Co-Encapsulation of Multiple Polyphenols in Plant-Based Milks: Formulation, Gastrointestinal Stability, and Bioaccessibility
Abstract
:1. Introduction
- Protocol 1: All three powdered polyphenols were dissolved in the same alkaline solution, which was then mixed with the soymilk ((CQR)M). This approach has the advantage that multiple polyphenols can be simultaneously encapsulated into the soymilk, thereby simplifying the process;
- Protocol 2: Each powdered polyphenol was dissolved in a different alkaline solution, which was then mixed with the soymilk ((C+Q+R)M)). This approach may be useful in situations where different polyphenols require different pH conditions to be fully dissolved and remain stable;
- Protocol 3: Each powdered polyphenols was dissolved in a different alkaline solution and then loaded into soymilk individually, and then the three different polyphenol-loaded soymilks were combined (CM+QM+RM). This approach may be useful for controlling the ratio of different polyphenols required in a soymilk product, which could be advantageous for personalized nutrition applications.
2. Materials and Methods
2.1. Materials
2.2. Formulations of Multiple Polyphenols Fortified Soymilk
- Crystalline: Crystalline curcumin (C), quercetin (Q), and resveratrol (R) were dispersed in water.
- (C+Q+R)M: Crystalline curcumin (C), quercetin (Q), and resveratrol (R) were individually dissolved in separate alkaline solutions that were then introduced into the soymilk. A NaOH solution of pH 12 was used to prepare all samples in this study.
- (CQR)M: The three nutraceuticals were all dissolved in the same alkaline solution before being introduced into the soymilk.
- CM+QM+RM: Each of the three nutraceuticals was dissolved in a separate alkaline solution and separately loaded into soymilk before the three soymilks were combined together.
2.3. Color and Appearance
2.4. In Vitro Gastrointestinal Model
2.5. Particle Dimensions and Charge
2.6. Encapsulation Efficiency, GIT Stability, and Bioaccessibility of Polyphenols
2.7. Statistical Analysis
3. Results and Discussion
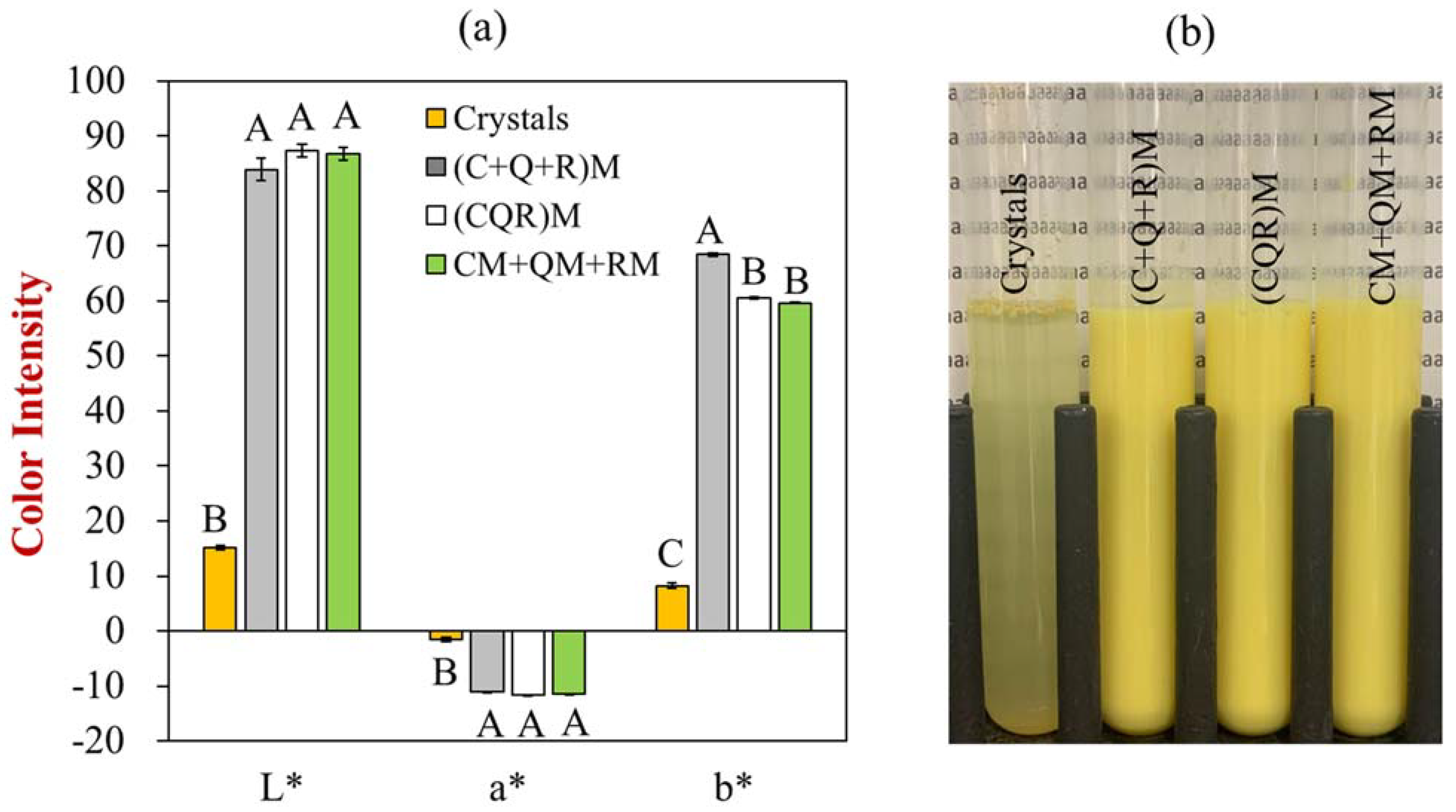
3.1. Color and Appearance of Polyphenol-Fortified Soymilk
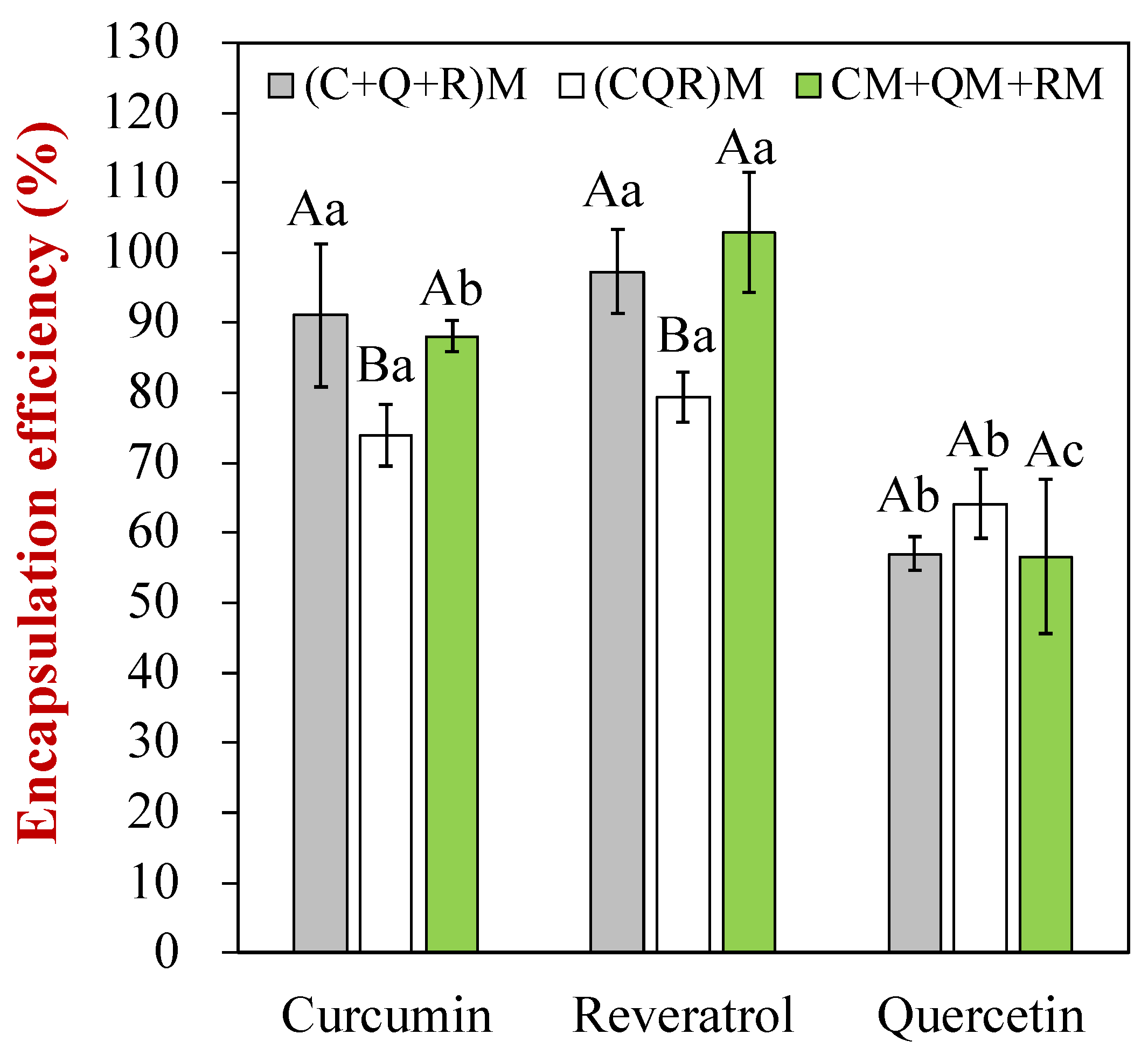
3.2. Encapsulation Efficiency
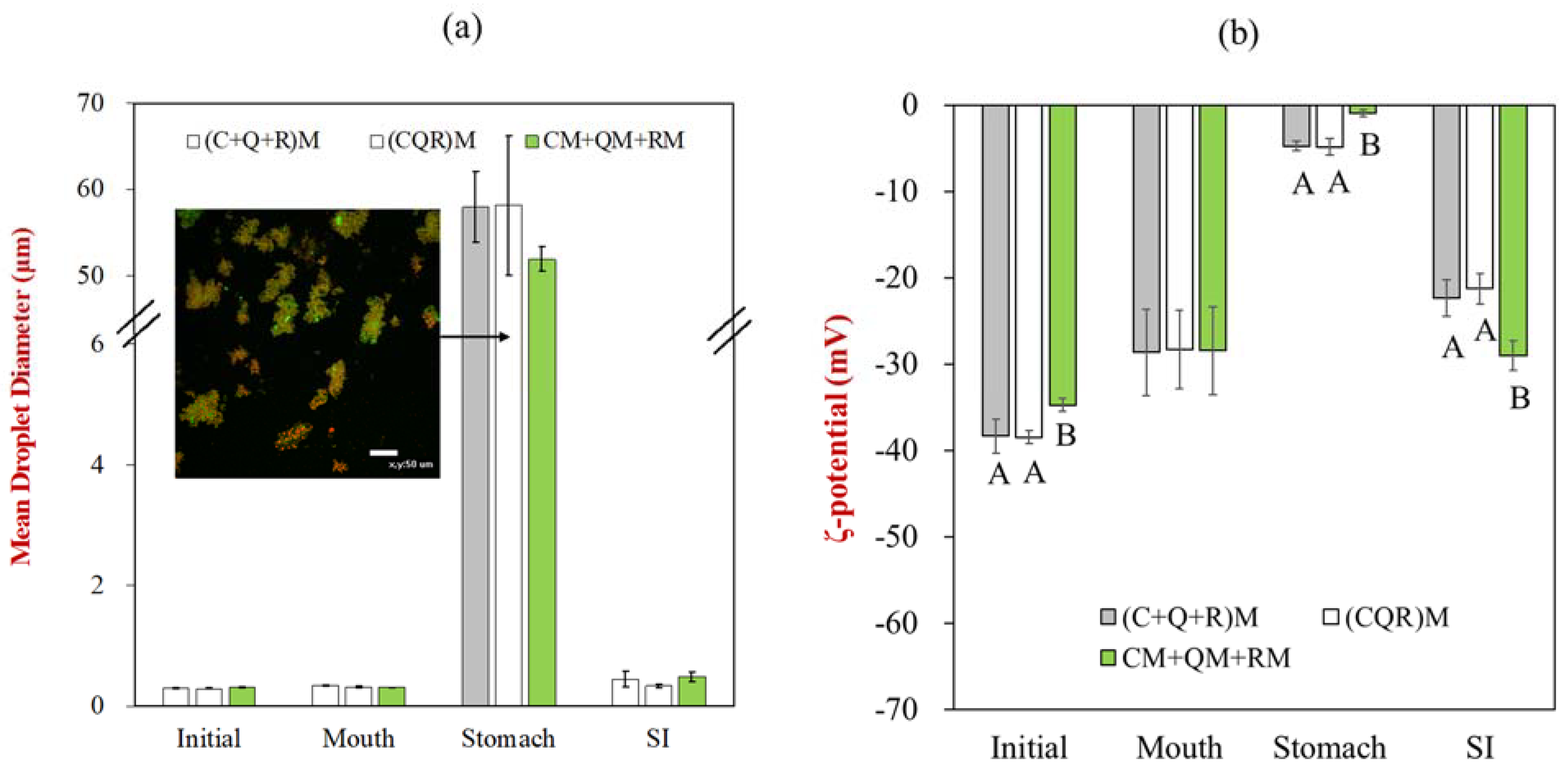
3.3. Particle Size and Zeta Potential
3.4. Gastrointestinal Stability
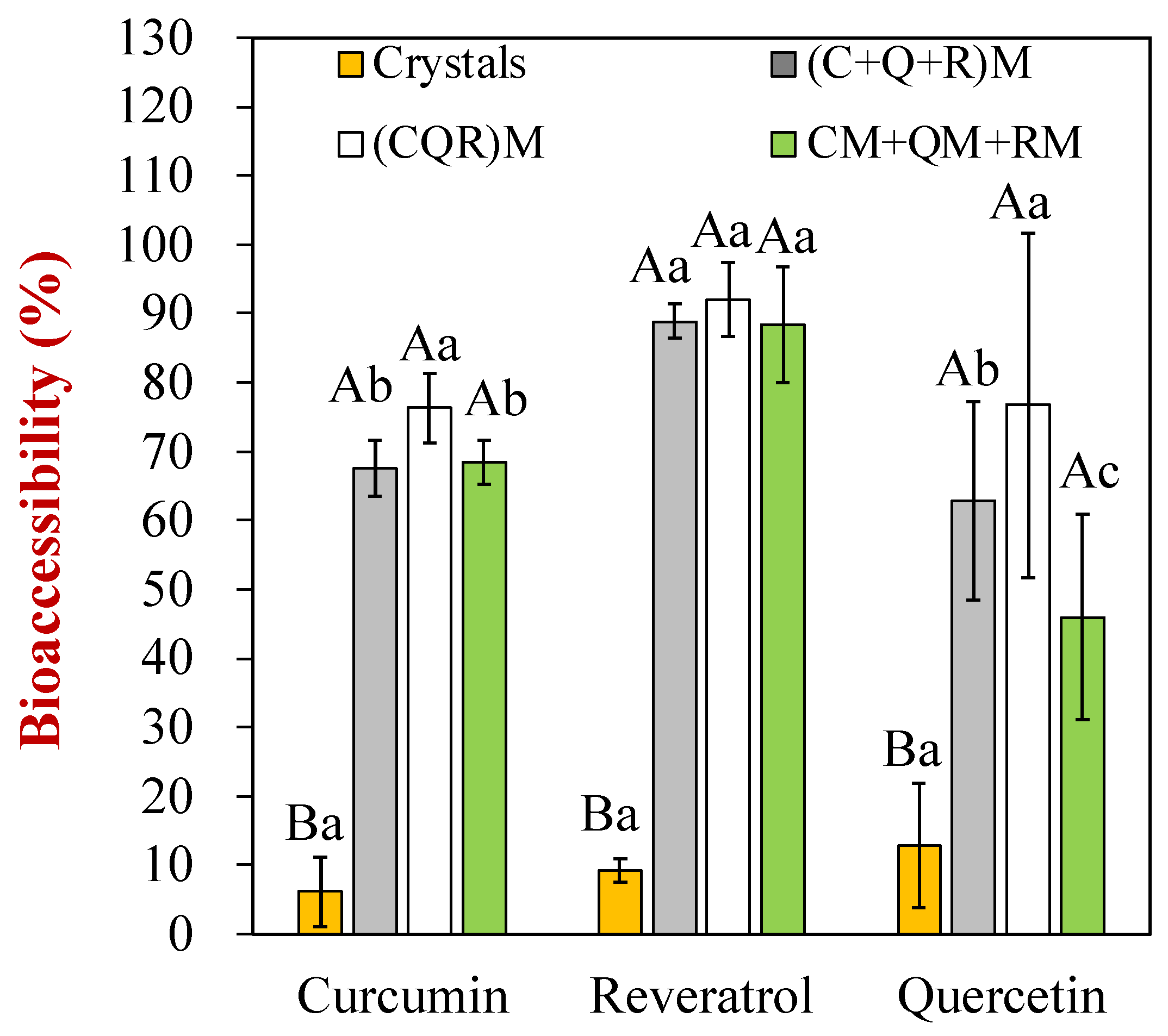
3.5. Bioaccessibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. Advance in Dietary Polyphenols as Aldose Reductases Inhibitors: Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2015, 55, 16–31. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Camp, J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef]
