Enhancing the Flavor Profile of Summer Green Tea via Fermentation with Aspergillus niger RAF106
Abstract
:1. Introduction
2. Materials and Methods
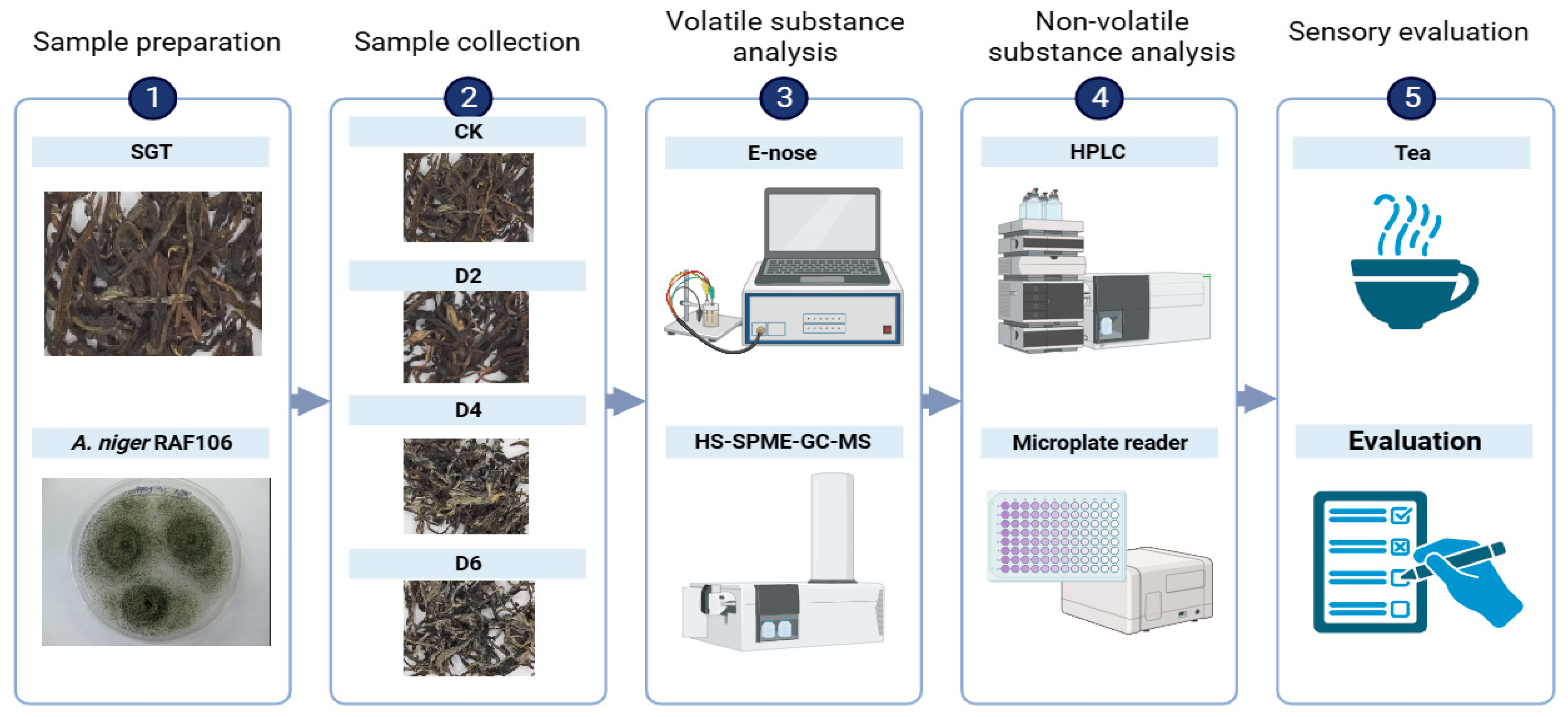
2.1. Schematic Overview of the Experimental Program
2.2. Chemicals
2.3. Preparation of Tea Samples
2.4. Volatile Substance Extraction and Analysis
2.4.1. E-Nose Analysis
2.4.2. HS-SPME-GC-MS Analysis
2.5. Non-Volatile Extraction and Analysis
2.5.1. Quantitative Analysis of Catechins and Gallic Acid
2.5.2. Quantitative Analysis Organic Acid
2.5.3. Analysis of Tea Polyphenols
2.5.4. Analysis of Total Free Amino Acids
2.5.5. Analysis of General Flavone
2.5.6. Quantitative Analysis of Caffeine
2.5.7. Analysis of Soluble Sugars via Ultraviolet Spectrophotometry
2.6. Sensory Evaluation
2.7. Data Analysis
3. Results
3.1. Changes of Tea Volatiles during Fermentation
3.1.1. E-Nose Evaluation
3.1.2. Volatile Compounds Identified by HS-SPME-GC-MS
3.2. Changes of Non-Volatile Components in Light Fermentation Tea
3.3. Sensory Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jeyaraj, A.; Yu, H.; Wang, Y.; Ma, Q.; Chen, X.; Sun, H.; Zhang, H.; Ding, Z.; Li, X. Metabolic Regulation Profiling of Carbon and Nitrogen in Tea Plants [Camellia sinensis (L.) O. Kuntze] in Response to Shading. J. Agric. Food Chem. 2020, 68, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Yu, Z.Y.; Xu, X.J.; You, Q.L.; Zhang, H.M. Study on the relationship between tea polyphenol content and catechin content. Chem. Biol. Eng. 2007, 11, 73–75. (In Chinese) [Google Scholar]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted Analysis Using Ultraperformance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Lee, L.S.; Choi, J.H.; Son, N.; Kim, S.H.; Park, J.D.; Jang, D.J.; Jeong, Y.; Kim, H.J. Metabolomic Analysis of the Effect of Shade Treatment on the Nutritional and Sensory Qualities of Green Tea. J. Agric. Food Chem. 2013, 61, 332–338. [Google Scholar] [CrossRef]
