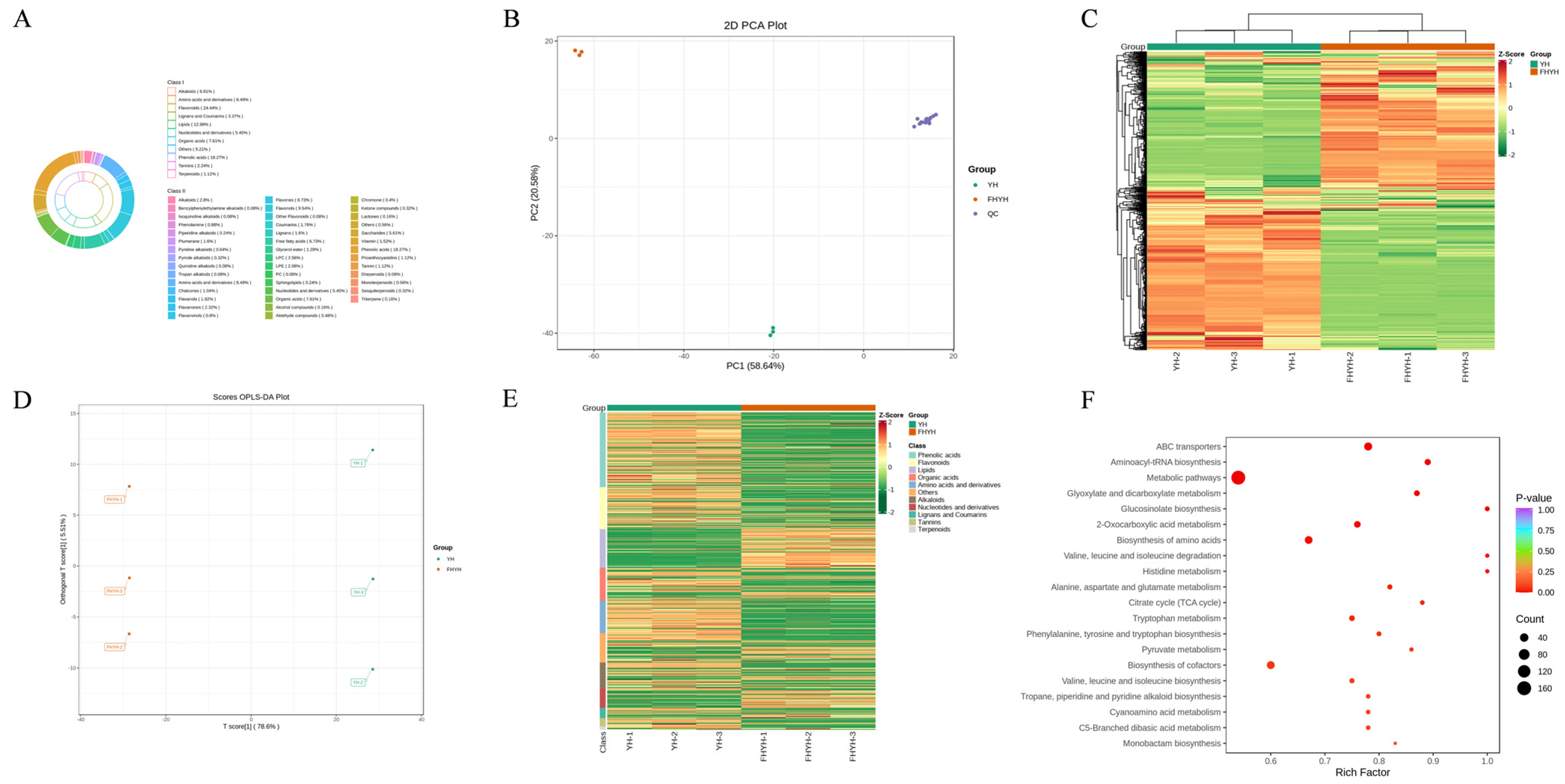
3.2. Stuck Fermentation Altered the Profile of Non-Volatile Metabolites in Summer Black Tea
3.2.1. Amino Acids and Derivatives
3.2.2. Phenolic Acids
3.2.3. Flavonoids
3.2.4. Organic Acids
3.2.5. Alkaloids
3.2.6. Saccharides
3.2.7. Lipids
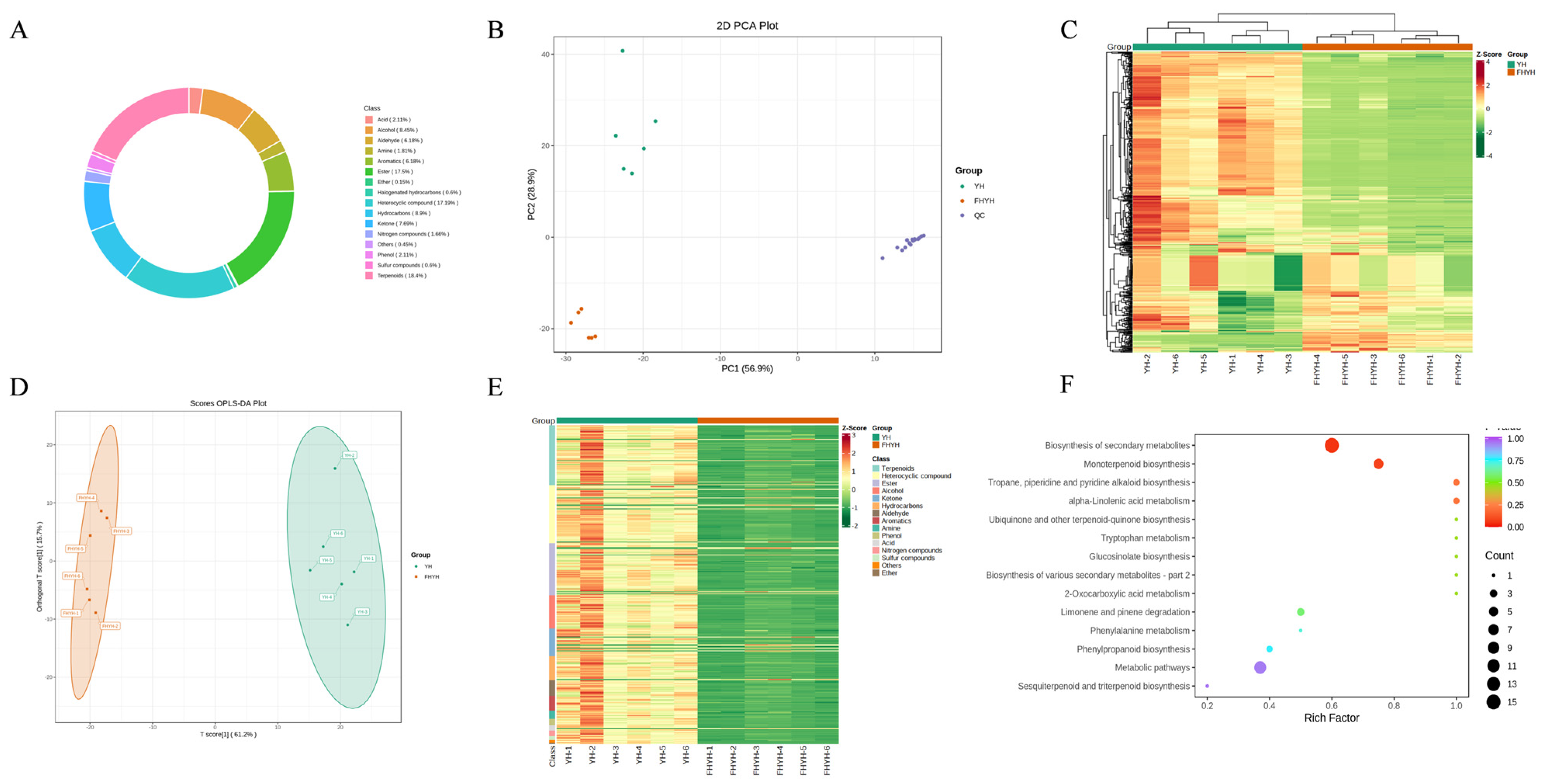
3.3. Stuck Fermentation Altered the Profile of Volatile Metabolites in Summer Black Tea
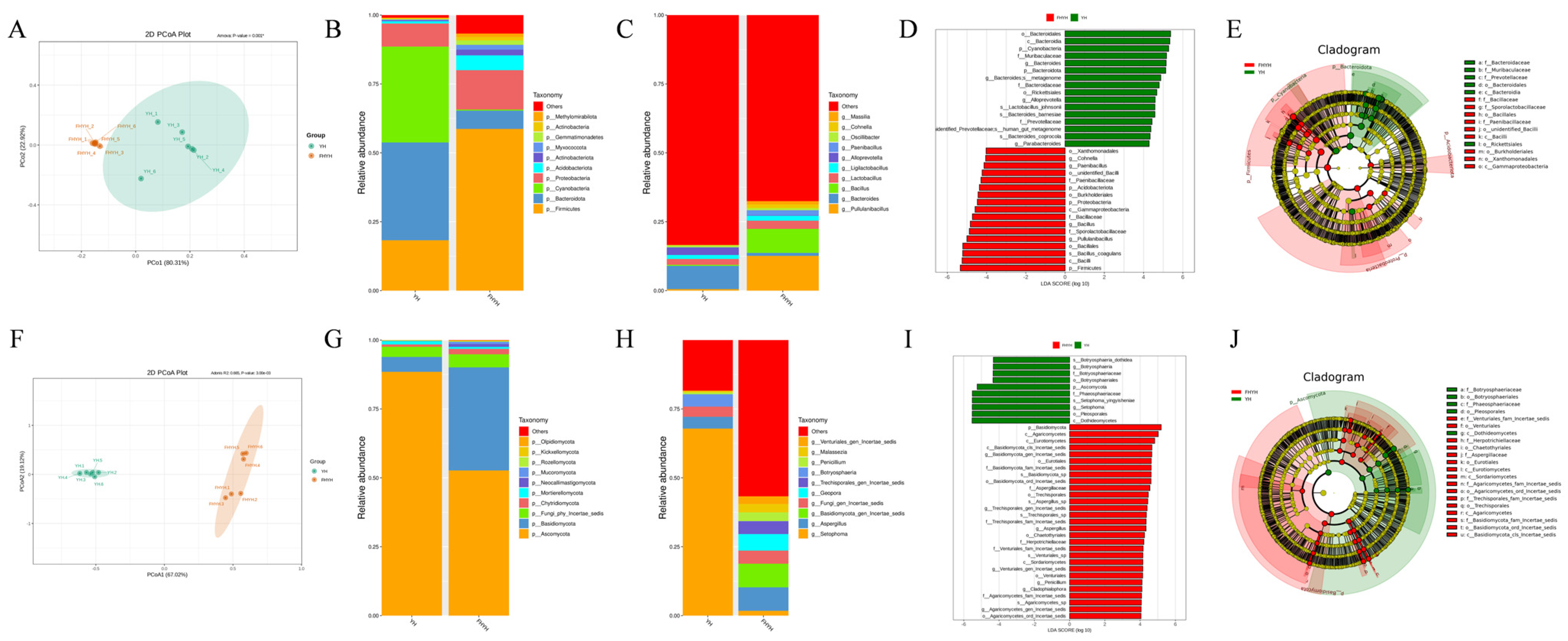
3.4. Stuck Fermentation Altered the Microbial Diversity in Summer Black Tea
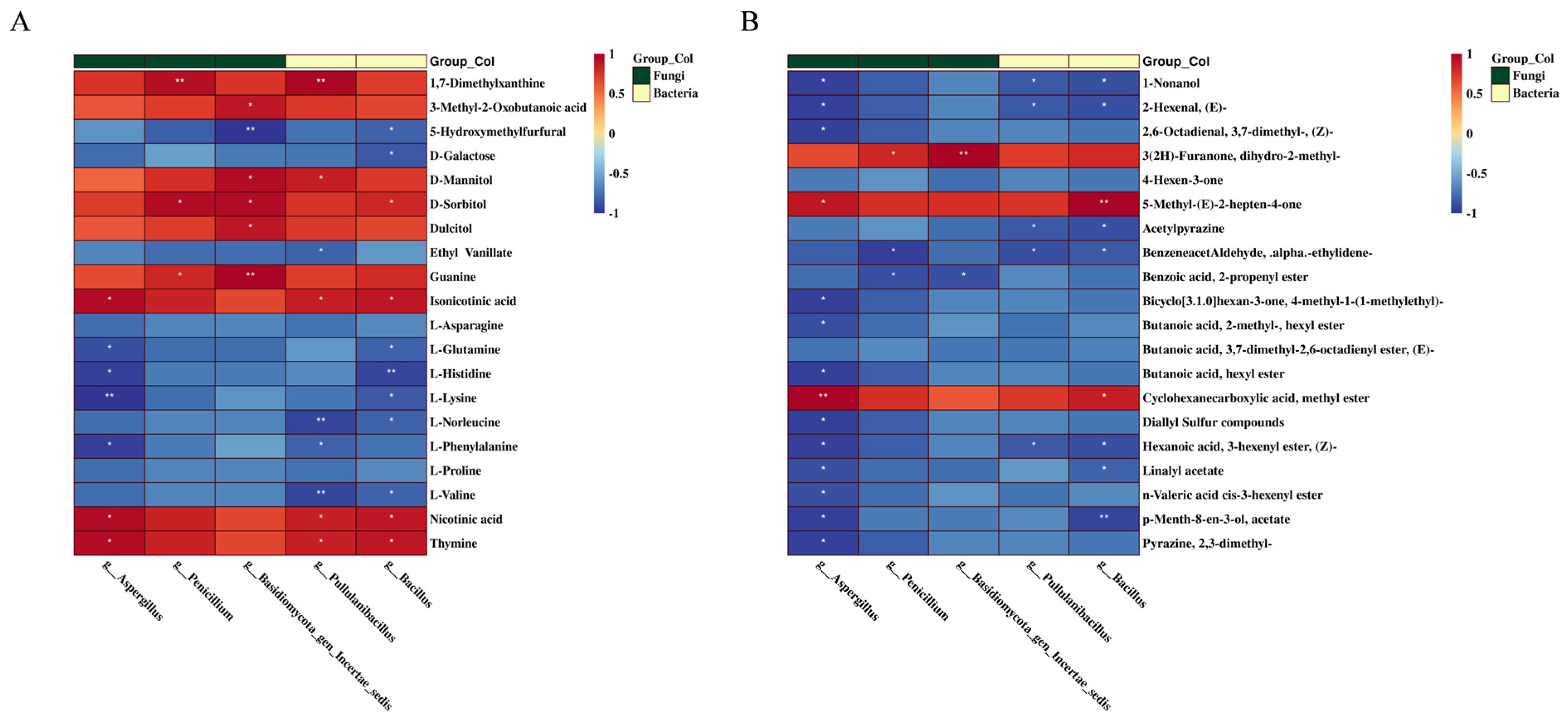
3.5. Correlation Analysis of Core Microorganisms and Principal Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fu, X.; Liao, Y.; Cheng, S.; Xu, X.; Grierson, D.; Yang, Z. Nonaqueous fractionation and overexpression of fluorescent-tagged enzymes reveals the subcellular sites of L-theanine biosynthesis in tea. Plant Biotechnol. J. 2021, 19, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, Q.; Li, Q.; Lin, H.; Li, J.; Zhu, M.; Liu, Z.; Wang, K. Integrative analysis of transcriptome and metabolome reveals the mechanism of foliar application of Bacillus amyloliquefaciens to improve summer tea quality (Camellia sinensis). Plant Physiol. Biochem. 2022, 185, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee flavour modification through controlled fermentations of green coffee beans by Saccharomyces cerevisiae and Pichia kluyveri: Part, I. Effects from individual yeasts. Food Res. Int. 2020, 136, 109588. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, M.; Liu, Y.; Xu, S.; Zhong, K.; Wu, Y.; Gao, H. The effect of Eurotium cristatum (MF800948) fermentation on the quality of autumn green tea. Food Chem. 2021, 358, 129848. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.; Chen, M.; Li, M.; Zhang, H.; Song, P.; An, T.; Yue, P.; Gao, X. Influence of Eurotium cristatum and Aspergillus niger individual and collaborative inoculation on volatile profile in liquid-state fermentation of instant dark teas. Food Chem. 2021, 350, 129234. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, Y.; Zhong, K.; Wu, Y.; Gao, H. Delving into the Biotransformation Characteristics and Mechanism of Steamed Green Tea Fermented by Aspergillus niger PW-2 Based on Metabolomic and Proteomic Approaches. Foods 2022, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Shen, S.; Zu, Z.; Chen, M.; Wen, Y.; Chen, X.; Chen, Q.; Wang, Y.; Wang, S.; Gao, X. Changes in the volatile compounds and characteristic aroma during liquid-state fermentation of instant dark tea by Eurotium cristatum. Food Chem. 2023, 410, 135462. [Google Scholar] [CrossRef]
- Zhu, M.-Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.-M.; Xu, W.; Li, J.; Lin, H.-Y.; Zhang, Z.; Xiao, J.-B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhang, P.; Le, M.-M.; Qi, Y.; Yang, Z.; Hu, F.-L.; Ling, T.-J.; Bao, G.-H. Improving flavor of summer Keemun black tea by solid-state fermentation using Cordyceps militaris revealed by LC/MS-based metabolomics and GC/MS analysis. Food Chem. 2023, 407, 135172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, S.; Yu, Q.; Wang, J.; Deng, Y.; Hua, J.; Zhou, Q.; Yuan, H.; Jiang, Y. Chemical profile of a novel ripened Pu-erh tea and its metabolic conversion during pile fermentation. Food Chem. 2022, 378, 132126. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic Changes in the Metabolite Profile and Taste Characteristics of Fu Brick Tea during the Manufacturing Process. Food Chemistry 2021, 344, 128576. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. Imeta 2023, 2, e85. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media (Second Enlarged and Revised Edition); Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; ISBN 978-7-03-058320-8. [Google Scholar]
- Van Gemert, L.J. Compilations of Flavour Threshold Values in Water and Other Media (Second Enlarged and Revised Edition); Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; ISBN 978-7-03-045311-2. [Google Scholar]
- An, T.; Chen, M.; Zu, Z.; Chen, Q.; Lu, H.; Yue, P.; Gao, X. Untargeted and targeted metabolomics reveal changes in the chemical constituents of instant dark tea during liquid-state fermentation by Eurotium cristatum. Food Res. Int. 2021, 148, 110623. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; He, C.; Li, Y.; Yu, Z.; Chen, Y.; Wang, Y.; Ni, D. Changes of fungal community and non-volatile metabolites during pile-fermentation of dark green tea. Food Res. Int. 2021, 147, 110472. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hua, J.; Yu, Q.; Li, J.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, T.; Li, W.; Jiang, Z.; Zhou, T.; Zhang, L.; Yu, Y. Widely targeted metabolomics using UPLC-QTRAP-MS/MS reveals chemical changes during the processing of black tea from the cultivar Camellia sinensis (L.) O. Kuntze cv. Huangjinya. Food Res. Int. 2022, 162, 112169. [Google Scholar] [CrossRef]