- McClements, D.J. Modeling the rheological properties of plant-based foods: Soft matter physics principles. Sustain. Food Proteins 2023, 1, 101–132. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.-W.; Huang, Q. Enhancing Stability and Oral Bioavailability of Polyphenols Using Nanoemulsions. In Micro/Nanoencapsulation of Active Food Ingredients; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1007, pp. 198–212. [Google Scholar]
- Zheng, B.; Lin, H.; Zhang, X.; McClements, D.J. Fabrication of Curcumin-Loaded Dairy Milks Using the pH-Shift Method: Formation, Stability, and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 12245–12254. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Lin, H.; McClements, D.J. Loading natural emulsions with nutraceuticals using the pH-driven method: Formation & stability of curcumin-loaded soybean oil bodies. Food Funct. 2019, 10, 5473–5484. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Mo, L.; Bai, X.; Wang, H. pH-shifting formation of goat milk casein nanoparticles from insoluble peptide aggregates and encapsulation of curcumin for enhanced dispersibility and bioactivity. LWT 2022, 154, 112753. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Zhou, W.; Liu, W.; Liu, C.; McClements, D.J. Encapsulation of Lipophilic Polyphenols into Nanoliposomes Using pH-Driven Method: Advantages and Disadvantages. J. Agric. Food Chem. 2019, 67, 7506–7511. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, M.; Xu, Y.; Wang, D. Construction of biopolymer-based nanoencapsulation of functional food ingredients using the pH-driven method: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 5724–5738. [Google Scholar] [CrossRef]
- Wang, T.; Wu, J.; Wang, R.; Zhong, Q. Nanostructures self-assembled from food-grade molecules with pH-cycle as functional food ingredients. Trends Food Sci. Technol. 2022, 120, 36–47. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of Curcumin Bioavailability by Encapsulation in Sophorolipid-Coated Nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, L.; Cai, Q.; Zou, L.; Liu, C.; Liu, W.; McClements, D.J. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocoll. 2020, 107, 105963. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Fabrication and Characterization of Curcumin-Loaded Liposomes Formed from Sunflower Lecithin: Impact of Composition and Environmental Stress. J. Agric. Food Chem. 2018, 66, 12421–12430. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ma, M.; Zhang, S.; Wang, D.; Xu, Y. pH-driven self-assembly of alcohol-free curcumin-loaded propylene glycol alginate nanoparticles. Int. J. Biol. Macromol. 2022, 195, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhou, H.; McClements, D.J. Nutraceutical-fortified plant-based milk analogs: Bioaccessibility of curcumin-loaded almond, cashew, coconut, and oat milks. LWT 2021, 147, 111517. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Zhang, J.; Hassane Hamadou, A.; Chen, C.; Xu, B. Encapsulation of phenolic compounds within food-grade carriers and delivery systems by pH-driven method: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 63, 4153–4174. [Google Scholar] [CrossRef]
- Gao, H.; Cheng, C.; Fang, S.; McClements, D.J.; Ma, L.; Chen, X.; Zou, L.; Liang, R.; Liu, W. Study on curcumin encapsulated in whole nutritional food model milk: Effect of fat content, and partitioning situation. J. Funct. Foods 2022, 90, 104990. [Google Scholar] [CrossRef]
- Hajirostamloo, B.; Mahastie, P. Comparison of nutritional and chemical parameters of soymilk and cow milk. World Acad. Sci. Eng. Technol. 2009, 57, 436–438. [Google Scholar]
- Zhou, H.; Zheng, B.; McClements, D.J. Utilization of pH-driven methods to fortify nanoemulsions with multiple polyphenols. Food Sci. Hum. Wellness 2023, in press. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, B.; McClements, D.J. In Vitro Gastrointestinal Stability of Lipophilic Polyphenols is Dependent on their Oil–Water Partitioning in Emulsions: Studies on Curcumin, Resveratrol, and Quercetin. J. Agric. Food Chem. 2021, 69, 3340–3350. [Google Scholar] [CrossRef]