- Xu, W.; Peng, Y.Q.; Zhang, T.; Kong, Y.; Xiao, W. Dynamic changes of main taste substances in green tea processing and their effects on green tea quality. Food Sci. 2019, 40, 36–41. (In Chinese) [Google Scholar]
- Zhu, Y.; Luo, Y.; Wang, P.; Zhao, M.; Li, L.; Hu, X.; Chen, F. Simultaneous determination of free amino acids in Pu-erh tea and their changes during fermentation. Food Chem. 2016, 194, 643–649. [Google Scholar] [CrossRef]
- Hu, T.; Shi, S.; Ma, Q. Modulation effects of microorganisms on tea in fermentation. Front. Nutr. 2022, 9, 931790. [Google Scholar] [CrossRef]
- Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Available online: https://www.mdpi.com/2076-3921/9/5/447 (accessed on 28 August 2023).
- Li, X.L.; Chen, H.H.; Zhang, J.L.; Wang, B.; Zhou, H.J. Effects of yeast strains on main functional components of Pu ‘er tea. Food Res. Dev. 2017, 38, 167–172. (In Chinese) [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, Y.; Liu, M.; Li, X.; Lai, Y.; Liu, X.; Tan, L.; Tang, Q.; Chen, W.; Li, D.; et al. Metagenomics reveal the role of microorganism and GH genes contribute to Sichuan South-road dark tea quality formation during pile fermentation. LWT 2023, 178, 114618. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhong, K.; Wu, Y.; Gao, H. Delving into the Biotransformation Characteristics and Mechanism of Steamed Green Tea Fermented by Aspergillus niger PW-2 Based on Metabolomic and Proteomic Approaches. Foods 2022, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Du, M.; Liu, T.; Fang, Q.A.; Liao, Z.; Zhong, Q.; Chen, J.; Meng, X.; Zhou, S.; Wang, J. Changes in the Biotransformation of Green Tea Catechins Induced by Different Carbon and Nitrogen Sources in Aspergillus niger RAF106. Front. Microbiol. 2019, 10, 2521. [Google Scholar] [CrossRef]
- GB/T 8313-2018; Method for the Determination of Tea Polyphenols and Catechins in Tea. China National Institute of Standardization (CNIS): Beijing, China, 2018.
