Recent Advances in the Determination of Veterinary Drug Residues in Food
Abstract
:1. Introduction
2. Regulatory Overview of Drug Residues in Food
3. Drug Residues in Food
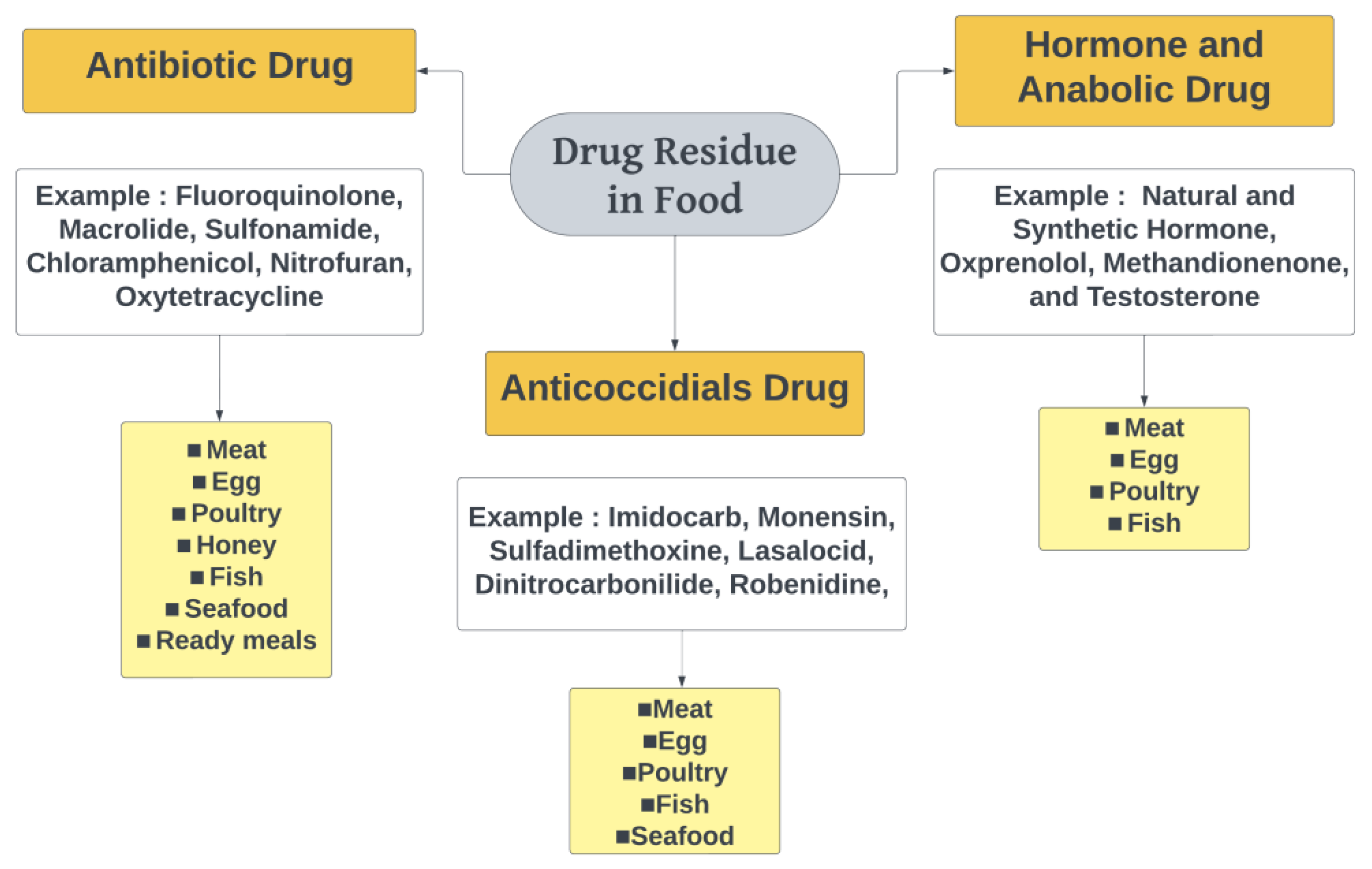
3.1. Classification of Drug Residues in Food
3.1.1. Antibiotics
3.1.2. Anticoccidial Drugs
3.1.3. Hormones and Anabolic Drugs
3.2. Type of Food That Usually Contains Drug Residues
3.2.1. Meat
3.2.2. Egg
3.2.3. Poultry
3.2.4. Honey
3.2.5. Fish
3.2.6. Seafood
3.2.7. Ready Meal
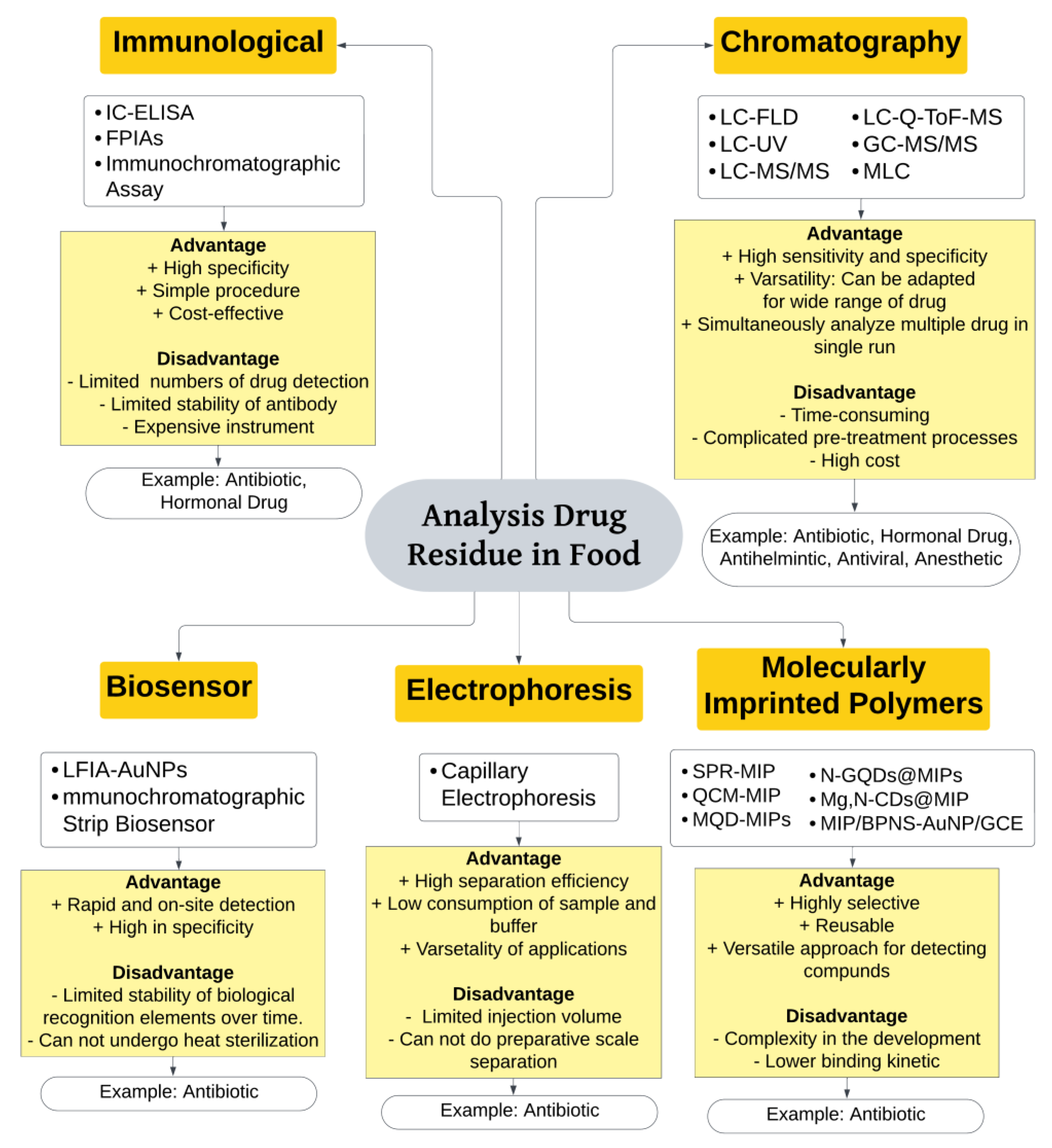
4. Analysis of Drug Residues in Food
4.1. Immunological Technique
4.2. Chromatographic Technique
4.2.1. Liquid Chromatography (LC)
4.2.2. Gas Chromatography
4.3. Biosensor
4.4. Electrophoresis
4.5. Molecularly Imprinted Polymers (MIP)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Zhang, Q.; Chen, M.; Zhang, X.; Liu, P. Determination of Veterinary Drug Residues in Food of Animal Origin: Sample Preparation Methods and Analytical Techniques. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 701–724. [Google Scholar] [CrossRef]
- Food and Drug Administration. CFR—Code of Federal Regulations Title 21. PART 556: Tolerances for Residues of New Animal Drugs in Food; Food and Drug Administration: Silver Spring, MD, USA, 2023.
- Commission European. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Official Journal of the European Union. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:015:0001:0072:en:PDF (accessed on 5 July 2023).
- Khaled, A.; Gionfriddo, E.; Acquaro, V.; Singh, V.; Pawliszyn, J. Development and Validation of a Fully Automated Solid Phase Microextraction High Throughput Method for Quantitative Analysis of Multiresidue Veterinary Drugs in Chicken Tissue. Anal. Chim. Acta 2019, 1056, 34–46. [Google Scholar] [CrossRef]
- Marazuela, M.D. Determination of Veterinary Drug Residues in Foods by Liquid Chromatography-Mass Spectrometry: Basic and Cutting-Edge Applications. In Liquid Chromatography; Elsevier: Amsterdam, The Netherlands, 2017; pp. 539–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.Q.; Li, H.M.; Zhang, Q.H.; Gao, Y.; Li, X.J. Antibiotic Residues in Honey: A Review on Analytical Methods by Liquid Chromatography Tandem Mass Spectrometry. TrAC Trends Anal. Chem. 2019, 110, 344–356. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-Free Optical Biosensors for Food and Biological Sensor Applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Wang, B.; Xie, K.; Lee, K. Veterinary Drug Residues in Animal-Derived Foods: Sample Preparation and Analytical Methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef]