- Liu, W.; Lou, H.; Ritzoulis, C.; Chen, X.; Shen, P.; Lu, Y.; Wu, K.; Dong, L.; Zhu, H.; Han, J. Structural characterization of soybean milk particles during in vitro digestive/non-digestive simulation. LWT 2019, 108, 326–331. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, B.; McClements, D.J. Encapsulation of lipophilic polyphenols in plant-based nanoemulsions: Impact of carrier oil on lipid digestion and curcumin, resveratrol and quercetin bioaccessibility. Food Funct. 2021, 12, 3420–3432. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Zhou, H.; McClements, D.J. Co-Encapsulation of Multiple Polyphenols in Plant-Based Milks: Formulation, Gastrointestinal Stability, and Bioaccessibility. Foods 2023, 12, 3432. https://doi.org/10.3390/foods12183432
Zheng B, Zhou H, McClements DJ. Co-Encapsulation of Multiple Polyphenols in Plant-Based Milks: Formulation, Gastrointestinal Stability, and Bioaccessibility. Foods. 2023; 12(18):3432. https://doi.org/10.3390/foods12183432
Chicago/Turabian StyleZheng, Bingjing, Hualu Zhou, and David Julian McClements. 2023. "Co-Encapsulation of Multiple Polyphenols in Plant-Based Milks: Formulation, Gastrointestinal Stability, and Bioaccessibility" Foods 12, no. 18: 3432. https://doi.org/10.3390/foods12183432