- 15. GB/T 8314-2013; Determination of total free amino acids. China National Institute of Standardization (CNIS): Beijing, China, 2013.
- Haldar, D.; Sen, D.; Gayen, K. Development of Spectrophotometric Method for the Analysis of Multi-component Carbohydrate Mixture of Different Moieties. Appl. Biochem. Biotechnol. 2017, 181, 1416–1434. [Google Scholar] [CrossRef] [PubMed]
- GB/T 23776-2018; Methodology for sensory evaluation. China National Institute of Standardization (CNIS): Beijing, China, 2018.
- Xiao, Y.; Tan, H.; Huang, H.; Yu, J.; Zeng, L.; Liao, Y.; Wu, P.; Yang, Z. Light synergistically promotes the tea green leafhopper infestation-induced accumulation of linalool oxides and their glucosides in tea (Camellia sinensis). Food Chem. 2022, 394, 133460. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.P.; Zhang, Y.; Dai, W.D.; Guo, L.; Tan, J.F.; Peng, Q.H.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.P.; Dai, W.D.; Guo, L.; Tan, J.F.; Zhang, Y.; Yu, F.L.; Shao, C.Y.; Peng, Q.H.; Lin, Z. Separation of aroma components in Xihu Longjing tea using simultaneous distillation extraction with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Sep. Purif. Technol. 2016, 164, 146–154. [Google Scholar] [CrossRef]
- Li, M.Y.; Liu, H.Y.; Wu, D.T.; Kenaan, A.; Geng, F.; Li, H.B.; Gunaratne, A.; Li, H.; Gan, R.Y. L-Theanine: A Unique Functional Amino Acid in Tea (Camellia sinensis L.) With Multiple Health Benefits and Food Applications. Front. Nutr. 2022, 9, 853846. [Google Scholar] [CrossRef]
- Wu, H.Y.; Liu, X.H.; Luo, L.X.; He, Q.X. Study on aroma components of 12 kinds of single cluster tea. Food Ind. Sci. Technol. 2019, 40, 234–239. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, M.; Su, X.Q.; Nian, B.; Chen, L.J.; Zhang, D.L.; Duan, S.M.; Wang, L.Y.; Shi, X.Y.; Jiang, B.; Jiang, W.W.; et al. Integrated Meta-omics Approaches To Understand the Microbiome of Spontaneous Fermentation of Traditional Chinese Pu-erh Tea. Msystems. 2019, 4, e00680-19. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, Y.H.; Zhang, F.; Yang, Q.M.; Weng, H.F.; Xiao, Q.; Xiao, A.F. Thermostable Tannase from Aspergillus Niger and Its Application in the Enzymatic Extraction of Green Tea. Molecules 2020, 25, 952. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Liu, T.R.; Fan, F.Y.; Gong, S.Y.; Song, C.J. Optimization of HPLC method for determination of organic acids and its application in detection of white tea. Chin. J. Food Sci. 2021, 21, 320–327. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, L.; Huang, J.A.; Gong, Z.H.; Chen, J.H.; Li, Y.H.; Yuan, Y.; Xiao, W.J. Establishment and application of reversed-phase high performance liquid chromatography for organic acid analysis of Fuzhuan tea. Chin. Agric. Sci. Bull. 2011, 27, 246–252. (In Chinese) [Google Scholar]