- Fei, A.; Liu, Q.; Huan, J.; Qian, J.; Dong, X.; Qiu, B.; Mao, H.; Wang, K. Label-Free Impedimetric Aptasensor for Detection of Femtomole Level Acetamiprid Using Gold Nanoparticles Decorated Multiwalled Carbon Nanotube-Reduced Graphene Oxide Nanoribbon Composites. Biosens. Bioelectron. 2015, 70, 122–129. [Google Scholar] [CrossRef]
- Du, L.; Liu, W. Occurrence, Fate, and Ecotoxicity of Antibiotics in Agro-Ecosystems. A Review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Leibovici, L.; Paul, M.; Garner, P.; Sinclair, D.J.; Afshari, A.; Pace, N.L.; Cullum, N.; Williams, H.C.; Smyth, A.; Skoetz, N.; et al. Addressing Resistance to Antibiotics in Systematic Reviews of Antibiotic Interventions. J. Antimicrob. Chemother. 2016, 71, 2367–2369. [Google Scholar] [CrossRef]
- Tadesse, T.; Tadesse, T. Public Health Impacts of Antibiotic Residues in Foods of Animal Origin: A Review. Public Health 2017, 7, 6–11. [Google Scholar]
- Turnipseed, S.B.; Jayasuriya, H. Analytical Methods for Mixed Organic Chemical Residues and Contaminants in Food. Anal. Bioanal. Chem. 2020, 412, 5969–5980. [Google Scholar] [CrossRef]
- Hajrulai-Musliu, Z.; Uzunov, R.; Jovanov, S.; Jankuloski, D.; Stojkovski, V.; Pendovski, L.; Sasanya, J.J. A New LC–MS/MS Method for Multiple Residues/Contaminants in Bovine Meat. BMC Chem. 2021, 15, 62. [Google Scholar] [CrossRef]
- Beyene, T. Veterinary Drug Residues in Food-Animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol. 2015, 7, 285. [Google Scholar] [CrossRef]
- Piñeiro, S.A.; Cerniglia, C.E. Antimicrobial Drug Residues in Animal-derived Foods: Potential Impact on the Human Intestinal Microbiome. J. Vet. Pharmacol. Ther. 2021, 44, 215–222. [Google Scholar] [CrossRef]
- Mensah, S.E.; Koudandé, O.D.; Sanders, P.; Laurentie, M.; Mensah, G.A.; Abiola, F.A. Antimicrobial Residues in Foods of Animal Origin in Africa: Public Health Risks. Rev. Sci. Tech. 2014, 33, 987–996. [Google Scholar] [PubMed]
- Bayou, K.; Haile, N. Review on Antibiotic Residues in Food of Animal Origin: Economic and Public Health Impacts. Appl. J. Hyg. 2017, 6, 1–8. [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China. Maxium Residue Level of Veterinary Drugs in Food of Animal Origin; Ministry of Agriculture: Beijing, China, 2002.