- Wang, H.; Teng, J.; Huang, L.; Wei, B.; Xia, N. Determination of the variations in the metabolic profile and sensory quality of Liupao tea during fermentation through UHPLC–HR–MS metabolomics. Food Chem. 2023, 404, 134773. [Google Scholar] [CrossRef]
- Chen, S.; Fu, Y.; Bian, X.; Zhao, M.; Zuo, Y.; Ge, Y.; Xiao, Y.; Xiao, J.; Li, N.; Wu, J.-L. Investigation and dynamic profiling of oligopeptides, free amino acids and derivatives during Pu-erh tea fermentation by ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2021, 371, 131176. [Google Scholar] [CrossRef]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.-T.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y.; et al. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Han, Z.; Cui, Y.; Ho, C.-T.; Wan, X.; Zhang, L. Identification of 4-O-p-coumaroylquinic acid as astringent compound of Keemun black tea by efficient integrated approaches of mass spectrometry, turbidity analysis and sensory evaluation. Food Chem. 2022, 368, 130803. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Tan, S.; Zheng, Q.; Zhao, X.; Gao, X.; Lu, Y. Citric acid-enhanced dissolution of polyphenols during soaking of different teas. J. Food Biochem. 2019, 43, e13046. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, F.; Kang, J.; Li, Q.; Warusawitharana, H.K.; Li, B. Quality Chemistry, Physiological Functions, and Health Benefits of Organic Acids from Tea (Camellia sinensis). Molecules 2023, 28, 2339. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Ren, X.; Xia, T.; Li, X. LC–MS/MS-based metabolomic analysis of caffeine-degrading fungus Aspergillus sydowii during tea fermentation. J. Food Sci. 2020, 85, 477–485. [Google Scholar] [CrossRef]