- Namal Senanayake, S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, B.; Long, F.; Wei, J.; Zhang, Y.; Cui, Y.; Yuan, Y.; Yue, T. Comparison of chemical constituents of Eurotium cristatum-mediated pure and mixed fermentation in summer-autumn tea. LWT 2021, 143, 111132. [Google Scholar] [CrossRef]
- Mao, A.; Su, H.; Fang, S.; Chen, X.; Ning, J.; Ho, C.; Wan, X. Effects of roasting treatment on non-volatile compounds and taste of green tea. Int. J. Food Sci. Technol. 2018, 53, 2586–2594. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Ren, X.; Xia, T.; Li, X. LC–MS/MS-based metabolomic analysis of caffeine-degrading fungus Aspergillus sydowii during tea fermentation. J. Food Sci. 2020, 85, 477–485. [Google Scholar] [CrossRef]
- Zheng, W.; Wan, X.; Bao, G. Brick dark tea: A review of the manufacture, chemical constituents and bioconversion of the major chemical components during fermentation. Phytochem. Rev. 2015, 14, 499–523. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, S.M.; Lee, J.H.; Lim, S.T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.I.; Zhang, N.A.; Cao, Q.; Schwab, W.; Li, D.; Song, C. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Zhao, J.; Wang, Y.H.; Tang, Q.; Khan, I.A. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef]
- Wallace, M.T. Multisensory Perception: The Building of Flavor Representations. Curr. Biol. 2015, 25, R986–R988. [Google Scholar] [CrossRef] [PubMed]


| No. | Volatile Compounds | Relative Content (%) | Odor Description | |||
|---|---|---|---|---|---|---|
| CK | D2 | D4 | D6 | |||
| Alcohol | ||||||
| 1 | Linalool | 10.61 ± 0.29 c | 13.28 ± 0.19 b | 14.34 ± 0.37 a | 13.95 ± 0.55 ab | Floral, sweet 1 |
| 2 | Dihydrolinalool | 10.41 ± 0.42 a | 4.65 ± 0.22 b | 3.24 ± 0.09 c | 2.38 ± 0.11 d | Rose, wood, fruity 2 |
| 3 | (E)-Oxidized linalool (Furan type) | 4.1 ± 0.12 d | 4.98 ± 0.27 c | 7.87 ± 0.11 b | 13.33 ± 0.39 a | Floral 1,3 |
| 4 | Oxidized linalool (Pyranoid type) | 3.79 ± 0.07 b | 2.73 ± 0.1 c | 3.32 ± 0.88 bc | 6.47 ± 0.27 a | Floral 1,3 |
| 5 | α-Terpinol | 3.08 ± 0.03 | ND | ND | ND | - |
| 6 | 1-Octene-3-ol | 2.76 ± 0.19 | ND | ND | ND | Mushroom flavor 1 |