- Van Norman, G.A. Drugs and Devices. JACC Basic Transl. Sci. 2016, 1, 399–412. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Veterinary Drug Residues in Food: Sixty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2006; Volume 66. [Google Scholar]
- Joppi, R.; Bertele, V.; Vannini, T.; Garattini, S.; Banzi, R. Food and Drug Administration vs. European Medicines Agency: Review Times and Clinical Evidence on Novel Drugs at the Time of Approval. Br. J. Clin. Pharmacol. 2020, 86, 170–174. [Google Scholar] [CrossRef]
- Ture, M.; Fentie, T.; Regassa, B. Veterinary Drug Residue: The Risk, Public Health Significance and Its Management. Vet. Sci. J. 2019, 13, 555–856. [Google Scholar]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar]
- Hosain, M.Z.; Kabir, S.M.L.; Kamal, M.M. Antimicrobial Uses for Livestock Production in Developing Countries. Vet. World 2021, 14, 210–221. [Google Scholar] [CrossRef]
- Rybicki, M. Coccidiostats in Treating Coccidiosis. Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2020, 125, 127–137. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing Veterinary Drug Residues in Animal Products: A Review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef]
- Tuck, S.; Furey, A.; Danaher, M. Analysis of Anthelmintic and Anticoccidial Drug Residues in Animal & Derived Foods. In Chemical Analysis of Non & Antimicrobial Veterinary Drug Residues in Food; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 245–309. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kang, D.; Lim, M.-W.; Kang, C.S.; Sung, H.J. Risk Assessment of Growth Hormones and Antimicrobial Residues in Meat. Toxicol. Res. 2010, 26, 301–313. [Google Scholar] [CrossRef]
- Tan, X.; Li, Z.; Deng, L.; Zhao, S.; Wang, M. Analysis of 13 Kinds of Steroid Hormones in Raw Milk Using Modified QuEChERS Method Combined with UPLC-QTOF-MS. J. Integr. Agric. 2016, 15, 2163–2174. [Google Scholar] [CrossRef]
- Woodward, K.N. The Toxicity of Particular Veterinary Drug Residues. In Pesticide, Veterinary and Other Residues in Food; CAB International: Wallingford, UK, 2004; pp. 175–223. [Google Scholar]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Hirpessa, B.B.; Ulusoy, B.H.; Hecer, C. Hormones and Hormonal Anabolics: Residues in Animal Source Food, Potential Public Health Impacts, and Methods of Analysis. J. Food Qual. 2020, 2020, 5065386. [Google Scholar] [CrossRef]
- Donovan, C. If FDA Does Not Regulate Food, Who Will? A Study of Hormones and Antibiotics in Meat Production. Am. J. Law Med. 2015, 41, 459–482. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef]
- Karunarathna, N.B.; Perera, I.A.; Nayomi, N.T.; Munasinghe, D.M.S.; Silva, S.S.P.; Strashnov, I.; Fernando, B.R. Occurrence of Enrofloxacin and Ciprofloxacin Residues in Broiler Meat Sold in Sri Lanka. J. Natl. Sci. Found. 2021, 49, 479. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Guo, L.; Xu, X.; Wu, A.; Kuang, H.; Sun, L.; Song, S.; Xu, C. Sensitive Lateral Flow Immunoassay for the Residues of Imidocarb in Milk and Beef Samples. ACS Omega 2021, 6, 2559–2569. [Google Scholar] [CrossRef]
- Temerdashev, A.; Dmitrieva, E.; Azaryan, A.; Gashimova, E. Determination of Oxprenolol, Methandienone and Testosterone in Meat Samples by UHPLC-Q-ToF. Heliyon 2023, 9, e13260. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, X.; Lu, Y.; Guo, Y.; Xie, K.; Chen, L.; Chen, J.; He, Z.; Guan, F.; Gao, P.; et al. Qualitative and Quantitative Determination of Tilmicosin in Poultry Eggs by Gas Chromatography Tandem Mass Spectrometry after Derivatization with Acetic Anhydride. Food Chem. 2022, 384, 132572. [Google Scholar] [CrossRef] [PubMed]
- Vandenberge, V.; Delezie, E.; Huyghebaert, G.; Delahaut, P.; Pierret, G.; De Backer, P.; Croubels, S.; Daeseleire, E. Transfer of the Coccidiostats Monensin and Lasalocid from Feed at Cross-Contamination Levels to Whole Egg, Egg White and Egg Yolk. Food Addit. Contam. Part A 2012, 29, 1881–1892. [Google Scholar] [CrossRef]
- Mi, X.; Li, S.; Li, Y.; Wang, K.; Zhu, D.; Chen, G. Quantitative Determination of 26 Steroids in Eggs from Various Species Using Liquid Chromatography–Triple Quadrupole-Mass Spectrometry. J. Chromatogr. A 2014, 1356, 54–63. [Google Scholar] [CrossRef]
- Feddern, V.; Gressler, V.; Surek, D.; Bedendo, G.C.; Scheuermann, G.N.; Cunha-Junior, A. Determination of Nicarbazin as Dinitrocarbanilide Residues in Chicken Feed, Breast And Litter. In Proceedings of the 7° Simpósio de Segurança Alimentar, Online, 27–29 October 2020. [Google Scholar]
- Morariu, I.D. Immunochemical Assay of Chloramphenicol in Honey. Farmacia 2019, 67, 235–239. [Google Scholar] [CrossRef]
- Melekhin, A.O.; Tolmacheva, V.V.; Shubina, E.G.; Dmitrienko, S.G.; Apyari, V.V.; Grudev, A.I. Determination of Nitrofuran Metabolites in Honey Using a New Derivatization Reagent, Magnetic Solid-Phase Extraction and LC–MS/MS. Talanta 2021, 230, 122310. [Google Scholar] [CrossRef]
- Jayasinghe, G.; Szpunar, J.; Lobinski, R.; Edirisinghe, E. Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Fishes 2023, 8, 154. [Google Scholar] [CrossRef]