- Bian, X.; Miao, W.; Zhao, M.; Zhao, Y.; Xiao, Y.; Li, N.; Wu, J.-L. Microbiota drive insoluble polysaccharides utilization via microbiome-metabolome interplay during Pu-erh tea fermentation. Food Chem. 2022, 377, 132007. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Zheng, C.; Zhou, B.; Xu, C.; Xia, T. Comparison of characteristic components in tea-leaves fermented by Aspergillus pallidofulvus PT-3, Aspergillus sesamicola PT-4 and Penicillium manginii PT-5 using LC-MS metabolomics and HPLC analysis. Food Chem. 2021, 350, 129228. [Google Scholar] [CrossRef] [PubMed]
- Scharbert, S.; Hofmann, T. Molecular Definition of Black Tea Taste by Means of Quantitative Studies, Taste Reconstitution, and Omission Experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Rong, Y.; Qian, M.C.; Liu, F.; Hua, J.; Zhou, Q.; Deng, Y.; Zeng, J.; Jiang, Y. Lipid metabolic characteristics and marker compounds of ripened Pu-erh tea during pile fermentation revealed by LC-MS-based lipidomics. Food Chem. 2023, 404, 134665. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, C.; Gong, J. Effects of enzymatic action on the formation of theabrownin during solid state fermentation of Pu-erh tea. J. Sci. Food Agric. 2011, 91, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, W.; Zhu, P.; Peng, Z.; Zheng, T.; Li, D.; Li, J.; Zhou, G.; Du, G.; Zhang, J. Profiling the role of microorganisms in quality improvement of the aged flue-cured tobacco. BMC Microbiol. 2022, 22, 197. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Luo, Y.; Xiao, L.; Wang, K.; Huang, J.; Liu, Z. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. Lwt 2020, 127, 109355. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, Y.; Shi, J.; Wang, J.; Wang, M.; Shao, C.; Yan, H.; Lin, Z.; Lv, H. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chem. 2020, 346, 128906. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, Y.; Ma, S.; Shi, J.; Yan, H.; Lin, Z.; Lv, H. Aroma characterisation of Liu-pao tea based on volatile fingerprint and aroma wheel using SBSE-GC–MS. Food Chem. 2023, 414, 135739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, J.; Li, Z.; Li, Q.; Lai, X.; Sun, L.; Chen, R.; Wen, S.; Sun, S.; Lai, Z. HS-SPME and GC/MS volatile component analysis of Yinghong No. 9 dark tea during the pile fermentation process. Food Chem. 2021, 357, 129654. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, R.; Ai, Z.; Shi, J.Y.Z.C.; Desk, S. Characteristics of Keemun Black Tea, Dark Tea and Green Tea Processed from C. Sinensis Var. Zhuye. J. Food Sci. Technol. 2020, 5, 111–124. [Google Scholar]
- Wang, Z.; Li, H.; Huang, W.; Duan, S.; Yan, Y.; Zeng, Z.; Fang, Z.; Li, C.; Hu, B.; Wu, W.; et al. Landscapes of the main components, metabolic and microbial signatures, and their correlations during pile-fermentation of Tibetan tea. Food Chem. 2024, 430, 136932. [Google Scholar] [CrossRef]
- Li, J.; Xu, R.; Zong, L.; Brake, J.; Cheng, L.; Wu, J.; Wu, X. Dynamic Evolution and Correlation between Metabolites and Microorganisms during Manufacturing Process and Storage of Fu Brick Tea. Metabolites 2021, 11, 703. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Y.; Li, F.; Weng, X.; Wei, X. Regulation of fungal community and the quality formation and safety control of Pu-erh tea. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4546–4572. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Factories 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Chu, J.; Cai, W.; Ma, H.; Zhu, W.; Zhang, X.; Ren, J.; Xiao, L.; Liu, D.; Liu, X. Microbial Succession and Interactions During the Manufacture of Fu Brick Tea. Front. Microbiol. 2022, 13, 892437. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Billet, K.; Torres-Cobos, B.; Vichi, S.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Use of a Minimal Microbial Consortium to Determine the Origin of Kombucha Flavor. Front. Microbiol. 2022, 13, 836617. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Luo, Y.; Zhang, Y.; Chen, Y.; Lin, H.; Wang, K.; Huang, J.; Liu, Z. Shifts in diversity and function of the bacterial community during the manufacture of Fu brick tea. Food Microbiol. 2019, 80, 70–76. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Sun, L.; Chen, R.; Li, Q.; Lai, X.; Cao, J.; Lai, Z.; Zhang, Z.; Li, Q.; Song, G.; et al. Metabolome and Microbiome Analysis to Study the Flavor of Summer Black Tea Improved by Stuck Fermentation. Foods 2023, 12, 3414. https://doi.org/10.3390/foods12183414
Wen L, Sun L, Chen R, Li Q, Lai X, Cao J, Lai Z, Zhang Z, Li Q, Song G, et al. Metabolome and Microbiome Analysis to Study the Flavor of Summer Black Tea Improved by Stuck Fermentation. Foods. 2023; 12(18):3414. https://doi.org/10.3390/foods12183414
Chicago/Turabian StyleWen, Lianghua, Lingli Sun, Ruohong Chen, Qiuhua Li, Xingfei Lai, Junxi Cao, Zhaoxiang Lai, Zhenbiao Zhang, Qian Li, Guang Song, and et al. 2023. "Metabolome and Microbiome Analysis to Study the Flavor of Summer Black Tea Improved by Stuck Fermentation" Foods 12, no. 18: 3414. https://doi.org/10.3390/foods12183414