| 7 | (Z)-α,α,5-Trimethyl-5-vinyltetrahydrofuran-2-methanol | 1.61 ± 0.01 d | 2.41 ± 0.15 c | 3.72 ± 0.1 b | 5.32 ± 0.09 a | Floral 2 |
| 8 | 1-Methylcycloheptanol | 1.17 ± 0.02 | ND | ND | ND | - |
| 9 | Palmityl alcohol | 0.98 ± 0.01 b | 1.01 ± 0.01 a | 0.47 ± 0.02 c | 0.41 ± 0.03 d | Floral, oily 2 |
| 10 | Nerol | 0.78 ± 0.18 | ND | ND | ND | Floral, fresh, citrus 1 |
| 11 | 3-Furanyl alcohol | ND | 0.79 ± 0.12 a | 0.64 ± 0.1 a | ND | - |
| 12 | Geraniol | ND | 0.26 ± 0.37 | ND | ND | Rose 1,3 |
| 13 | Benzyl alcohol | ND | 2.2 ± 0.24 a | 1.34 ± 0.49 a | ND | Floral 1,3 |
| 14 | Phenylethanol | ND | 4.45 ± 1.4 b | 8.05 ± 1.06 a | 4.77 ± 0.99 b | Rose-like 1 |
| 15 | 2,6-Dimethyl-3,7-octadiene-3,6-diol | ND | ND | ND | 1.66 ± 0.03 | - |
| 16 | (+)-α-Terpinol | ND | 2.19 ± 0.14 c | 2.8 ± 0.03 b | 3.13 ± 0.08 a | Floral 2 |
| 17 | Ionol | ND | 2.84 ± 0.09 c | 3.1 ± 0.22 b | 3.38 ± 0.05 a | Violet-like 2 |
| Alkanes | - | |||||
| 1 | Norphytane | 3.61 ± 0.59 a | 1.61 ± 0.35 b | 0.77 ± 0.32 b | 1.06 ± 0.38 b | Alkane-like 1 |
| 2 | 3-Methylpentadecane | 2.88 ± 0.07 c | 4.39 ± 0.09 a | 5.29 ± 0.51 a | 3.18 ± 0.12 b | - |
| 3 | 2,6,10-Trimethylpentadecane | 1.72 ± 0.15 a | 0.58 ± 0.14 b | 0.29 ± 0.23 b | 0.54 ± 0.13 b | Alkane-like 1 |
| 4 | 4-Methyltetradecane | 1.39 ± 0.03 a | 1.3 ± 0.01 b | 1.03 ± 0.04 c | 0.85 ± 0.01 d | Alkane-like 1 |
| 5 | N-Dodecane | 1.2 ± 0.05 d | 6.72 ± 0.22 a | 5.94 ± 0.13 b | 5.04 ± 0.12 c | Honeysuckle-like 1 |
| 6 | Theospiran | 1.17 ± 0.02 | ND | ND | ND | Pine wood-like 2 |
| 7 | 5-Methylpentadecane | 1.08 ± 0.01 | ND | ND | ND | Alkane-like 1 |
| 8 | Tridecane | 0.93 ± 0.03 a | ND | ND | 0.35 ± 0.02 b | Alkane-like 1 |
| 9 | 3-Methyltetradecane | 0.89 ± 0.04 | ND | ND | ND | Alkane-like 1 |
| 10 | 5-Methyltridecane | 0.57 ± 0.04 a | ND | ND | 0.46 ± 0.11 a | - |
| 11 | Cyclotetradecane | ND | 0.46 ± 0.02 a | ND | 0.91 ± 0.01 a | - |
| 12 | 9-Methylnonadecane | ND | 1.09 ± 0.02 b | 1.31 ± 0.05 a | 1.22 ± 0.09 ab | Alkane-like 1 |
| 13 | 9-Methyl-octadecane | ND | 0.55 ± 0.05 a | 0.47 ± 0.02 a | 0.48 ± 0 a | Alkane-like 1 |
| 14 | 9-Methyl-eicosane | ND | ND | ND | 0.5 ± 0.04 | - |
| 15 | 7-Methylheptadecane | ND | 1.65 ± 0.08 a | 1.13 ± 0.02 b | 0.89 ± 0.01 c | - |
| 16 | 6-Hexylpentadecane | ND | ND | ND | 0.36 ± 0.07 | - |
| 17 | 4-Ethyltetradecane | ND | 0.59 ± 0.01 a | ND | 0.5 ± 0.02 b | - |
| 18 | 4-Methylhexadecane | ND | 0.72 ± 0.01 a | ND | ND | - |
| 19 | 4,6-Dimethyldodecane | ND | ND | ND | 0.5 ± 0.01 | - |