- Chafi, S.; Ballesteros, E. A Simple, Efficient, Eco-Friendly Sample Preparation Procedure for the Simultaneous Determination of Hormones in Meat and Fish Products by Gas Chromatography—Mass Spectrometry. Foods 2022, 11, 3095. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Cheng, B.; Dong, J.; Xu, N.; Zhou, S.; Yang, Q.; Ai, X. Development and Validation of a HPLC-HESI-MS/MS Method for Simultaneous Determination of Robenidine Hydrochloride and Its Metabolites in Fish and Exploration of Their Kinetic Regularities in Grass Carp. Food Anal. Methods 2020, 13, 516–529. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Y.; Lin, H.; Sun, Y.; Pan, Y.; Qiao, J.; Lian, H.; Xu, C. Residue Screening and Analysis of Enrofloxacin and Its Metabolites in Real Aquatic Products Based on Ultrahigh-Performance Liquid Chromatography Coupled with High Resolution Mass Spectrometry. Food Chem. 2023, 404, 134757. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, S.; Wu, X.; Liu, L.; Kuang, H.; Xiao, J.; Xu, C. Determination of Robenidine in Shrimp and Chicken Samples Using the Indirect Competitive Enzyme-Linked Immunosorbent Assay and Immunochromatographic Strip Assay. Analyst 2021, 146, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Frida, M.; Resto, M.; Faith, M.; Kennedy, C. A Simple and Sensitive Method for the Detection of Oxytetracycine Levels in Ready-to-Eat Beef by Liquid Chromatography-Mass Spectrometry. Afr. J. Pharm. Pharmacol. 2016, 10, 571–578. [Google Scholar] [CrossRef]
- Wolecki, D.; Caban, M.; Pazdro, K.; Mulkiewicz, E.; Stepnowski, P.; Kumirska, J. Simultaneous Determination of Non-Steroidal Anti-Inflammatory Drugs and Natural Estrogens in the Mussels Mytilus Edulis Trossulus. Talanta 2019, 200, 316–323. [Google Scholar] [CrossRef]
- Di Donna, L.; Benabdelkamel, H.; Taverna, D.; Indelicato, S.; Aiello, D.; Napoli, A.; Sindona, G.; Mazzotti, F. Determination of Ketosteroid Hormones in Meat by Liquid Chromatography Tandem Mass Spectrometry and Derivatization Chemistry. Anal. Bioanal. Chem. 2015, 407, 5835–5842. [Google Scholar] [CrossRef] [PubMed]
- Galarini, R.; Fioroni, L.; Moretti, S.; Pettinacci, L.; Dusi, G. Development and Validation of a Multi-Residue Liquid Chromatography–Tandem Mass Spectrometry Confirmatory Method for Eleven Coccidiostats in Eggs. Anal. Chim. Acta 2011, 700, 167–176. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Zhao, X.; Xie, K.; Diao, Z.; Zhang, G.; Zhang, T.; Dai, G. Determination of Eight Coccidiostats in Eggs by Liquid–Liquid Extraction–Solid-Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2020, 25, 987. [Google Scholar] [CrossRef]
- Ji, X.; Yang, H.; Wang, J.; Zhou, W.; Wang, X.; Qian, M. Evaluation of Tilmicosin Contamination in Eggs Following Its Administration to Laying Hens and Subsequent Assessment of Dietary Risks to Chinese Consumers. J. Food Sci. 2019, 84, 3054–3062. [Google Scholar] [CrossRef]
- Patel, T.; Marmulak, T.; Gehring, R.; Pitesky, M.; Clapham, M.O.; Tell, L.A. Drug Residues in Poultry Meat: A Literature Review of Commonly Used Veterinary Antibacterials and Anthelmintics Used in Poultry. J. Vet. Pharmacol. Ther. 2018, 41, 761–789. [Google Scholar] [CrossRef]
- Mund, M.D.; Khan, U.H.; Tahir, U.; Mustafa, B.-E.-; Fayyaz, A. Antimicrobial Drug Residues in Poultry Products and Implications on Public Health: A Review. Int. J. Food Prop. 2017, 20, 1433–1446. [Google Scholar] [CrossRef]
- Lawal, J.R.; Jajere, S.M.; Geidam, Y.A.; Bello, A.M.; Wakil, Y.; Mustapha, M. Antibiotic Residues in Edible Poultry Tissues and Products in Nigeria: A Potential Public Health Hazard. Int. J. Anim. Vet. Adv. 2015, 7, 55–61. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Msagati, T.A.M.; Williams, A.B.; Benson, N.U. Detection and Quantification of Multiclass Antibiotic Residues in Poultry Products Using Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Detection. Heliyon 2021, 7, e08469. [Google Scholar]
- El Hawari, K.; Mokh, S.; Doumyati, S.; Al Iskandarani, M.; Verdon, E. Development and Validation of a Multiclass Method for the Determination of Antibiotic Residues in Honey Using Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A 2017, 34, 582–597. [Google Scholar] [CrossRef]
- İsmail Emir, A.; Ece, Y.K.; Sinem, R.; Sezer, A.; Özge, E. Validation of Two UHPLC-MS/MS Methods for Fast and Reliable Determination of Quinolone Residues in Honey. Food Addit. Contam. Part A 2021, 38, 807–819. [Google Scholar] [CrossRef]
- Morariu, I.D. Estimation of Quinolones, Ceftiofur, and Thiamphenicol Residues Levels in Honey. Farmacia 2021, 69, 515–520. [Google Scholar] [CrossRef]
- Shendy, A.H.; Al-Ghobashy, M.A.; Gad Alla, S.A.; Lotfy, H.M. Development and Validation of a Modified QuEChERS Protocol Coupled to LC–MS/MS for Simultaneous Determination of Multi-Class Antibiotic Residues in Honey. Food Chem. 2016, 190, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ladan, A.A.; Okolocha, E.C.; Kabir, J.; Bolorunduro, P.I.-O.; Barde, I.J. Assessment of Antimicrobial Drugs Use and Their Residue in the Farmed Fish of Kaduna, Nigeria. Glob. J. Fish. Sci. 2021, 3, 15–26. [Google Scholar] [CrossRef]
- Turnipseed, S.B.; Storey, J.M.; Wu, I.-L.; Gieseker, C.M.; Hasbrouck, N.R.; Crosby, T.C.; Andersen, W.C.; Lanier, S.; Casey, C.R.; Burger, R. Application and Evaluation of a High-Resolution Mass Spectrometry Screening Method for Veterinary Drug Residues in Incurred Fish and Imported Aquaculture Samples. Anal. Bioanal. Chem. 2018, 410, 5529–5544. [Google Scholar] [PubMed]
- Abou-Raya, S.H.; Shalaby, A.R.; Salama, N.A.; Emam, W.H.; Mehaya, F.M. Effect of Ordinary Cooking Procedures on Tetracycline Residues in Chicken Meat. J. Food Drug Anal. 2013, 21, 7. [Google Scholar]
- Nguyen, V. Effect of Cooking Methods on Tetracycline Residues in Pig Meat. Afr. J. Pharm. Pharmacol. 2013, 7, 1448–1454. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Lightfield, A.R. Extract-and-Inject Analysis of Veterinary Drug Residues in Catfish and Ready-to-Eat Meats by Ultrahigh-Performance Liquid Chromatography—Tandem Mass Spectrometry. J. AOAC Int. 2020, 103, 584–606. [Google Scholar] [CrossRef]
- Song, W.; Luo, M.; Li, H.; Xiao, J.; He, X.; Liang, J.; Peng, D. A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues. Foods 2022, 11, 3305. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, H.; Zhang, Y.; Li, Q.; Li, P.; Mari, G.M.; Eremin, S.A.; Shen, J.; Wang, Z. A Robust Homogeneous Fluorescence Polarization Immunoassay for Rapid Determination of Erythromycin in Milk. Foods 2023, 12, 1581. [Google Scholar] [CrossRef]