| 20 | 3-Methylundecane | ND | 2.07 ± 0.36 a | 1.88 ± 0.08 a | 1.33 ± 0.05 b | - |
| 21 | 3-Methyltridecane | ND | 5.9 ± 0.09 a | ND | 4.14 ± 0.08 a | Alkane-like 1 |
| 22 | 3-Methylheptadecane | ND | 0.41 ± 0.03 a | 0.38 ± 0.02 a | 0.31 ± 0.03 b | Alkane-like 1 |
| 23 | 2,3-Dimethylundecane | ND | ND | ND | 0.22 ± 0.04 | - |
| 24 | 10-Methylnonadecane | ND | ND | 1.13 ± 0.06 a | 0.68 ± 0.02 b | - |
| 25 | 10-Methyleicosane | ND | 0.64 ± 0 a | 0.51 ± 0.02 b | 0.4 ± 0.01 c | - |
| 26 | Decane | ND | ND | 0.44 ± 0.04 a | 0.39 ± 0.01 a | - |
| ketone | ||||||
| 1 | Ethyl ionone | 8.99 ± 0.12 a | 2.3 ± 0.06 b | 1.69 ± 0.01 c | 1 ± 0.07 d | Violet-like 2 |
| 2 | Geranyl acetone | 4.02 ± 0.13 a | 0.44 ± 0.05 c | 0.6 ± 0 b | ND | Fruital 2 |
| 3 | Ionone | 2.11 ± 0.01 a | 0.36 ± 0 b | ND | ND | Floral, violet 1,3 |
| 4 | (Z)-1-Methyldicyclodecane-2,10-dione | 1.92 ± 0.09 a | 0.7 ± 0.03 b | ND | ND | - |
| 5 | 2,2,6-Trimethylcyclohexanone | 1.19 ± 0.05 | ND | ND | ND | - |
| 6 | Jasmonone | 0.93 ± 0.04 b | 1.37 ± 0.09 a | 0.31 ± 0.02 c | ND | Floral 2 |
| 7 | Isopropylidene acetone | 0.8 ± 0.03 | ND | ND | ND | Mint, honey-like 2 |
| 8 | 1,5-Dihydro-3,4-dimethyl-2h-pyrrolo-2-one | 0.69 ± 0.02 a | 0.45 ± 0.04 b | ND | ND | White bread crust 2 |
| 9 | Phytoketone | 0.63 ± 0.04 a | ND | 0.22 ± 0.16 b | 0.16 ± 0.01 b | Oily, woody 2 |
| 10 | Methyl octyl ketone | 0.32 ± 0.04 | ND | ND | ND | Floral 2 |
| 11 | Tetrahydrofuran-2,2,6-trimethyl-6-ethylene-3-pyranone | ND | ND | ND | 0.42 ± 0.04 | Floral, honey-like 2 |
| Olefins | ||||||
| 1 | (R)-1-Methyl-5-(1-Methylvinyl) cyclohexene | 2.09 ± 0.02 | ND | ND | ND | - |
| 2 | β-Caryophyllene | 1.5 ± 0.05 | ND | ND | ND | Floral 1 |
| 3 | 1-Isopropyl, 2,3, 5,6,8-4,7-dimethyl -1a-hexahydronaphthalene | 1.4 ± 0.01 | ND | ND | ND | Herbal, woody 2 |
| 4 | (E)-5-Eicosene | 1 ± 0.01 | ND | ND | ND | - |
| 5 | (3E,7E)-4,8,12-Trimethyltridecyl-1,3, 7,11-tetraene | 0.88 ± 0.03 | ND | ND | ND | - |
| 6 | α-Farnesene | 1.07 ± 0.19 | ND | ND | ND | Citrus, herbal 2 |
| 7 | Ocimene | 0.58 ± 0.04 a | ND | ND | 0.42 ± 0.01 b | Citrus, sweet 2 |
| 8 | Artemisintriene | ND | ND | 0.52 ± 0.02 | ND | - |
| 9 | 2,4,6-Trimethyl-1,3,6-heptriene | ND | 0.73 ± 0.06 | ND | ND | - |
| 10 | 1-Tetradecene | ND | ND | 1.04 ± 0.01 | ND | - |
| 11 | 2,4-Dimethyl-2-pentene | ND | ND | ND | 0.12 ± 0.02 | - |
| 12 | (-)-β-Pinene | ND | 0.5 ± 0.03 a | 0.38 ± 0.02 b | 0.35 ± 0.03 b | - |
| 13 | (+)-Limonene | ND | 1.39 ± 0.14 a | 1.39 ± 0.02 a | 1.09 ± 0.03 b | Fruity, lemon-like 2 |
| 14 | 2-Ethyl-1-dodecene | ND | 1.7 + 0.15 a | 1.26 + 0.04 b | 0.96 + 0.04 c | - |
| 15 | Neophytadiene | ND | ND | ND | 0.13 + 0.04 | - |
| 16 | 4-Hydroxymethyl, alcohol-3-cyclohexene | ND | ND | 1.3 ± 0.18 b | 5.22 ± 0.26 a | - |
| Aldehydes | ||||||
| 1 | β-Cyclocitral | 2.3 ± 0.06 c | 0.67 ± 0.12 a | 0.54 ± 0.03 a | 0.41 ± 0.02 b | Fruity 2 |
| 2 | Safranal | 1.48 ± 0.03 a | 0.85 ± 0.27 b | ND | ND | Woody, spicy, phenolic 1 |
| 3 | Phenylacetaldehyde | 0.87 ± 0.03 | ND | ND | ND | Honey 1 |
| 4 | 2-Methylbutyraldehyde | 0.78 ± 0.04 | ND | ND | ND | Fruity 1 |
| 5 | Benzaldehyde | 0.6 ± 0.03 | ND | ND | ND | Caramel, fruity, bitter almond, burnt sugar 1 |
| 6 | Isobutyraldehyde | 0.28 ± 0.03 | ND | ND | ND | - |
| 7 | Isovaleraldehyde | 0.23 ± 0.04 | ND | ND | ND | - |
| Esters | ||||||
| 1 | Dihydrokiwifolactone | 2.61 ± 0.05 a | 0.91 ± 0.04 b | 0.49 ± 0.02 c | ND | Sweet 2 |
| 2 | Methyl salicylate | 0.87 ± 0.07 b | 1.11 ± 0.03 a | 0.76 ± 0.11 b | 0.52 ± 0.05 c | Peppermint 1,3 |
| 3 | Methyl palmitate | ND | ND | 0.37 ± 0.02 a | 0.43 ± 0.1 a | Oily, waxy, fatty 1 |
| Oxynitride | ||||||
| 1 | Caffeine | 1.62 ± 0.21 a | 0.55 ± 0 b | 0.84 ± 0.95 ab | 1.19 ± 0.31 a | - |
| 2 | N-Ethylsuccinimide | 1.21 ± 0.03 a | ND | ND | 0.86 ± 0.39 a | - |
| 3 | Theapyrrole | 1.11 ± 0.02 c | 6.27 ± 0.48 b | 7.81 ± 0.42 a | 6.9 ± 0.52 ab | - |
| Other | ||||||
| 1 | 2-N-Amylfuran | 0.88 ± 0.13 | ND | ND | ND | - |
| 2 | 3,7-Octanediene-2,6-diol, 2,6-dimethyl | ND | 2.89 ± 0.13 a | 2.07 ± 0.05 b | ND | - |
| 3 | 3,4-Diethylbiphenyls | 0.58 ± 0.03 | ND | ND | ND | - |
| Compound | Content (%) | |||
|---|---|---|---|---|
| CK | D2 | D4 | D6 | |
| Catechins and gallic acid * | ||||
| EGC | 1.46 ± 0.089 c | 2.79 ± 0.153 b | 3.15 ± 0.239 a | 3.26 ± 0.101 a |
| C | 0.45 ± 0.027 a | 0.52 ± 0.031 a | 0.45 ± 0.008 a | 0.41 ± 0.062 a |
| EC | 1.67 ± 0.092 b | 3.96 ± 0.143 a | 4.09 ± 0.196 a | 3.90 ± 0.18 a |
| Non-ester catechins | 3.58 ± 0.123 c | 7.27 ± 0.181 b | 7.69 ± 0.437 a | 7.57 ± 0.161 ab |
| EGCG | 3.88 ± 0.236 a | 1.32 ± 0.08 b | 0.89 ± 0.025 c | 0.65 ± 0.034 c |
| ECG | 4.27 ± 0.208 a | 1.86 ± 0.103 b | 1.13 ± 0.044 c | 0.88 ± 0.006 c |
| Ester catechins | 8.15 ± 0.441 a | 3.17 ± 0.181 b | 2.02 ± 0.066 c | 1.53 ± 0.033 d |
| GA | 0.12 ± 0.003 c | 3.35 ± 0.086 b | 4.30 ± 0.218 a | 4.65 ± 0.139 a |
| Organic acid | ||||
| Oxalic acid | 1.04 ± 0.074 a | 0.42 ± 0.097 c | 0.87 ± 0.161 ab | 0.66 ± 0.055 bc |
| Quinic acid | 3.73 ± 0.272 a | 3.14 ± 0.299 b | 2.22 ± 0.103 d | 2.57 ± 0.154 c |