- He, S.; Liang, D.; Xiong, J.; Wang, Z.; Zheng, P.; Zhang, H.; Ren, Z.; Jiang, H. Development of a Sensitive and Rapid Fluorescence Polarization Immunoassay for High Throughput Screening Eight Glucocorticoids in Beef. J. Pharm. Biomed. Anal. 2022, 214, 114719. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Bao, H.; Xing, K.; Liu, J.; Xia, J.; Lai, W.; Peng, J. Immunochromatographic Assay Based on Time-resolved Fluorescent Nanobeads for the Rapid Detection of Sulfamethazine in Egg, Honey, and Pork. J. Sci. Food Agric. 2021, 101, 684–692. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-Linked Immunosorbent Assay for the Quantitative/Qualitative Analysis of Plant Secondary Metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Shah, K.; Maghsoudlou, P. Enzyme-Linked Immunosorbent Assay (ELISA): The Basics. Br. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef]
- He, T.; Liu, J.; Wang, J.P. Development of a Dihydropteroate Synthase-Based Fluorescence Polarization Assay for Detection of Sulfonamides and Studying Its Recognition Mechanism. J. Agric. Food Chem. 2021, 69, 13953–13963. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, X.; Wen, K.; Zhang, S.; Li, C.; Shen, J. A Highly Sensitive and Class-Specific Fluorescence Polarisation Assay for Sulphonamides Based on Dihydropteroate Synthase. Biosens. Bioelectron. 2015, 70, 1–4. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Coskun, O. Separation Tecniques: Chromatography. North Clin. Istanb. 2016, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, P.; Guo, Y.; He, Z.; Dong, Y.; Tang, Y.; Guan, F.; Zhang, T.; Xie, K. Determination of Levamisole and Mebendazole and Its Two Metabolite Residues in Three Poultry Species by HPLC-MS/MS. Foods 2021, 10, 2841. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, S.; Wu, L.; Li, Y.; Sun, C. Determination of Six Tetracyclines in Eggs and Chicken by Dispersive Liquid-Liquid Microextraction Combined with High-Performance Liquid Chromatography. J. AOAC Int. 2021, 104, 1549–1558. [Google Scholar] [CrossRef]
- Li, Y.; Bu, N.; Chen, J.; Ma, Y.; Yang, Q. Determination of 80 Veterinary Drug Residues and Their Metabolites in Beef by HPLC-MS/MS. J. Food Sci. Technol. 2022, 40, 140–149. [Google Scholar]
- Ye, H.; Li, S.; Xi, Y.; Shi, Y.; Shang, X.; Huang, D. Highly Sensitive Determination of Antibiotic Residues in Aquatic Products by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Antibiotics 2022, 11, 1427. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, J.; Cui, Y.; Wu, G. Determination of Nine Antiviral Drug Residues in Animal Origin Foods by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Sci. Technol. Food Ind. 2023, 44, 332–337. [Google Scholar] [CrossRef]
- Moon, H.; Nam, A.; Muambo, K.E.; Oh, J.-E. Simultaneous Multi-Residue Analytical Method for Anesthetics and Sedatives in Seafood Samples by LC-ESI/MSMS. Food Chem. 2023, 404, 134157. [Google Scholar] [CrossRef]
- Cai, C.; Xiang, Y.; Tian, S.; Hu, Z.; Hu, Z.; Ma, B.; Wu, P. Determination of Β2-Agonist Residues in Fermented Ham Using UHPLC-MS/MS after Enzymatic Digestion and Sulfonic Resin Solid Phase Purification. Molecules 2023, 28, 2039. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Ma, Y.; Dong, S.; Wang, B.; Zhang, Y. Determination of Nine Food-Borne Stimulant Drug Residues in Pork, Egg, and Milk by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Chin. J. Chromatogr. 2022, 40, 148–155. [Google Scholar] [CrossRef]
- Guo, Y.; He, Z.; Gao, P.; Liu, S.; Zhu, Y.; Xie, K.; Dong, Y. Concurrent Determination of Tigecycline, Tetracyclines and Their 4-Epimer Derivatives in Chicken Muscle Isolated from a Reversed-Phase Chromatography System Using Tandem Mass Spectrometry. Molecules 2022, 27, 6139. [Google Scholar] [CrossRef]
- Liu, B.; Xie, J.; Zhao, Z.; Wang, X.; Shan, X. Simultaneous Determination of 11 Prohibited and Restricted Veterinary Drugs and Their Metabolites in Animal-Derived Foods by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with Solid Phase Extraction. Chin. J. Chromatogr. 2021, 39, 406–414. [Google Scholar] [CrossRef]
- Yoo, K.-H.; Park, D.-H.; Abd El-Aty, A.M.; Kim, S.-K.; Jung, H.-N.; Jeong, D.-H.; Cho, H.-J.; Hacimüftüoğlu, A.; Shim, J.-H.; Jeong, J.H.; et al. Development of an Analytical Method for Multi-Residue Quantification of 18 Anthelmintics in Various Animal-Based Food Products Using Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Anal. 2021, 11, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, X.; Diao, Z.; Wang, Y.; Wang, B.; Xie, K.; Wang, X.; Zhang, P. Detection and Determination of Spectinomycin and Lincomycin in Poultry Muscles and Pork by ASE-SPE-GC–MS/MS. J. Food Compos. Anal. 2021, 101, 103979. [Google Scholar] [CrossRef]
- Pawar, R.-P.; Durgbanshi, A.; Bose, D.; Peris-Vicente, J.; Albiol-Chiva, J.; Esteve-Romero, J.; Carda-Broch, S. Determination of Albendazole and Ivermectin Residues in Cattle and Poultry-Derived Samples from India by Micellar Liquid Chromatography. J. Food Compos. Anal. 2021, 103, 104111. [Google Scholar] [CrossRef]
- Meng, H.-L.; Chen, G.-H.; Guo, X.; Chen, P.; Cai, Q.-H.; Tian, Y.-F. Determination of Five Quinolone Antibiotic Residues in Foods by Micellar Electrokinetic Capillary Chromatography with Quantum Dot Indirect Laser-Induced Fluorescence. Anal. Bioanal. Chem. 2014, 406, 3201–3208. [Google Scholar] [CrossRef]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of Pesticides and Veterinary Drug Residues in Food by Liquid Chromatography-Mass Spectrometry: A Review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef]