| Malic acid | 4.04 ± 0.394 a | 3.03 ± 0.201 b | 1.74 ± 0.074 c | 1.12 ± 0.086 d |
| Lactic acid | 4.63 ± 0.71 c | 24.83 ± 4.184 b | 28.89 ± 1.083 a | 24.1 ± 3.075 b |
| Acetic acid | 3.13 ± 0.476 d | 16 ± 0.991 a | 13.05 ± 1.316 b | 9.48 ± 1.532 c |
| Fumaric acid | 0.36 ± 0.043 b | 0.34 ± 0.061 b | 0.5 ± 0.033 a | 0.2 ± 0.017 c |
| Citric acid | 0.21 ± 0.017 c | 0.23 ± 0.03 c | 0.86 ± 0.064 b | 1.38 ± 0.073 a |
| Others | ||||
| Tea polyphenols | 18.34 ± 0.33 a | 20.59 ± 0.644 a | 19.66 ± 1.032 a | 20 ± 0.692 a |
| Amino acid | 4.73 ± 0.138 c | 5.11 ± 0.181 bc | 5.18 ± 0.071 ab | 5.59 ± 0.137 a |
| Caffeine | 3.86 ± 0.015 a | 3.82 ± 0.044 a | 3.93 ± 0.023 a | 3.57 ± 0.023 b |
| Soluble sugar | 0.68 ± 0.044 c | 1.29 ± 0.085 ab | 1.33 ± 0.045 a | 1.16 ± 0.01 b |
| Total flavone | 0.75 ± 0.06 c | 1.04 ± 0.074 ab | 1.16 ± 0.08 a | 0.84 ± 0.03 bc |
| Sample | Color 1 | Aroma 1 | Taste 1 | Total Ccore 2 | General Evaluation 3 |
|---|---|---|---|---|---|
| CK | 81.5 ± 4.44 | 81.63 ± 9.395 | 88.38 ± 3.377 | 84.97 ± 4.90 | Dull yellow, slightly harsh and stale odour, plain and thin |
| D2 | 90.3 ± 4.41 | 86.83 ± 2.63 | 87.83 ± 5.07 | 88.03 ± 2.81 | Bright yellow, fragrant, mellow and normal |
| D4 | 89.83 ± 4.7 | 85.33 ± 4.03 | 89.17 ± 2.78 | 88.15 ± 2.44 | Bright yellow, fragrant, sweet after taste |
| D6 | 90.01 ± 5.014 | 88.63 ± 6.39 | 92.38 ± 2.875 | 90.92 ± 2.855 | Bright yellow, fragrant, mellow and sweet after taste, moderate acidity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Huang, L.; Dong, S.; Diao, N.; Ye, W.; Peng, Z.; Fang, X. Enhancing the Flavor Profile of Summer Green Tea via Fermentation with Aspergillus niger RAF106. Foods 2023, 12, 3420. https://doi.org/10.3390/foods12183420
Cai M, Huang L, Dong S, Diao N, Ye W, Peng Z, Fang X. Enhancing the Flavor Profile of Summer Green Tea via Fermentation with Aspergillus niger RAF106. Foods. 2023; 12(18):3420. https://doi.org/10.3390/foods12183420
Chicago/Turabian StyleCai, Minyu, Liyan Huang, Sashuang Dong, Nanxin Diao, Weilian Ye, Zhiye Peng, and Xiang Fang. 2023. "Enhancing the Flavor Profile of Summer Green Tea via Fermentation with Aspergillus niger RAF106" Foods 12, no. 18: 3420. https://doi.org/10.3390/foods12183420
APA StyleCai, M., Huang, L., Dong, S., Diao, N., Ye, W., Peng, Z., & Fang, X. (2023). Enhancing the Flavor Profile of Summer Green Tea via Fermentation with Aspergillus niger RAF106. Foods, 12(18), 3420. https://doi.org/10.3390/foods12183420