- Greer, B.; Chevallier, O.; Quinn, B.; Botana, L.M.; Elliott, C.T. Redefining Dilute and Shoot: The Evolution of the Technique and Its Application in the Analysis of Foods and Biological Matrices by Liquid Chromatography Mass Spectrometry. TrAC Trends Anal. Chem. 2021, 141, 116284. [Google Scholar] [CrossRef]
- Furi, M.; Sinaga, S.M.; Putra, E.D.L. Analysis of Amoxicillin and Tetracycline Residues in Chicken Meat Using High Performance Liquid Chromatography-Mass Spectrometry. Indones. J. Pharm. Clin. Res. 2018, 1, 14–20. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-QTOF-MS as a Powerful Analytical Tool for Characterising Phenolic Compounds in Olive-Leaf Extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef]
- Prasad Pawar, R.; Mishra, P.; Durgbanshi, A.; Bose, D.; Albiol-Chiva, J.; Peris-Vicente, J.; García-Ferrer, D.; Esteve-Romero, J. Use of Micellar Liquid Chromatography to Determine Mebendazole in Dairy Products and Breeding Waste from Bovine Animals. Antibiotics 2020, 9, 86. [Google Scholar] [CrossRef]
- Oubahmane, M.; Mihucz, V.G.; Vasanits, A. Recent Trends in the Determination of Organic UV Filters by Gas Chromatography-Mass Spectrometry in Environmental Samples. TrAC Trends Anal. Chem. 2023, 161, 116995. [Google Scholar] [CrossRef]
- Wu, D.; Du, D.; Lin, Y. Recent Progress on Nanomaterial-Based Biosensors for Veterinary Drug Residues in Animal-Derived Food. TrAC Trends Anal. Chem. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, M.O. Aptamer-based Ellipsometric Sensor for Ultrasensitive Determination of Aminoglycoside Group Antibiotics from Dairy Products. J. Sci. Food Agric. 2020, 100, 3386–3393. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Jiménez, Y.; Montoya, Á.; Juan-Borrás, M.; Pascual, N.; Arnau, A.; Escriche, I. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCMD) Immunosensor for Detection of Sulfathiazole in Honey. Food Control 2020, 115, 107296. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Xu, X.; Guo, L.; Xu, L.; Sun, M.; Hu, S.; Kuang, H.; Xu, C.; Li, A. An Overview for the Nanoparticles-Based Quantitative Lateral Flow Assay. Small Methods 2022, 6, 2101143. [Google Scholar]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral Flow Assays: Principles, Designs and Labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef]
- Alhammadi, M.; Yoo, J.; Sonwal, S.; Park, S.Y.; Umapathi, R.; Oh, M.-H.; Huh, Y.S. A Highly Sensitive Lateral Flow Immunoassay for the Rapid and On-Site Detection of Enrofloxacin in Milk. Front. Nutr. 2022, 9, 1036826. [Google Scholar] [CrossRef]
- Lei, X.; Xu, X.; Liu, L.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. Gold-Nanoparticle-Based Multiplex Immuno-Strip Biosensor for Simultaneous Determination of 83 Antibiotics. Nano Res. 2023, 16, 1259–1268. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Gammelgaard, B.; Halvorsen, T.G. Introduction to Pharmaceutical Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kośka, I.; Purgat, K.; Głowacki, R.; Kubalczyk, P. Simultaneous Determination of Ciprofloxacin and Ofloxacin in Animal Tissues with the Use of Capillary Electrophoresis with Transient Pseudo-Isotachophoresis. Molecules 2021, 26, 6931. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ma, S.; Zhu, K.; Wang, M.; Li, J.; Arabi, M.; Liu, H.; Li, Y.; Chen, L. Simultaneous Enrichment/Determination of Six Sulfonamides in Animal Husbandry Products and Environmental Waters by Pressure-Assisted Electrokinetic Injection Coupled with Capillary Zone Electrophoresis. J. Food Compos. Anal. 2020, 88, 103462. [Google Scholar] [CrossRef]
- Semail, N.-F.; Abdul Keyon, A.S.; Saad, B.; Kamaruzaman, S.; Mohamad Zain, N.N.; Lim, V.; Miskam, M.; Wan Abdullah, W.N.; Yahaya, N.; Chen, D.D.Y. Simultaneous Preconcentration and Determination of Sulfonamide Antibiotics in Milk and Yoghurt by Dynamic PH Junction Focusing Coupled with Capillary Electrophoresis. Talanta 2022, 236, 122833. [Google Scholar] [CrossRef] [PubMed]
- Gattu, S.; Crihfield, C.L.; Lu, G.; Bwanali, L.; Veltri, L.M.; Holland, L.A. Advances in Enzyme Substrate Analysis with Capillary Electrophoresis. Methods 2018, 146, 93–106. [Google Scholar] [CrossRef]
- Sastre Toraño, J.; Ramautar, R.; de Jong, G. Advances in Capillary Electrophoresis for the Life Sciences. J. Chromatogr. B 2019, 1118–1119, 116–136. [Google Scholar] [CrossRef]
- Maráková, K.; Opetová, M.; Tomašovský, R. Capillary Electrophoresis-mass Spectrometry for Intact Protein Analysis: Pharmaceutical and Biomedical Applications (2018–March 2023). J. Sep. Sci. 2023, 46, 2300244. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.; Ren, J. Pressure-Assisted Electrokinetic Injection Stacking for Seven Typical Antibiotics in Waters to Achieve Μg/L Level Analysis by Capillary Electrophoresis with UV Detection. Microchem. J. 2019, 146, 1295–1300. [Google Scholar] [CrossRef]
- Shamsi, S.A.; Patel, J. Advances and Strategies for Capillary Electrophoresis in the Characterization of Traditional Chinese Medicine: A Review of the Past Decade (2011–2021). Front. Anal. Sci. 2023, 3, 1059884. [Google Scholar] [CrossRef]
- Britz-McKibbin, P.; Chen, D.D.Y. Selective Focusing of Catecholamines and Weakly Acidic Compounds by Capillary Electrophoresis Using a Dynamic PH Junction. Anal. Chem. 2000, 72, 1242–1252. [Google Scholar] [CrossRef]
- Li, M.; Fan, L.-Y.; Zhang, W.; Cao, C.-X. Stacking and Quantitative Analysis of Lovastatin in Urine Samples by the Transient Moving Chemical Reaction Boundary Method in Capillary Electrophoresis. Anal. Bioanal. Chem. 2007, 387, 2719–2725. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, K.; Wang, L.; Tong, W.; Chen, D.D.Y. Quantitative Analysis of Microcystin Variants by Capillary Electrophoresis Mass Spectrometry with Dynamic PH Barrage Junction Focusing. Electrophoresis 2019, 40, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Tůma, P.; Opekar, F. Detectors in Capillary Electrophoresis. In Analytical Separation Science; Wiley: Hoboken, NJ, USA, 2015; pp. 607–628. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Bereli, N.; Çimen, D.; Hüseynli, S.; Denizli, A. Detection of Amoxicillin Residues in Egg Extract with a Molecularly Imprinted Polymer on Gold Microchip Using Surface Plasmon Resonance and Quartz Crystal Microbalance Methods. J. Food Sci. 2020, 85, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Fang, G.; Deng, J.; Wang, S. A Molecularly Imprinted Polymer Capped Nitrogen-Doped Graphene Quantum Dots System for Sensitive Determination of Tetracycline in Animal-Derived Food. ChemistrySelect 2020, 5, 839–846. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Dong, J.; Liu, C.; Qi, Y.; Fang, G.; Wang, S. A Strong Blue Fluorescent Nanoprobe Based on Mg/N Co-Doped Carbon Dots Coupled with Molecularly Imprinted Polymer for Ultrasensitive and Highly Selective Detection of Tetracycline in Animal-Derived Foods. Sens. Actuators B Chem. 2021, 338, 129809. [Google Scholar] [CrossRef]
- Chen, S.; Su, X.; Yuan, C.; Jia, C.Q.; Qiao, Y.; Li, Y.; He, L.; Zou, L.; Ao, X.; Liu, A. A Magnetic Phosphorescence Molecularly Imprinted Polymers Probe Based on Manganese-Doped ZnS Quantum Dots for Rapid Detection of Trace Norfloxacin Residual in Food. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 253, 119577. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, Label-Free Voltammetric Determination of Norfloxacin Based on Molecularly Imprinted Polymers and Au Nanoparticle-Functionalized Black Phosphorus Nanosheet Nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
| FDA | EU | |
|---|---|---|
| Regulatory approach |
|
|
| Requirement for marketing |
|
|
| Safety and effectiveness assessment |
|
|
| Food Type | Analyte | Method | Concentration (μg/kg) | Maximum Residue Limit (MRL) (μg/kg) | Reference |
|---|---|---|---|---|---|
| Meat | Enrofloxacin and Ciprofloxacin | HPLC-FLD | Enrofloxacin: 130.3–578.6 Ciprofloxacin: 15.7–28.8 | 100 | [37] |
| Imidocarb | Immunochromatography Assay | 50.61 | 50 | [38] | |
| Oxprenolol, Methandienone, and Testosterone | UHPLC-Q-ToF-MS | Oxprenolol: 0.007 Methandienone: 0.03 Testosterone: 0.004 | - | [39] | |
| Egg | Tilmicosin | GC-MS/MS | 18.9 | 75 | [40] |
| Monensin, Salinomycin, and Lasalocid | HPLC-MS/MS and UPLC-MS/MS | HPLC-MS/MS: 0.82–1.73 UPLC-MS/MS: 0.81–1.25 | - | [41] | |
| Pregnenolone, Progesteron, Testosteron, Androstenedione, and Estrone | LC-MS/MS | 19–116.03, 9–89.8, 0.04–0.5, 0.05–21, 1.83–9.3 | - | [42] | |
| Poultry | Enrofloxacin, Sulfadimethoxin, and Tylosin | HPLC-DAD | 371 ± 139, 3750 ± 2180, 4492 ± 1383 | Enrofloxacin: 100 Sulfadimethoxine: 100 Tylosin: 200 | [37] |
| Dinitrocarbanilide | LC-MS/MS | 99 | 200 | [43] | |
| Honey | Chloromaphenicol | Immunochemical assay | <0.9 | 1 | [44] |
| Nitrofurans metabolite | LC-MS/MS | 0.12–0.74 | Nitrofurans: 1.00 | [45] | |
| Fish | Sulfacetamide and Sulfamethoxypyridazine | UPLC-MS/MS | 4.31 ± 0.70 0.75 ± 0.15 | 100 | [46] |
| Natural and Synthetic hormones | GC–MS | 0.030–1.9 | 0.1–10 | [47] | |
| Robenidine Hydrochloride | HPLC-HESI-MS/MS | 4.63 | 5 | [48] | |
| Seafood | CAP TAP, FF, FFA * | HPLC-MS/MS | 0.834–1.81, 0.0615–107, 0.261–243, no detect | 1000 | [49] |
| Robenidine | Ic-ELISA and Immunochromatographic strip assay | 10 | 100 | [50] | |
| Ready meal | Oxytetracycline | LC-MS/MS | 251.40 | 200 | [51] |
| Analyte | Sample | Analytical Method | Sample Extraction | Linearity (Correlation Coefficient) | LOD (µg/kg) | LOQ (µg/kg) | Recovery (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Quinoxaline | Pork, | Ic-ELISA and LC-MS/MS | - | - | 0.48–0.58 | 0.61–0.90 | 73.7–107.8 | - | [70] |
| Swine liver, | |||||||||
| Swine kidney, Chicken, and Chicken liver | |||||||||
| Robenidine | Shrimp and Chicken | Ic-ELISA and immunochromatographic strip assay | Centrifugation | 0.99932 | - | - | 87.8–102.0 | 3.2–5.9 | [50] |
| Erythromycin | Milk | FPIAs | Precipitation | - | 14.08 | - | 96.08–107.77 | - | [71] |
| DMS, BMS, PNS, HCS, BCMS, CS, 6-α-MNPS, and HFCS * | Beef | FPIAs | - | - | 0.23, 0.36, 0.75, 3.57, 0.31, 1.59, 2.26, and 0.74 | 76.5–91.7 | 1.2–7.3 | [72] | |
| - | |||||||||
| Sulfamethazine | Egg, Honey and Pork | Immunochromatographic assay (ICA) | Centrifugation | - | 0.016, 0.049, 0.029 | - | 90.5–113.9 82.4–112.0 79.8–93.4 | - | [73] |
| Imidocarb | Meat | Immunochromatographic assay | - | - | 0.45 | - | 84.5–101.2 | - | [38] |
| Analyte | Sample | Analytical Method | Sample Extraction | Linearity (Correlation Coefficient) | LOD (µg/kg) | LOQ (µg/kg) | Recovery (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Levamisole, Mebendazole, 5-hydroxymebendazole, 2-amino-5-benzoylbenzimidazole | Poultry eggs (Hen, Duck, and Goose) | HPLC-MS/MS | Dispersive liquid–liquid microextraction (DLLME) | ≥0.9990 | 0.04–0.30 | 0.12–0.80 | 86.77–96.94 | 2.06–4.22 | [80] |
| Tetracycline | Eggs and Chicken | HPLC-UV | Dispersive liquid–liquid microextraction (DLLME) | 0.9986–1.000 | 0.219–1.42 | 0.731–4.72 | 87.1–104 | 0.853–8.62 | [81] |
| Veterinary drug | Beef | HPLC-MS/MS | Oasis PRiME HLB solid phase extraction | ≥0.9990 | - | 2.0–5.0 | 60–103 | <20 | [82] |
| Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicolamine | Aquatic products | HPLC-MS/MS | - | >0.992 | <0.01 | 0.02 | 84.0–105 | 0.769–13.7 | [83] |
| Nevirapine, Famciclovir, Abidox, Acyclovir, Imiquimod, Memantine, Amantadine, Oseltamivir and Morpholinoguanidine | Meat, Egg and Milk | HPLC-MS/MS | PRiME HLB solid phase extraction | 0.9991–0.9998 | 0.1–0.5 | 0.3–1.5 | 82.3- 95.7 | 3.2~5.9 | [84] |
| Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol amine | Aquatic product | HPLC-MS/MS | Solid phase extraction (SPE) | >0.992 | 0.01 | 0.02 | 84–105 | 0.769~13.7 | [83] |
| Robenidine hydrochloride | Fish | HPLC-HESI-MS/MS | liquid–liquid extraction (LLE) | ≥0.9985 | <2.5 | <5.0 | 85.8–108.2 | 12.4 | [54] |
| Anesthetics and Sedatives | Flatfish, Eels and Shrimp | LC-ESI/MSMS | Acetonitrile (ACN) only (for flatfish and eel) and 0.1% ammonium acetate in ACN (for shrimp) | >0.98 | 0.2–2.0 | 0.5–5.0 | 64.7–112.5 | 1.0–8.6 | [85] |
| β2-agonists (Clenbuterol, Ractopamine, Salbutamol, and Terbutaline) | Fermented ham | UHPLC-MS/MS | Solid phase extraction (SPE) | 0.997–1.000 | 0.1 | 0.3 | 76.0–102.0 | 1.8–13.3 | [86] |
| Stimulant drugs | Pork, Egg and Milk | UPLC-MS/MS | Hydrolyzed with β-glucuronidase/aryl sulfate esterase in pH 5. 2 ammonium acetatebuffer | >0.99 | 0.3–0.6 | 1.0–2.0 | 65.2–117.0 | 1.3–14.4 | [87] |
| Sulfacetamide, Sulfamethoxypyridazine, Sulfapyridine, Sulfadoxine | Fish and Shrimp | UPLC-MS/MS | Solid-liquid extraction (SLE) | >0.99 | Fish: 0.07–0.42 Shrimp: 0.13–0.48 | Fish: 0.24–1.32 Shrimp: 0.42–1.62 | 75–105 | <20 | [46] |
| Tigecycline, Four tetracyclines and Their three 4-epimer derivatives | Chicken muscle | HPLC-MS/MS | Solid-phase extraction (SPE) | - | 0.06–0.09 | 200 | 89–98 | 5.0 and 6.9 | [88] |
| Chloramphenicols, Nitroimidazoles, Lincosamides, and Macrolides | Eggs, Liquid Milk, Chicken and Freshwater fish | UPLCMS/MS | Solid phase extraction (SPE) | >0.99 | 0.050–0.500 | 0.20–1.5 | 65.3–108.0 | 0.40–21 | [89] |
| Anthelmintics (including Benzimidazoles, Macrocyclic Lactones, Salicylanilides, Substituted Phenols, Tetrahydropyrimidines, and Imidazothiazoles) | Chicken muscle, Pork, Beef, Milk and Egg | LC-MS/MS | Liquid–liquid extractions (LLE) | ≥0.9752 | 0.02–5.50 | 0.06–10 | 61.2–118.4 | ≤19.9 | [90] |
| Natural and Synthetic Hormones | Meat and Fish | GC–MS | Solid phase extraction (SPE) | ≥0.996 | 0.4–15.0 | - | 90–105 | ≤7 | [47] |
| Enrofloxacin Ciprofloxacin | Meat | HPLC-FLD | - | >0.998 |
0.5 2.2 |
1.6 7.5 | 62.0.0–63.3 58.6–60.9 | 11.2–12.5 4.9–6.9 | [37] |
| Oxprenolol, Methandienone and Testosterone | Meat | UHPLC-Q-ToF-MS | Solid phase extraction (SPE) | 0.998, 0.995, 0.996 |
0.25, 1.25, 0.50 |
0.50, 2.50, 1.25 | 89–96 | - | [39] |
| Nitrofurans metabolite | Honey | LC-MS/MS | Magnetic Solid phase extraction (MSPE) | 0.99 | 0.1–0.3 | 0.3–1.0 | >85 | <12 | [45] |
| Spectinomycin and Lincomycin | Poultry (Chicken, Duck and Goose) | GCMS/MS | Accelerated solvent extraction (ASE) and and solid-phase extraction (SPE) | 0.9992–0.9998 | 2.5–4.6 | 5.7–7.6 | 79.7–94.2 | 1.2–3.5 | [91] |
| Tilmicosin | Chicken, Goose, Duck eggs | GC-MS/MS | Liquid–liquid extraction (LLE) dan Solid phase extraction (SPE) | 0.9990 |
3.8, 4.6, 5.6 |
8.4, 9.6, 10.5 | 78.11, 72.80, 74.82 | 1.75, 1.62, 1.46 | [40] |
| Albendazole and Ivermectin | Cattle and Poultry | MLC | Batch stirring-assisted solid-to-liquid extraction (BSASLE) | >0.999 | 10 | 25 | 86.3–105.6 | <12.2 | [92] |
| Analyte | Sample | Analytical Method | Linearity (R2) | LOD (ng/mL) | LOQ | Recovery (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Enrofloxacin | Milk | AuNPs | 0.9266 | 20 | - | - | - | [107] |
| Antibiotic drug | Aquaculture fish | ISB multiplex | 0.9981 | - | - | 87.5–115.2 | <9.5 | [108] |
| Analyte | Sample | Analytical Method | Sample Extraction | Linearity (R2) | LOD (µg/kg) | LOQ (µg/kg) | Recovery (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin Ofloxacin | Meat tissues (Chicken liver, Chicken kidneys, Duck liver, and Turkey liver) | CE-UV | Liquid–liquid extraction | 0.9988 0.9987 | 0.0994 0.0361 | 0.27 0.11 | 85–115 | <15 | [110] |
| SMZ, SMR, SMM, SDZ, SMX, SFA * | Milk, Pork, and Egg | CE-UV | - | 0.9931–0.9994 | 1.8–16.3 8.3–63.8 5.2–47.8 | 6.1–50.3, 25.3–182.6, 16.4–147.5 | 89–107, 96–113, 95–109 | 1.7–6.7, 2.2–7.3, 1.6–6.8 | [111] |
| SMX, SMZ, SDZ, SMM, SDM, SAc * | Milk and Yoghurt | CE-DAD | Liquid–liquid extraction | ≥0.9940 | 4.1–6.3 | 12.9–19.8 | 81.2–106.9 | 5.3–13.7 | [112] |
| Analyte | Sample | Analytical Method | Linearity (R2) | LOD (µg/kg) | LOQ (µg/kg) | Recovery (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Amoxicillin | Egg | SPR * | - | 0.0005 | 0.0019 | 97.50–98.75 | 0.916 | [123] |
| QCM * | - | 0.0023 | 0.0076 | 96.00–99.00 | 1.664 | |||
| Tetracycline | Milk, Honey, Egg, Chicken muscle | N-GQDs@MIPs * | 0.9980, 0.9982, 0.9993, 0.9997 | 1.41, 1.26, 2.97, 2.46 | - | 92.6–111.2 | 4.6 | [124] |
| Milk, Egg, Pork | Mg,N-CDs@MIP * | 0.9976 | 0.79 | - | 78.6–98.7 | 3.3–5.0 | [125] | |
| Norfloxacin | Spiked fish and Milk | MQD-MIPs * | 0.9993 | 0.80 | - | 90.92–111.53 | <7 | [126] |
| Milk | MIP/BPNS-AuNP/GCE * | 0.988 | 0.000003832 | - | 99.36–105.2 | 2.35–5.80 | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, R.; Ramadhanti, S.P.; Amatulloh, A.; Megantara, S.; Subra, L. Recent Advances in the Determination of Veterinary Drug Residues in Food. Foods 2023, 12, 3422. https://doi.org/10.3390/foods12183422
Pratiwi R, Ramadhanti SP, Amatulloh A, Megantara S, Subra L. Recent Advances in the Determination of Veterinary Drug Residues in Food. Foods. 2023; 12(18):3422. https://doi.org/10.3390/foods12183422
Chicago/Turabian StylePratiwi, Rimadani, Shinta Permata Ramadhanti, Asyifa Amatulloh, Sandra Megantara, and Laila Subra. 2023. "Recent Advances in the Determination of Veterinary Drug Residues in Food" Foods 12, no. 18: 3422. https://doi.org/10.3390/foods12183422
APA StylePratiwi, R., Ramadhanti, S. P., Amatulloh, A., Megantara, S., & Subra, L. (2023). Recent Advances in the Determination of Veterinary Drug Residues in Food. Foods, 12(18), 3422. https://doi.org/10.3390/foods12183422





